Abstract
Bacterial wilt, caused by Ralstonia solanacearum species complex, is one of the most serious and widespread diseases across the globe. No single control method has yet been effective against this pathogen despite the use of several control strategies. The best approach to manage this disease is breeding for resistant varieties, which requires the identification of resistant sources and continuous pathogen screening. There is a lack of knowledge about resistance to bacterial wilt in the available pepper germplasm in India, despite the availability of identified molecular markers for marker-assisted selection of resistance against bacterial wilt. The objective of this study was to assess the degrees of resistance of 21 bell pepper genotypes grown in R. solanacearum contaminated field soils by evaluating the disease incidence per genotype and validate the results by PCR using specific SSR primers associated with bacterial wilt resistance, marker CAMS 451. Those genotypes were previously developed through hybridization with bacterial wilt resistant lines. Two parental resistant lines were included as resistant controls and "California Wonder" variety as susceptible control. Sixteen of the genotypes evaluated showed variable high-level resistance evidenced by disease incidences below 13.33%, while four lines were completely resistant with zero disease incidence. The genotypes were confirmed by PCR. Further, the fruit yield data for the mentioned genotypes was assessed under field conditions which showed a heterotic effect in the recombinants over the parental genotypes. In summary, these genotypes could be used as donors in developing new disease-resistant bell pepper varieties which contribute to the availability of resistant bell pepper germplasm to bacterial wilt.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bell pepper (Capsicum annuum L. var. grossum Sendt.), also known as sweet pepper or capsicum, is a Solanaceous vegetable crop cultivated worldwide for its delicious taste, pleasant flavour, wide range of colours and rich nutritional profile (Devi et al. 2015). It is a rich source of vitamins A (370 IU/100 g) and C (99.5 mg/100 g) (USDA 2022) and also has great therapeutic value as it reduces cholesterol, improves blood circulation, strengthens immunity and prevents arthritis (Thakur et al. 2019).
As per the latest data from FAO (2021), China leads the worldwide capsicum production (46%) with 36 million tonnes from 2 million hectares area under its cultivation followed by Mexico, Indonesia and Turkey. In India, 563 thousand tonnes of capsicum is produced from an area of 37 thousand hectares (NHB 2020). Bell pepper cultivation contributes towards the economy of developing countries like India as a source of income as it is labour intensive and offers many employment opportunities in smallholder farms. Wide cultivation of capsicum predisposes the crop towards an extensive spectrum of pathogens including fungi, bacteria and viruses (Dhaliwal 2015).
Ralstonia solanacearum species complex (RSCC), the causative organism of bacterial wilt, is one of the most devastating pathogen in pepper-growing areas resulting in disease incidence varying from 9 to 50% in hot and sweet peppers posing a major constraint in the productivity (Denny 2007; Tan et al. 2014; Aslam et al. 2015). This disease is common in wet tropics, sub-tropics and warm temperate regions (Liao 2005). The pathogen is known to infect more than 450 plant species from 54 families, with Solanaceous crops being its most susceptible hosts (Wicker et al. 2007; Kurabachew and Ayana 2016).
RSCC is highly diversified with five races and six biovars which are distributed over different geographical locations throughout the world (Xue et al. 2011; Chandrashekara et al. 2012; Mamphogoro et al. 2020). The Solanaceous crops are commonly infected by the races 1 and 3, of which race 1 is prevalent in sub-tropical and tropical regions, while race 3 is restricted to temperate regions (Kim-Lee et al. 2005). Based on the ancestral relationship and geographical distribution of the pathogen, these bacterial strains have recently been grouped into four phylotypes (I: Asia, II: America, III: Africa and IV: Indonesia and Australia) (Safni et al. 2014; Prior et al. 2016). In India, four out of six races have been reported in different states with the dominant prevalence of biovar 3 (a member of race 1 and phylotype I) in North Western Himalayan region including Himachal Pradesh (Sinha 1985; Singh et al. 2018) with a disease incidence ranging from 0 to 45% (Aggarwal et al. 2006) leading to a significant loss in the bell pepper productivity in the region.
