Abstract
Surveys were conducted during November – February (2015–16 to 2017–18) to collect Sclerotinia sclerotiorum infected plant parts of various hosts from different agro-ecological regions of Assam, Mizoram, Nagaland, Sikkim and West Bengal. Thirty-seven S. sclerotiorum cultures were isolated from the infected plant parts collected from the crop fields with disease incidence ranging from 3.3–20.0%. Rumex scutatus was recorded as a host of the fungus for the first time in the world and five other plant genera were identified as new hosts in India. Phenotypic variability was observed for growth pattern, morphological characteristics, sclerotial characteristics and pathogenic diversity. Isolates varied on the basis of the colony colour of which 83.3% were light-brown to whitish and the rest were brown to black. Majority of the isolates (58.33%) exhibited medium growth rate, whereas, 36.1 and 5.57% were having low and high growth rate, respectively. Nearly, 89% of the isolates belonged to moderate and low sclerotia forming groups. Almost 44.4, 38.9 and 16.7% of the isolates belonged to less, moderately and highly aggressive groups, respectively. Growth rate and biomass conjointly explained 42.0% of the variation in the virulence of the isolates. Further, pectinase and oxalic acid production could explain 54.9% of the variation in the virulence of the isolates. Eleven MCGs were identified and isolates with similar aggressiveness mostly belonged to the same group. S. sclerotiorum isolates exhibited significant variations in morphological, cultural, biochemical and pathogenic variability with potential to emerge as an important pathogen in the eastern and north eastern region of the country.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sclerotinia sclerotiorum is primarily a soil borne pathogen which survives through sclerotia in soil. It is also air borne as aerial dispersal and infections are caused by ascospores. Soil borne pathogens with their characteristic attributes such as wide host range, scarce resistant source and long period of survival; make their management an arduous task. The fungus is designated cosmopolitan, necrotrophic and host nonspecific as its occurrence is worldwide infecting more than 400 plant species in 75 families (Purdy 1979; Boland and Hall 1994). S. sclerotiorum mostly inhabits the cool as well as moist, temperate and subtropical regions (Willets and Wong 1980; Saharan and Mehta 2008). It also occurs in the hot as well as dry areas, emphasising its wider level of genetic diversity and energized adaptability (Manjunatha et al. 2014; Sharma et al. 2015). In temperate climatic zones the S. sclerotiorum population is predominantly clonal while in the subtropical and tropical climatic areas it is sexual reproduction (Atallah et al. 2004; Malvarez et al. 2007). Isolates of the pathogen exhibit significant variations in aggressiveness or severity of disease symptoms on hosts (Garg et al. 2010; Upadhyay et al. 2015; Kamvar et al. 2017).
Production of several crops is known to be affected by S. sclerotiorum since 1915 (Mehta 2014; Sharma et al. 2015), however, the impact is felt much more in recent years especially in the subtropical winter cropping, probably with the emergence of more virulent forms of the pathogen along with favorable shift in the climatic conditions towards proliferation of the pathogen (Dutta et al. 2016). Till late 80s, 30 host species were reported under 19 genera from North East India and the host list is ever increasing since its first report from Assam (Chowdhury, 1946). The pathogen was first recorded on Motihari tobacco from West Bengal in 1988 (Monga 1988). However, more than 25 hosts of S. sclerotiorum were reported from West Bengal after 2000 and this pathogen has become an emerging one in vegetables, pulses, oilseeds and flower crops in eastern and north-eastern regions of India (Dutta et al. 2016). Despite the substantial impact on agricultural production, variations within the fungus in the eastern and north-eastern region of the country have been scantily explored. The present research was undertaken to know the existing variability of the pathogen in terms of morphology, pathogenicity and host range of S. sclerotiorum in the eastern and north eastern states of India.
Materials and methods
Survey, collection, isolation, purification and identification of the pathogen.
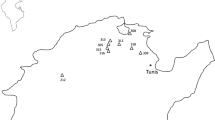
Surveys were conducted during winter months (November – February) of 2015–16, 2016–17 and 2017–18 to collect S. sclerotiorum infected plant parts of various hosts from different agro-ecological zones of four North Eastern states viz. Assam, Mizoram, Nagaland, Sikkim and the Eastern state of West Bengal. Diseased plant parts i.e., fruits, stem, leaves and inflorescence of different hosts showing typical symptoms along with sclerotia were collected for isolation of the causal pathogen. The diseased plant tissue (cut into 1 to 1.5 cm length) or individual sclerotium were surface sterilized with 1% sodium hypochlorite solution for 1 min, washed for three times with sterile distilled water and blot dried under aseptic condition. These bits or single sclerotium were then placed on Petri dish containing PDA medium and incubated at 22 ± 2ºC for 2 to 3 days. Fine radiating hyphal tips from the edge of the infected bits or sclerotium were transferred aseptically to PDA slants in test tubes to obtain pure cultures of the fungus. After preliminary microscopic examination, these pure cultures were maintained in refrigerator at 4ºC with periodical sub-culturing at monthly interval. Pathogenic nature of the isolates (especially for new hosts) was ascertained by proving the Koch’s postulates after inoculating them on the respective hosts.
