Abstract
The inhibition effects of ten eugenol derivatives against Fusarium graminearum Q1 strain were determined using the mycelia growth rate process. Our results revealed that the toxicities of various derivatives differed substantially as compared to that of eugenol. Isoeugenol had the highest toxicity, about 4.2 times that of eugenol, with the EC50 value of 279.7 µM. Based on the toxicity ratio (TR) and synergistic coefficient (SR), the optimum mixing mass ratios of isoeugenol to phenamacril and isoeugenol to tebuconazole against F. graminearum were 7:1 and 6:1, respectively. Based on their additive effects, these mixtures could be used as potential alternatives to control F. graminearum resistance against phenamacril and tebuconazole due to different mechanism of action.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fusarium head blight of wheat (FHB) caused by Fusarium graminearum is a devastating disease of cereal crops worldwide (Bai and Shaner 2004; Goswami and Kistler 2004). In addition to reducing grain yield and quality, F. graminearum produces harmful mycotoxins in infected grain (Sutton 1982; Bottalico 1998; Varga et al. 2015). The most favourable scenario to avoid deoxynivalenol (DON) contamination (ploughing, moderately resistant variety, triazole application at heading) reduced the DON content by 97% compared to the worst one (direct sowing, susceptible variety, no fungicide application) (Blandino et al. 2012). The most successful single measure was tested triazole fungicides, applied at the time of wheat anthesis, which lowered DON content to 53.4 percent than the grain harvested from untreated control plots (Beyer et al. 2006). Phenamacril (2-cyano-3-amino-3-phenylancryic acetate, JS399-19) and tebuconazole were the two most effective fungicides being used. Phenamacril could reduce both the FHB index and mycotoxin level by 80% (Chen and Zhou 2009; Zhang et al. 2010). Phenamacril is a novel cyanoacrylate fungicide introduced by Jiangsu Branch of China National Pesticide Research & Department South Center. This fungicide was demonstrated to have activity against Fusarium spp, especially against F. graminearum, and had reasonable control of FHB in the field (Li et al. 2008). So far, registered phenamacril fungicidal products including 15% SC and 25% SC in China to control FHB, rice bakanae disease and strawberry fusarium wilt (http://www.chinapesticide.org.cn/hysj/index.jhtml). FRAC has given phenamacril a separate classification code B6 for the mechanism of action and resistance risk in 2015 (FRAC 2020). Tebuconazole has played an essential role in the management of FHB for more than ten years (Mesterhazy et al. 2003; Muellenborn et al. 2008), and its EC50 value against F. graminearum was 0.41828 mg/L (Lei et al. 2018). However, long-term application has resulted in poor effectiveness against FHB (Chen et al. 2008), and the mechanism of resistance to phenamacril and tebuconazole in F. graminearum revealed the risks of continuing applying them. Mutations in myosin-5 conferred phenamacril resistance in F. graminearum, and tebuconazole resistance was linked to the fungal sterol 14α-demethylase (CYP51) gene, and a Tyr137 amino acid mutation in the FgCYP51B gene could contribute to tebuconazole resistance (Zheng et al. 2015; Qian et al. 2018).
Syzygium aromaticum has the highest antifungal effect among 17 medicine plants against 8 tested phytopathogenic fungi, including Rhizoctonia cerealis, F. graminearum, Gaeumannomyces graminis, F. oxysporum f. sp. vasinfectum, Valsa mali, Colletotrichum gloeosporioides, F. oxysporum f. sp. cucumebrium and Colletotrichum lagenarium (Yang et al. 2020). Eugenol (4-allyl-2-methoxyphenol) as an active substance in S. aromaticum was traced and isolated using sensitive indicator F. graminearum, with EC50 value of 190.58 mg/L (Yang et al. 2020). Eugenol contains three functional groups: phenolic hydroxyl, allyl, and methoxy. The antifungal activities of eugenol and its analogues against phytopathogenic and human fungi were reported, and their structure–activity relationships were observed that activities were the influence of C4-allyl, C1-OH, and C2-OCH3, or one/two NO2 in various positions of the phenyl ring (Carrasco et al. 2012). The activities of a series of phenylpropanoids derived from eugenol against mycelial growth of a virulent and multi-resistant isolate of Botrytis cinerea were strongly related to their chemical structures, and increasing activity has been obtained by isomerization of the double bond or introduction of a nitro group on the aromatic ring (Olea et al. 2019).