RSCC being a diverse soil borne pathogen, having broad host range, ability to survive for longer periods in soil, is quite difficult to control despite the use of various physical, cultural, chemical and biological control practices (Mbega et al. 2013). However, the pathogen is able to survive through continuous evolution rendering these strategies less effective. Hence, for sustainable control of this diseases, more plant centric approaches need to be explored. Resistance breeding has emerged as the best alternative for effective and eco-friendly disease management. Many bell pepper accessions including chilli have been reported to carry resistance to bacterial wilt (Devi et al. 2021; Chae et al. 2022). Marker-assisted selection (MAS) can lead to swift development of such elite genotypes with durable resistance against bacterial wilt. However, the information on markers linked with bacterial wilt resistance is scant, limiting the use of MAS in resistance breeding against this disease.
Keeping the aforesaid information in view, the present study was undertaken to assess the bacterial wilt resistance among indigenously developed bell pepper genotypes in naturally contaminated fields and further validate the results by PCR using specific SSR primers.
Materials and methods
Plant material and experimental conditions
The experimental material comprised 24 genotypes including 21 stable recombinants, one susceptible (California Wonder) and two resistant parental genotypes (EC-464107 and EC-464115) as controls (Table 1). The genotypes were evaluated under natural epiphytotic conditions in R. solanacearum (biovar 3 of race 1 and phylotype I) contaminated fields (sick fields) at the Vegetable Research Farm, Department of Vegetable Science and Floriculture, Himachal Pradesh Agricultural University, Palampur, India during the summer-rainy season for 3 consecutive years (2020-2022) (Fig. 1, Supplementary file S1). The genotypes under investigation were indigenously developed at the Department of Vegetable Science and Floriculture through hybridization programmes involving bacterial wilt resistant lines introduced from World Vegetable Center, Taiwan (Devi et al. 2015), followed by rigorous selections in consecutive segregating generations over the years to obtain stable lines.
The nursery was sown in plastic pro-trays and the healthy, disease-free seedlings were transplanted in well prepared sick fields (naturally containing the inoculum in soil) in a completely randomized block design (CRBD) with three replications. In a block, each genotype was planted in two rows consisting of 10 plants in each replication with inter- and intra-row spacing of 60 cm and 45 cm, respectively. One row each of susceptible and resistant parental genotypes (controls) was included at every tenth row to ensure the uniform presence of bacterial wilt disease inoculum in the experimental field. Well-decomposed farmyard manure (FYM) @ 20 tonnes per hectare was applied along with the chemical fertilizers (90 kg N, 75 kg P2O5 and 50 kg K2O per hectare). During anthesis and fruit development, 5 sprays of urea @ 1.5% were given at weekly intervals to the plants to enhance their vegetative growth and vigour. Proper drainage channels were prepared throughout the experimental field to drain excess water during the rainy season.
Sampling and data collection
The data were recorded on all the plants in each replication at weekly intervals and the disease intensity was recorded under sick fields maintained at the farm as per the scale of Winstead and Kelman (1952) (Table 2). To confirm bacterial wilt, all the plants showing wilting symptoms were subjected to a bacterial ooze test. The disease incidence (%) was calculated by using the formula:
Then, from the disease incidence (%), we calculated the plant survival (%) as:
The genotypes were then characterized following the scale of Mew and Ho (1976) (Table 3).
Furthermore, the data for marketable fruit yield per plant (g) were recorded on five competitive plants selected at random from each genotype in each replication (excluding border plants).
Molecular studies
The molecular work involving DNA extraction and further validation of results by PCR was performed in the Molecular Laboratory of the Department of Genetics and Plant Breeding.