The purpose of this study was to identify eugenol derivatives with higher toxicity against F. graminearum and mix them with phenamacril or tebuconazole. Currently, phenamacril and tebuconazole are the most effective fungicides for controlling FHB in China. The optimal mixtures screened out could be used as potential alternatives to regulate F. graminearum resistance against phenamacril or tebuconazole, as well as improve FHB control efficiency.
Materials and methods
Eugenol and its derivatives
Eugenol and its ten derivatives were listed in Table 1.
Fungicides used for mixing
98.5% phenamacril TC, supplied by the Pesticide Laboratory, College of Plant Health and Medicine, Qingdao Agricultural University, China. 97.2% tebuconazole TC, supplied by Wuhu Duowei Agricultural Chemical Co. LTD.
Origin of F. graminearum
The tested F. graminearum Q1 strain was isolated from the infected wheat plant in 2016, subcultured and grown at potato dextrose agar (PDA) medium in 4°C at the Pesticide Laboratory, College of Plant Health and Medicine, Qingdao Agricultural University, China.
Toxicity determination of compounds
Different doses of each compound dissolved in 100 µL of dimethyl formamide (DMF) were added to 30 mL of sterilized melted PDA medium to obtain desired concentration. The concentration gradients of each compound were set as follows: eugenol (2436, 1218, 609, 305, and 153 µM), isoeugenol (609, 305, 153, 77, and 39 µM), 4-ethyl-2-methoxyphenol(657, 328, 164, 82, and 41 µM), 2-methoxyphenol (6444, 3222, 1611, 805, and 403 µM), 2-methoxy-4-vinylphenol (3996, 2664, 1332, 666, and 333 µM), 4-bromo-2-methoxyphenol (985, 492, 246, 123, and 62 µM), 2-methoxyl-5-nitrophenol (1182, 591, 296, 148, and 74 µM), methyleugenol (2244, 1122, 560, 280, and 140 µM), eugenol acetate (970, 485, 242, 141, and 70 µM), phenol (4255, 2128, 1064, 832, and 416 µM), 2-allylphenol (745, 372, 186, 93, and 47 µM), phenamacril (15.4, 7.7, 3.8, 1.9, and 1.0 µM) and tebuconazole (5.2, 2.6, 1.3, 0.6, and 0.3 µM). These melted PDA were poured into Petri dishes at 10 mL per dish, with three replicates. Parallel control was maintained to mix 100 µL of DMF with PDA medium. At the center of each Petri plate fungal mycelia block (0.6 cm in diameter) was inoculated, then incubated at 27 ± 2 °C in the dark. All colony diameters were measured when the control colony had almost covered the Petri dishes for 4 days after inoculation. Colony growth inhibition rate was calculated from the formula: Colony growth inhibition rate (%) = [(DC-DT) / (DC-0.6)] × 100, where DC and DT were average diameters of control and treatment colonies, respectively. The concentrations of all tested compounds inhibiting fungal colony growth by 50% (EC50) on F. graminearum were calculated using probit analysis.
Mixed ratio screening of the highly active eugenol derivative with fungicides
The highly active eugenol derivative against F. graminearum was screened out based on the EC50 value. Then, the mixture of a highly active derivative with phenamacril or tebuconazole was further studied to inhibit F. graminearum.
The optimum mixing ratio was screened by cross-determination method (Wu and Si 2006). Based on the EC50 values of the selected highly active derivative, phenamacril and tebuconazole, 11 concentration gradients of each mixing combination (the highly active derivative and phenamacril or the highly active derivative and tebuconazole) were set, and the volume ratio of two single ingredient solution in each concentration gradient was 10:0; 9:1; 8:2; 7:3; 6:4; 5:5; 4:6; 3:7; 2:8; 1:9 and 0:10 (each approximate EC50 was 100%, and the mass ratio of in different volume proportions could be calculated), respectively. Antifungal activities were tested as mentioned above. The actual inhibition rate (AIR), theoretical inhibition rate (TIR) and toxicity ratio (TR) were calculated according to the following formula: TIR = AIR of highly active derivative EC50 × volume ratio of highly active derivative + AIR of phenamacril or tebuconazole × volume ratio of phenamacril or tebuconazole. TR = AIR/TIR (TR > 1.25, synergistic effect; TR < 0.75, antagonistic effect; TR≈1.00, additive effect) (Wu and Si 2006).
EC50 and synergistic coefficient determination of optimum mixtures
Based on TR, the optimum ratio of the highly active eugenol derivative to phenamacril or to tebuconazole was screened out, and its EC50 value was determinated and synergistic coefficient (SR) was calculated by Wadley method (SR < 0.5, antagonistic effect; 0.5 ≤ SR ≤ 1.5, additive effect; SR > 1.5, synergistic effect). SR = EC50(th)/EC50(ob), EC50(th) = (a + b)/(a/EC(A)50 + b/EC(B)50), Here, A and B are the single compound; a and b are the proportions of the corresponding compound in the mixture; EC50(th) is the theoretical EC50 value of mixture; and EC50(ob) is the measured EC50 value of mixture (Lei et al. 2018).
Statistical analysis
Data were subjected to statistical analysis of variance (ANOVA) using IBM SPSS Statistics Software, ver. 20. Duncan test was used to analyze the significant differences among treatments at P < 0.05.
Results
Antifungal activity of eugenol and its derivatives against F. graminearum
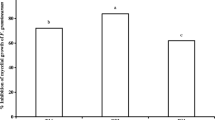
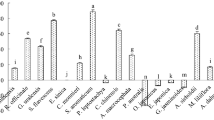
According to EC50, the toxicities of derivatives against F. graminearum were stronger than that of eugenol except for 2-methoxy-4-vinylphenol, phenol and 2-methoxyphenol (Table 2). The toxicity of isoeugenol was about 4.2 times higher than that of eugenol, with an EC50 value of 279.7 µM. The EC50 values of 2-methoxy-5-nitrophenol, methyleugenol, 4-ethyl-2-methoxyphenol, 4-bromo-2-methoxyphenol, eugenol acetate and 2-allylphenol were 758.9 µM, 539.6 µM, 508.0 µM, 471.0 µM, 467.0 µM and 395.7 µM, respectively, about 1.5 ~ 3.0 times higher than that of eugenol. The EC50 value of eugenol was 1163.9 µM, and the EC95 value was 9427.0 µM, indicating that the sensitivity of F. graminearum to eugenol was far lower than their derivatives. Based on the antifungal activity, isoeugenol was screened out for mixing with phenamacril or tebuconazole for FHB control.
Toxicity of two fungicides against F. graminearum
Phenamacril and tebuconazole are the two most effective fungicides used to regulate FHB in China at present, which had potent toxicity in vitro against F. graminearum, with EC50 values 3.194 µM and 2.749 µM, respectively (Table 3).
Screened mixing ratios of isoeugenol with two fungicides by TR
According to TR, the EC50 volume ratios of 3:7, 2:8 and 1:9 of isoeugenol to phenamacril all had additive effects on F. graminearum, but the other ratios performed antagonistic effects with TR < 0.75 (Table 4). When the EC50 volume ratio was 2:8 (mass ratio: 16:1), it had the maximum TR of 0.94. But the volume ratio of 1:9 (mass ratio of 7:1) had the highest inhibition rate of 53.76%, and the TR was 0.93. There was no significant difference in TR between EC50 volume ratio of 2:8 and 1:9. Therefore, the toxicities of both ratios of isoeugenol to phenamacril would be measured simultaneously to determine the final mixing ratio.