DNA extraction
Young leaves of 15 days old seedlings measuring up to 250-500 mg (multiple individuals of same genotype) were pooled for DNA extraction. The genomic DNA was extracted following CTAB method described by Doyle and Doyle (1987) with minor modifications. Leaf tissues were ground to a fine powder in liquid nitrogen and transferred to 1.5 ml Eppendorf tubes containing 750 µl of 2% CTAB extraction buffer. The tubes were incubated at 60 °C for one hour in a water bath and gently shaken after every 15 minutes. Then, 750 µl of chloroform: isoamyl alcohol (24:1) was added to the tubes and they were incubated at 60 °C for 30 minutes. The tubes were centrifuged at 12,000 rpm for 10 minutes. The supernatant (upper aqueous phase) was transferred to a fresh tube and 700 µl of chilled isopropanol was added. The samples were gently mixed and stored overnight at -20 °C. Next day, the tubes were centrifuged at 12,000 rpm for 10 minutes. The supernatant was discarded and the DNA pellets were washed with 700 µl of 70% ethanol and the tubes were centrifuged at 10,000 rpm for 5 minutes. The DNA pellets were allowed to air dry. The pellet was then dissolved in 50 µl of TE buffer and stored at -20 °C. The integrity and quantity of the extracted genomic DNA were determined on 0.8% agarose gel (Invitrogen UltraPureTM Agarose; catalog number 16500500). Approximately 50 ng of DNA was used as working template in PCR.
Selection of primer
Specific SSR primer associated with bacterial wilt resistance (CAMS 451, Forward 5’- TGCATTGGTGGGCTAACATA-3’ and Reverse 5’-GCTCTTGACACAACCCCAAT-3’) was used. The amplicon size for resistant genotypes ranged between 200 and 210 bp, whereas it ranged between 220 and 230 bp for susceptible genotypes (Mathew 2020). Nuclease free water was used to dilute the primer from a concentration of 100 µM to 10 µM.
DNA amplification and PCR reaction
For DNA amplification, each PCR reaction was performed in 0.2 ml PCR tubes containing 12.5 µl reaction mixture (1 µl template DNA, 2.5 µl 5X PCR buffer, 1.25 µl 25 mM MgCl2, 1.25 µl 2 mM dNTPs, 0.5 µl 10 µM forward primer, 0.5 µl 10 µM reverse primer, 0.2 µl Taq DNA polymerase (GeNeiTM Taq DNA Polymerase; catalog number 0601600051730) and 5.3 µl nuclease free water) in a Thermal Cycler (Bio-Rad S1000TM Thermal Cycler). The PCR regimen included initial denaturation for 5 minutes at 94 °C, followed by 35 cycles of 45 seconds at 94 °C, 45 seconds at 62 °C, 45 seconds at 72 °C and a final extension of 5 minutes at 72 °C.
Electrophoretic separation of the amplified PCR products
The amplified PCR products for each sample were separated on 3% agarose gel (Invitrogen UltraPureTM Agarose; catalog number 16500500), stained with 9 µl ethidium bromide (3 µl/100 ml agarose gel) in 0.5X TAE buffer at 80 V for 2 to 3 hours. DNA ladder (GeNeiTM StepUpTM 100 bp DNA ladder ready to use; catalog number 2662670501730) was loaded in the left well of the gel as a marker. The gel was visualized under a Gel Documentation Unit (UVITEC, Cambridge).
Data analysis
The mean values of plant survival (%) and marketable fruit yield per plant (g) obtained from the 24 genotypes were subjected to analysis of variance (ANOVA) using Microsoft Excel Data Analysis tools. Simultaneously, Bartlett’s test was applied for testing homogeneity of variance and validating ANOVA.
Results
Analysis of variance (ANOVA)
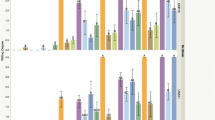
ANOVA revealed that mean sum of squares due to genotypes were significantly different for plant survival (%) as well as marketable fruit yield per plant (g) during all the three years, indicating the availability of sufficient genetic variability (Table 4).
The Bartlett’s test indicated that the variances were homogeneous for both the traits, thus, pooled ANOVA was done (Supplementary file S2). The pooled ANOVA over the years suggested that for both the traits only the mean squares due to genotypes or treatments were significantly different, indicating that the real differences exist among the treatments and were consistent during experiments.