Based on TR, all EC50 volume ratios of isoeugenol to tebuconazole had additive effects on F. graminearum, with TR of 1.08 ~ 1.12 (Table 5). All TR had no significant difference, but the volume ratio of 4:6 (mass ratio of 35:1) had the maximum TR of 1.12, and the volume ratio of 1:9 (mass ratio of 6:1) had the highest actual inhibition rate 67.71%. Similarly, the toxicities of both volume ratios of 4:6 and 1:9 of isoeugenol to tebuconazole would be measured simultaneously to determine the final mixing ratio.
Determined optimal mixing ratios of isoeugenol with fungicides
All mixture ratios screened by TR were further tested for toxicity, and SR was calculated. For mixtures of isoeugenol to phenamacril, volume ratio of 2:8 (mass ratio of 16:1) had the EC50 value of 61.96 µM and SR of 0.92, and volume ratio of 1:9 (mass ratio of 7:1) had the EC50 value of 26.71 µM and SR of 1.10 (Table 6). Therefore, the optimum ratio of isoeugenol to phenamacril against F. graminearum was mass ratio of 7:1.
As well, for mixtures of isoeugenol to tebuconazole, volume ratio of 1:9 (mass ratio of 6:1) had the EC50 value of 48.74 µM and SR of 0.62, and volume ratio of 4:6 (mass ratio of 35:1) had the EC50 value of 228.63 µM and SR of 0.48 (Table 6). So, the optimum ratio of isoeugenol to tebuconazole against F. graminearum was mass ratio of 6:1 (Supplementary material).
Discussion
Eugenol isolated from plants, was environmentally safe and easily biodegradable, and generally assumed to be more acceptable and less hazardous (Romanazzi 2012). Regarding eugenol, its concentration that causes no toxicological effects in rats is 500 mg/kg of body weight which is in contrast with 2.5 mg/kg of body weight as estimation of temporary acceptable daily intake for humans (Campaniello et al. 2010). Therefore, eugenol may be an ideal alternative to synthetic fungicides as an eco-friendly pesticide for plant disease control (Zaker 2016). Eugenol has a broad-spectrum of activities against plant pathogenic fungi, especially to F. graminearum with the EC50 value of 190.58 mg/L (Yang et al. 2020), and its derivatives had significantly different activities against F. graminearum.
The antifungal activity of eugenol was attributed to the destruction of membrane structure, which accumulated to the phospholipid bilayer through its lipotropism, interacted to change the fluidity and penetrability of the fungal membrane, and affected the activity of membrane binding enzymes or proteins (Gill and Holley 2006; Braga et al. 2007; Wang et al. 2010; Campaniello et al. 2010). Isoeugenol, as a double-bond isomer of eugenol, had almost no effect on its lipophilicity. However, this small structural modification increased the toxicity on F. graminearum by about 3.2 times by conjugation of the side chain of the double bond with aromatic system, and other possible mechanisms must exist. A possible explanation was the enhanced Michael-type reaction between the side propenyl chain and biological nucleophiles (Olea et al. 2019). The increase of 4-ethyl-2-methoxyphenol toxicity may also be consistent with this mechanism. When allyl was replaced by ethyl, the lipophilicity of side-chain increased, and the destruction of membrane structure was enhanced.
The compound position and orientation in the membrane were determined by the chemical structure of the adsorbed molecule. Compared with eugenol, 2-allylphenol was not conducive to the insertion of phospholipid bilayer into the membrane, but its toxicity to F. graminearum was increased by 1.9 times. This perhaps related to the metabolism of more active antifungal compounds from 2-allylphenol. Four hydroxylation metabolism products of 2-allylphenol were isolated and identified from its metabolites of Rhzoctonia solani (Qu et al. 2008). Among them, the activity of 2-(2-hydroxypropyl) phenol was about 2 times of matrix structure. Therefore, the inhibition effect of 2-allylphenol on R. solani did not decrease with the increase of drug degradation rate between 0 and 144 h (Qu et al. 2008).