Disease incidence
Screening under field conditions
The disease response of bell pepper genotypes to R. solanacearum is summarized in Table 5. The secretion of milky-white bacterial ooze from the surface of freshly-cut diseased stems confirmed the disease. The symptoms first appeared on the leaves and then progressed to other parts of the plant resulting in complete wilting of the susceptible plants. Brown discolouration in the vascular system of the cut stems was also observed (Fig. 2). On the other hand, there was no wilting in resistant plants.
The screening studies over three years revealed that the test genotypes and resistant controls involved in the study exhibited high degrees of resistance against bacterial wilt ranging from 86.67% to complete immunity, whereas the susceptible genotypes as well as control showed high disease incidence (63.33 to 68.89%). Among the 21 test genotypes, four genotypes (BWT-6-1, BWT-7, BWT-CP and BWT-PBC-631) had zero disease incidence, four genotypes (BWT-3Y-4L, BWT-39-DR, BWT-39-BR and Kandaghat Selection) showed 3.33% disease incidence, seven genotypes (BWT-1, BWT-2-16, BWT-3Y, BWT-3Y-3L, BWT-5Y, BWT-22-HY and BWT-39) exhibited 4.44% disease incidence, four genotypes (BWT-22, BWT-29, BWT-48-AC and BWT-49-AC) had a disease incidence of 7.78% one genotype (BWT-35) had 13.33% disease incidence, whereas the maximum incidence was found in BWT-Belle-1 (63.33%).
Thus, it was observed that all the genotypes except BWT-Belle-1 were phenotypically Resistant (R) against bacterial wilt, whereas BWT-Belle-1 was shown as Susceptible (S) to bacterial wilt.
Screening at molecular level
Molecular studies further validated the phenotypic observation as thermal cycling generated distinct, specific and polymorphic amplification bands in all the genotypes (Fig. 3). Two kinds of amplicons were observed among the genotypes using the SSR marker CAMS 451. Amplicon for the resistant genotypic reaction was observed at ~205-210 bp with resistant parental DNA, whereas the susceptible reaction at ~225-230 bp in susceptible parent. The allele at 205 bp was detected in 20 genotypes viz., BWT-1, BWT-2-16, BWT-3Y, BWT-3Y-4L, BWT-3Y-3L, BWT-5Y, BWT-6-1, BWT-7, BWT-22, BWT-22-HY, BWT-29, BWT-35, BWT-39, BWT-39-DR, BWT-39-BR, BWT-48-AC, Kandaghat Selection, BWT-49-AC, BWT-CP and BWT-PBC-631. These genotypes were characterized as Resistant (R). On the other hand, the allele at 225 bp was detected for only one genotype BWT-Belle-1, thus, characterized as Susceptible (S).
Thus, the observations recorded under field conditions were validated through molecular studies.
Marketable fruit yield per plant (g)
Marketable fruit yield per plant (g) is summarized in Table 6. Over three years, the top yielding genotypes were BWT-39-DR (588.33 g), BWT-39 (583.33 g), BWT-39-BR (570 g), Kandaghat Selection (521.11 g) and BWT-3Y-4L (486.11 g). These top yielding genotypes also exhibited high resistance to bacterial wilt.
Discussion
Capsicum cultivation has become a preferred source of income among the small and marginal farmers in the North Western Himalayan region due to its increasing demand in food industry. But the biotic constraints like bacterial wilt can pose a major bottleneck to its productivity owing to the significant damage caused to the crop. Hence, development of elite cultivars through resistant breeding programmes has emerged as a sustainable approach for combating various abiotic and biotic stresses.
The present study was executed at Palampur (HP, India) which is a mid hill sub-tropical region presenting a niche habitat for the crop as well as pathogen R. solanacearum for effective natural screening of resistant germplasm against bacterial wilt. Further CRBD experimental design was followed to negate errors arousing from the experimental, environmental as well as human interactions so as to have an appropriate idea of the host pathogen interaction leading to the development of disease.
Phenotypic evaluation of the tested genotypes against bacterial wilt revealed that all of them showed significantly high resistance except one i.e., BWT-Belle-1. The results from the present research are in conformity with those presented by Sood and Thakur (2017), Anuradha and Sood (2019) and Thakur et al. (2019) who evaluated bell pepper genotypes to assess bacterial wilt resistance in their respective studies and observed that most of the genotypes taken for the study were resistant or moderately resistant and can be used in breeding programmes as donors. Similarly, Dhillon et al. (2021) reported resistance for bacterial wilt in the crosses DPBWRC-6-1 × EC-464107, DPBWRC-1 × EC-464115, DPBWRC-29 × EC-464107 and DPBWRC-6-1 × DPBWRC-29. The study involved the same resistant genotypes as in the present investigation. Such high level disease resistance among the capsicum genotypes under field conditions may be attributed to the genetic architecture (from the resistant parental genotypes) and the genotype × environment (G × E) interactions during the cultivation period (Ganiyu et al. 2017; Guji et al. 2019).
Phenotypic evaluation was further validated by genotypic screening through PCR based amplification with a specific disease linked SSR marker CAMS 451 showing specific amplification in all the resistant as well as susceptible genotypes. The results of molecular screening are in concordance with those of Mathew (2020), who found the similar observations in six pepper varieties with SSR marker CAMS 451, showing the importance of MAS in durable crop improvement programmes. The robust nature of SSR marker CAMS 451 was also established during the investigation after repeating the experiments.
Moreover, the aim behind any crop improvement programme is to develop elite cultivars with high yields along with disease resistance. Hence, all the genotypes in this study were evaluated for their respective marketable fruit yields. Five genotypes viz., BWT-39-DR, BWT-39, BWT-39-BR, Kandaghat Selection and BWT-3Y-4L were observed to be the top yielding genotypes and produced higher yields than their respective resistant as well as susceptible parents thereby showing heterosis after hybridization. Similar results have been reported by Anuradha and Sood (2019), Thakur et al. (2019) and Dhillon et al. (2021) in their respective studies involving the similar genotypes as in this investigation. Such heterosis can be attributed to G × E interactions, soil conditions, plant genetic architecture and growing conditions (Ganiyu et al. 2017; Guji et al. 2019).
The results in the present investigation are quite encouraging showing the development of bacterial wilt resistant cultivars with higher yields. These high yielding genotypes can be released as varieties after multi-location trials to encourage their cultivation among the farmers. These can also be used as novel donor germplasm in future capsicum improvement programmes for sustainable disease management.
Data availability
All relevant data are within the paper and supplied as supplementary files along with the manuscript.
References
Aggarwal P, Sood AK, Kumar P (2006) Status of bacterial wilt of solanaceous vegetable crops in Himachal Pradesh. Indian Phytopath 59:231–233
Anuradha, Sood S (2019) Genetic assessment for fruit yield and horticultural traits in bacterial wilt tolerant genotypes of bell pepper (Capsicum annuum L. var. grossum Sendt.). Int J Curr Microbiol Appl Sci 8:872-888. https://doi.org/10.20546/ijcmas.2019.808.101
Aslam MN, Mukhtar T, Ashfaq M, Asad MJ, Hussain MA (2015) Incidence and prevalence of bacterial wilt of chili in Punjab, Pakistan. Mycopath 13:37–41