Considering that the polarity of nitro compounds is higher than that of related eugenol derivatives, the antifungal activity of these compounds, associated to lipophilic concentration in the membrane, should be lower than that of eugenol. But the toxicity of 4-bromo-2-methoxyphenol and 2-methoxy-5-nitrophenol against F. graminearum was 2.5 and 1.5 times than that of eugenol, respectively. Therefore, it was speculated that the nitro group may act directly on the double bond of the membrane as a strong electron-withdrawing group. Pal and Bandyopadhyay (2012) found that compounds containing nitro aromatic ring could produce reactive oxygen species (ROS) by an enzymatic process. Olea et al. (2019) further confirmed that production of ROS might be the primary mechanism, and production of ROS was consistent with activity.
Based on the existing studies, the antifungal effects of eugenol and its derivatives may be two different or parallel mechanisms: accumulation in the fungal membrane by lipophilic interaction and Michael-type reactions between eugenol derivatives and membrane components or ROS production by enzymatic reduction of nitro compounds (Wang et al. 2010; Marchese et al. 2017; Olea et al. 2019). Among all tested eugenol derivatives, isoeugenol had the hightest toxicity to F. graminearum, with the EC50 value of 279.7 µM. However, the mixtures of isoeugenol and phenamacril or isoeugenol and tebuconazole did not produce synergistic effects. The optimum mass ratios of isoeugenol to phenamacril or tebuconazole against F. graminearum were 7:1 (EC50 value of 26.71 µM and SR of 1.10) and 6:1 (EC50 value of 48.74 µM and SR of 0.62), respectively, and both them were additive effects. Nevertheless, isoeugenol is a natural product (Maurizio et al. 2020), and its mechanism of action was different with that of phenamacril and tebuconazole. Isoeugenol worked by destructing membrane structure and enhancing reaction between the side chain and the biological nucleophile (Olea et al. 2019). Phenamacril acted on cytoskeleton and motor protein, presumed to be a myosin-5 inhibitor. Myosin-5 is an actin-dependent ATPase motor in F. graminearum, and the mutations of myosin-5 might lead to the change in the expression of itself or other corresponding genes in the resistant strain (Zheng et al. 2015). Resistance to phenamacril in F graminearum had medium to high resistance risk (FRAC 2020). Tebuconazole inhibited sterol biosynthesis in membranes, with medium resistance risk (FRAC 2020). The Y137H mutation in the cytochrome P450 FgCYP51B protein conferred reduced sensitivity to tebuconazole in F. graminearum (Qian et al. 2018).
Globally, FHB was the most severe and hazardous floral disease of wheat (Dweba et al. 2017), and accumulated various mycotoxins in the grain (Varga et al. 2015). Chemical fungicides were still one of the most effective means to control it. Benzimidazole fungicides as carbendazol, and triazole fungicides as tebuconazole and phenamacril were mainly adopted, but F. graminearum had appeared serious resistance (Liu et al. 2014; Chen and Zhou 2009). Therefore, although the mixture of isoeugenol and phenamacril or tebuconazole only showed an additive effect, it could effectively control the generation and aggravation of resistance due to different mechanisms.
Conclusions
In vitro, the inhibitory activities of eugenol and its ten derivatives against F. graminearum were significantly different, and isoeugenol showed the highest toxicity with the EC50 value of 279.7 µM, which was about 4.2 times than that of eugenol. The toxicity differences among eugenol and its derivatives may be due to the dose accumulated in the cell membrane by lipophilic effect, and the ability to react with membrane components by nucleophilic reaction or to induce ROS production. The optimum mixing mass ratios of isoeugenol to phenamacril and isoeugenol to tebuconazole against F. graminearum were 7:1 and 6:1, respectively.