Chae SY, Lee K, Do JW, Hong SC, Lee KH, Cho MC, Yang EY, Yoon JB (2022) QTL mapping of resistance to bacterial wilt in pepper plants (Capsicum annuum) using genotyping-by-sequencing (GBS). Horticulturae 8:115. https://doi.org/10.3390/horticulturae8020115
Chandrashekara KN, Prasannakumar MK, Deepa M, Vani A (2012) A rapid, sensitive and reliable method for detecting Ralstonia solanacearum using fta (whatman) card. J Plant Pathol 94:219–221. https://doi.org/10.4454/jpp.fa.2012.012
Denny T (2007) Plant pathogenic Ralstonia species. In: Gnanamanickam SS (ed) Plant-associated bacteria. Springer, Dordrecht, pp 573-644. https://doi.org/10.1007/978-1-4020-4538-7_16
Devi J, Sagar V, Kaswan V, Ranjan JK, Kumar R, Mishra GP, Dubey RK, Verma RK (2021) Advances in breeding strategies of bell pepper (Capsicum annuum L. var. grossum Sendt.). In: Al-Khayri JM, Jain SM, Johnson DV (ed) Advances in plant breeding strategies: vegetable crops. Springer, Cham, pp 3-58. https://doi.org/10.1007/978-3-030-66961-4_1
Devi J, Sood S, Vidyasagar Singh Y (2015) Inheritance of bacterial wilt resistance and performance of horticultural traits in bell pepper (Capsicum annuum var. grossum). Indian J Agric Sci 85:1498–1503
Dhaliwal MS (2015) Breeding vegetables for biotic stress resistance. Horticulture for inclusive growth, vol 2. Crop Production. Westville Publishing House, Delhi, pp 478–491
Dhillon HK, Sood S, Sood VK, Chahota RK, Rana S (2021) Combining ability and gene action analysis of some bacterial wilt resistant intraspecific hybrids of bell pepper (Capsicum annuum var. grossum). Electron J Plant Breed 12:1413–1421. https://doi.org/10.37992/2021.1204.193
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
FAO (2021) Food and Agriculture Organization Corporate Statistical Database (FAOSTAT). https://www.fao.org/faostat/en/#data/QCL/visualize. Accessed 22 Feb 2023
Ganiyu SA, Popoola AR, Enikuomehin OA, Bodunde JG, Adedibu OB, Gurama AU (2017) Assessment of resistance status of some tomato genotypes to bacterial wilt disease and evaluation of SNP marker (LEOH19) for selection of BW resistant gene. Niger J Biotechnol 34:54–64. https://doi.org/10.4314/njb.v34i1.8
Guji MJ, Yetayew HT, Kidanu ED (2019) Yield loss of ginger (Zingiber officinale) due to bacterial wilt (Ralstonia solanacearum) in different wilt management systems in Ethiopia. Agric and Food Secur 8:1–11. https://doi.org/10.1186/s40066-018-0245-6
Kim-Lee H, Moon JS, Hong YJ, Kim MS, Cho HM (2005) Bacterial wilt resistance in the progenies of the fusion hybrids between haploid of potato and Solanum commersonii. Am J Potato Res. 82:129–137. https://doi.org/10.1007/BF02853650
Kurabachew H, Ayana G (2016) Bacterial wilt caused by Ralstonia solanacearum in Ethiopia: status and management approaches: a review. Int J Phytopathol 5:107–119. https://doi.org/10.33687/phytopath.005.03.1829
Liao B (2005) A broad review and perspective on breeding for resistance to bacterial wilt. In: Allen C, Prior P, Hayward AC (eds) Bacterial wilt disease and the Ralstonia solanacearum species complex. APS Press, St. Paul, MN, pp 225–238
Mamphogoro TP, Babalola OO, Aiyegoro OA (2020) Sustainable management strategies for bacterial wilt of sweet peppers (Capsicum annuum) and other solanaceous crops. J Appl Microbiol 129:496–508. https://doi.org/10.1111/jam.14653
Mathew D (2020) Analysis of QTL Bw1 and marker CAMS451 associated with the bacterial wilt resistance in hot pepper (Capsicum annuum L.). Plant Gene 24:100260. https://doi.org/10.1016/j.plgene.2020.100260
Mbega ER, Adriko J, Mortensen CN, Wulff EG, Lund OS, Mabagala RB (2013) Improved sample preparation for PCR-based assays in the detection of Xanthomonads causing bacterial leaf spot of tomato. Biotechnol J Int 3:556–574. https://doi.org/10.9734/BBJ/2013/3810
Mew TW, Ho WC (1976) Varietal resistance to bacterial wilt in tomato. Plant Disease Reporter 60:264–268