Based on different fungistatic mechanisms of isoeugenol, phenamacril and tebuconazole, and their additive effects, these mixtures could be used as the potential alternatives for the FHB control.
References
Bai GH, Shaner G (2004) Management and resistance in wheat and barley to Fusarium head blight. Annu Rev Phytopathol 42:135–161
Beyer M, Klix MB, Klink H, Verreet A (2006) Quantifying the effects of previous crop, tillage, cultivar and triazole fungicides on the deoxynivalenol content of wheat grain-a review. J Plant Dis Prot 113(6):241–246
Blandino M, Haidukowski M, Pascale M, Plizzari L, Scudellari D, Reyneri A (2012) Integrated strategies for the control of Fusarium head blight and deoxynivalenol contamination in winter wheat. Field Crop Res 133:139–149
Bottalico A (1998) Fusarium diseases of cereals: species complex and related mycotoxin profiles, in Europe. J Plant Pathol 80:85–103
Brage PC, Sasso MD, Culici M, Alfieri M (2007) Eugenol and thymol, alone or in combination, induce morphological alterations in the envelope of Candida albicans. Fitoterapia 78:396–400
Campaniello D, Corbo MR, Sinigaglia M (2010) Antifungal activity of eugenol against Penicillium, Aspergillus, and Fusarium Species. J Food Prot 73:1124–1128
Carrasco H, Raimondi M, Svetaz L, Di Liberto M, Rodriguez MV, Espinoza L, Madrid A, Zacchino S (2012) Antifungal activity of eugenol analogues. Influence of different substituents and studies on mechanism of action. Molecules 17:1002–1024
Chen Y, Li HK, Chen CJ, Zhou MG (2008) Sensitivity of Fusarium graminearum to fungicide JS399-19: in vitro determination of baseline sensitivity and the risk of developing fungicide resistance. Phytoparasitica 36:326–337
Chen Y, Zhou MG (2009) Characterization of Fusarium graminearum isolates resistant to both carbendazim and a new fungicide JS399-19. Phytopathology 99:441–446
Dweba CC, Figlan S, Shimelis HA, Motaung TE, Sydenham S, Mwadzingeni L, Tsilo TJ (2017) Fusarium head blight of wheat: pathogenesis and control strategies. Crop Prot 91:114–122
FRAC (2020) FRAC Code List ©*2020: Fungal control agents sorted by cross resistance pattern and mode of action (including FRAC Code numbering) https://www.frac.info/docs/default-source/publications/frac-code-list/frac-code-list-2020-finalb16c2b2c512362eb9a1eff00004acf5d.pdf?sfvrsn=54f499a_2
Gill AO, Holley RA (2006) Inhibition of membrane bound ATPase of Escherichia coli and Listeria monocytogenes by plant oil aromatics. Int J Food Microbiol 111:170–174
Goswami RS, Kistler HC (2004) Heading for disaster: Fusarium graminearum on cereal crops. Mol Plant Pathol 5:515–525
Lei CY, Yin JL, Fang ZW, Wang SP, Zhang X, Ma DF (2018) Ihhibitory effects of phenamacril, tebuconazole and their mixtures on Fusarium graminearum in wheat. Plant Diseases and Pests 9(3–4):14–17
Li HK, Diao YM, Wang JX, Chen CJ, Ni JP, Zhou MG (2008) JS399-19, a new fungicide against wheat scab. Crop Prot 27(1):90–95
Liu Y, Chen X, Jiang J, Hamada MS, Yin Y, Ma Z (2014) Detection and dynamics of different carbendazim resistance conferring β-tubulin variants of Gibberella zeae collected from infected wheat heads and rice stubble in China. Pest Manag Sci 70(8):1228–1236
Marchese A, Barbieri R, Coppo E, Orhan IE, Daglia M, Nabavi SF, Izadi M, Abdollahi M, Nabavi SM, Ajami M (2017) Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit Rev Microbiol 43(6):668–689