NHB (2020) Area and Production of Horticulture Crops for 2020-21 (Final Estimates). National Horticulture Board, Ministry of Agriculture and Farmers Welfare, Government of India, Gurugram, Haryana, India. http://agricoop.gov.in/en/StatHortEst#. Accessed 22 Feb 2023
Prior P, Ailloud F, Dalsing BL, Remenant B, Sanchez B, Allen C (2016) Genomic and proteomic evidence supporting the division of the plant pathogen Ralstonia solanacearum into three species. BMC Genom 17:90. https://doi.org/10.1186/s12864-016-2413-z
Safni I, Cleenwerck I, De Vos P, Fegan M, Sly L, Kappler U (2014) Polyphasic taxonomic revision of the Ralstonia solanacearum species complex: proposal to emend the descriptions of Ralstonia solanacearum and Ralstonia syzygii and reclassify current R. syzygii strains as Ralstonia syzygii subsp. syzygii subsp. nov., R. solanacearum phylotype IV strains as Ralstonia syzygii subsp. indonesiensis subsp. nov., banana blood disease bacterium strains as Ralstonia syzygii subsp. celebesensis subsp. nov. and R. solanacearum phylotype I and III strains as Ralstonia pseudosolanacearum sp. nov. Int J Syst Evol Microbiol 64:3087–3103. https://doi.org/10.1099/ijs.0.066712-0
Singh D, Chaudhary G, Yadav DK (2018) Characterisation and diversity of Indian isolates of Ralstonia solanacearum causing bacterial wilt of Capsicum annuum L. Arch Phytopathol Plant Prot 51:267–276. https://doi.org/10.1080/03235408.2018.1467620
Sinha SK (1985) Bacterial wilt disease in Asia and the South pacific. In: Persley GJ (ed) ACIAR Proceedings No. 13, pp 28-29
Sood S, Thakur M (2017) Screening for bacterial wilt resistance of bell pepper under sick field conditions and morphological characterization. J Hill Agric 8:442–448. https://doi.org/10.5958/2230-7338.2017.00087.8
Tan QQ, Yuan J, Yang XH, Chen X, Wang LS, Wu SP (2014) Identification of resistance to Phytophthora blight and bacterial wilt in pepper varieties in Guizhou Province Regional Trial. Seed 33:82–85
Thakur M, Sood S, Gupta N (2019) Genetic analysis of quantitative and quality traits in bacterial wilt resistant genotypes of bell pepper under sub-temperate conditions of North-Western Himalayas. J Pharmacogn Phytochem 1:66–73
USDA (2022) U.S. Department of Agriculture, Agricultural Research Service. FoodData Central, https://fdc.nal.usda.gov/fdc-app.html#/food-details/2258588/nutrients. Accessed 22 Feb 2023
Wicker E, Grassart L, Coranson-Beaudu R, Mian D, Guilbaud C, Fegan M, Prior P (2007) Ralstonia solanacearum strains from Martinique (French West Indies) exhibiting a new pathogenic potential. Appl Environ Microbiol 73:6790–6801. https://doi.org/10.1128/AEM.00841-07
Winstead NN, Kelman A (1952) Inoculation techniques for evaluating resistance to Pseudomonas solanacearum. Phytopathology 42:628–634
Xue QY, Yin YN, Yang W, Heuer H, Prior P, Guo JH, Smalla K (2011) Genetic diversity of Ralstonia solanacearum strains from China assessed by PCR-based fingerprints to unravel host plant- and site-dependent distribution patterns. FEMS Microbiol Ecol 75:507–519. https://doi.org/10.1111/j.1574-6941.2010.01026.x
Acknowledgements
The authors are grateful to the Department of Vegetable Science and Floriculture, HPAU, Palampur for providing the bell pepper germplasm for conducting the present investigation. The authors also acknowledge the Department of Genetics and Plant Breeding, HPAU, Palampur for providing the facilities for carrying out the molecular studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sood, T., Sood, S., Sood, V. et al. Assessment and validation of resistance to bacterial wilt (Ralstonia solanacearum) through field and molecular studies in bell pepper. J Plant Pathol 105, 849–857 (2023). https://doi.org/10.1007/s42161-023-01378-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42161-023-01378-1