Maurizio D., Richard L., Marisabel M., Rocco R., Vito Antonio R., Licia V., 2020. Fragrance components of gymnadenia conopsea and gymnadenia odoratissima collected at several sites in italy and germany. Natural Product Research, 1-5. http://dx.doi.org/10.1080/14786419.2020.1851227
Mesterhazy A, Bartok T, Lamper C (2003) Influence of wheat cultivar, species of Fusarium, and isolate aggressiveness on the efficiency of fungicides for control of Fusarium head blight. Plant Dis 87:1107–1115
Muellenborn C, Steiner U, Ludwig Mand Oerke E (2008) Effect of fungicides on the complex of Fusarium species and saprophytic fungi colonizing wheat kernels. Plant Pathol 120:157–166
Olea AF, Bravo A, Martínez R, Thomas M, Sedan C, Espinoza L, Carrasco H (2019) Antifungal activity of eugenol derivatives against Botrytis cinerea. Molecules 24(7):1239–1252
Pal C, Bandyopadhyay U (2012) Redox-active antiparasitic drugs. Antioxid Redox Signal 17:555–582
Qian HW, Du J, Chi MY, Sun XM, Liang WX, Huang JG, Li BD (2018) The Y137H mutation in the cytochrome P450 FgCYP51B protein confers reduced sensitivity to tebuconazole in Fusarium graminearum. Pest Manag Sci 74:1472–1477
Qu TL, Meng ZL, Li JQ (2008) Determination of degradation dynamics of fungicide 2-allylphenol in Rhizoctonia cerealis by high performance liquid Chromatography. Chin J Anal Chem 5:637–641 (in Chinese)
Romanazzi G, Lichter A, Gabler FM, Smilanick JL (2012) Recent advances on the use of natural and safe alternatives to conventional methods to control postharvest gray mold of table grapes. Postharvest Biol Technol 63(1):141–147
Sutton JC (1982) Epidemiology of wheat head blight and maize ear rot caused by Fusarium graminearum. Can J Plant Pathol 4:195–209
Varga E, Wiesenberger G, Hametner C, Ward TJ, Dong Y, Schöfbeck D, Krska R (2015) New tricks of an old enemy: isolates of Fusarium graminearum produce a type A trichothecene mycotoxin. Environ Microbiol 17(8):2588–2600
Wang CM, Zhang J, Chen H, Fan YJ, Shi ZQ (2010) Antifungal activity of eugenol against Botrytis cinerea. Tropical Plant Pathology 35(3):137–143
Wu RF, Si SY (2006) The screening of optimum ratio of abamectin and alphacypermethrin against Liriomyza sativae Blanchard. Modern Agrochemicals 5(3):49–51
Yang CJ, Gao Y, Du KY, Luo XY (2020) Screening of 17 Chinese medicine plants against phytopathogenic fungi and active component in Syzygium aromaticum. J Plant Dis Prot 127:237–244
Zaker M (2016) Natural plant products as eco-friendly fungicides for plant diseases control—a review. Agriculturists 14(1):134–141
Zhang YJ, Zhang X, Chen CJ, Zhou MG, Wang HC (2010) Effects of fungicides JS399-19, azoxystrobin, tebuconazloe, and carbendazim on the physiological and biochemical indices and grain yield of winter wheat. Pestic Biochem Physiol 98:151–157
Zheng ZT, Hou YP, Cai YQ, Zhang Y, Li YJ, Zhou MG (2015) Whole-genome sequencing reveals that mutations in myosin-5 confer resistance to the fungicide phenamacril in Fusarium graminearum. Sci Rep 5:8248–8256
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cui, W., Du, KY., Ling, YX. et al. Activity of eugenol derivatives against Fusarium graminearum Q1 strain and screening of isoeugenol mixtures. J Plant Pathol 103, 915–921 (2021). https://doi.org/10.1007/s42161-021-00875-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42161-021-00875-5