Cultural and morphological variability.
Isolates of the S. sclerotiorum were grown on PDA medium in Petri plates at 22 ± 2ºC until mycelial run reached periphery of the plates. Mycelial growth was measured periodically in centimeter (cm) scale at 24 h interval until complete growth of the mycelia and accordingly growth rate was calculated. Growth rate in three Petri plates were considered as three replications for each isolate. Clustering of the isolates were performed on the basis of growth rate into slow, medium and fast growing categories.
Mycelial growth habit and colony colour were recorded by visual observation of mycelia growth type and colour at 24 h interval up to 7–10 days after incubation. Two type of mycelial growth patterns were observed: surface (aerial mycelium does not obscure surface mycelium) and aerial hyphae (aerial mycelium obscures surface and contact the cover of the Petri dish).
For the determination of mycelial biomass production, potato dextrose broth (50 ml) was dispensed in conical flask (250 ml) and autoclaved at 15 lb psi. Four-day-old mycelial disc (5 mm size) of each S. sclerotiorum isolate was inoculated in each flask in three replications and incubated at 22 ± 2ºC. Fresh mycelial mat was harvested after 5 days of incubation. It was then washed properly with distilled water and separated from the mycelia disc. Excess moisture in the fungal mycelial mat was removed with the help of sterile blotting paper and weight of fresh mycelial mat was taken. On the basis of mycelial biomass production, isolates were categorized into three groups as high, moderate and low biomass producers.
Mycelial discs of 5 mm diameter from four-day-old cultures of each isolate were transferred to center of sterilized PDA plates and incubated at 22 ± 2 ºC for 10 days to obtain different sclerotial characteristics. The experiment was laid with three replications for each S. sclerotiorum isolate.
Time required for first sclerotia formation was recorded at 12 h intervals. On the basis of sclerotial location in Petri plates, isolates were grouped into central, peripheral and scattered. Sclerotial exudation was marked as present or absent. Number of sclerotia formed per Petri plate (in three replicates) were counted and average was considered. Fresh and dry weight of 10 randomly selected mature sclerotia for each isolate was recorded. The diameter of sclerotia was measured in randomly selected 10 sclerotia by digital slide calipers.
Extra-cellular enzymatic activities and oxalic acid production.
Cellulase activities of S. sclerotiorum isolates were measured by culturing them on Carboxy Methyl Cellulase (CMC) medium, where CMC was the only source of carbon. After 3 days of incubation at 24ºC, the culture plates in three replications for each isolate were flooded with 1% w/v of Congo red solution for 1 h at room temperature and excess stain was discarded. Culture plates were subsequently de-stained with 1 M NaCl solution. Plates were kept overnight at 4ºC and then examined for clear zone in the substrate around the point of inoculation and were compared with the control plate. Diameter of the clear zone was measured and recorded. Cellulase activity was expressed as ratio between the diameter of clear zone and the diameter of fungal growth in the control (Asoufi et al. 2007).
Each isolate was inoculated on three pectin agar media plates as replicates. The plates were incubated at 24ºC for three days and were stained by flooding with adequate volume of aqueous solution of tri-methyl ammonium bromide for 30 min. Pectinase activity was assessed by observing the development of colourless zone around the inoculated culture. Diameter of the clear zone was measured and recorded. Ratio between the diameter of the clear zone and diameter of fungal growth represented pectinase activity (Asoufi et al. 2007).
Isolates of S. sclerotiorum were examined for their ability to produce oxalic acid according to the procedure described by Godoy et al. (1990) with modifications. Mycelial discs from the margin of actively growing four-day-old cultures were placed in the center of Petri plates containing 20 ml PDA medium previously adjusted to pH 7.0 and amended with bromophenol blue (50 mg/l) as pH indicator dye. Oxalic acid production was examined after 48 h of incubation at 24 ºC. Appearance of characteristic yellow colour surrounding the inoculated culture indicated oxalic acid production. Oxalic acid production was expressed as ratio between the diameter of yellow zone and diameter of fungal growth.
Virulence test.
In vitro analysis of virulence of S. sclerotiorum isolates was conducted by mycelial plug inoculation technique in 30-day-old French bean (cv. Falguni) plants under growth chamber. Three plants were inoculated for each isolate as replications. Length of lesions were measured at 7 DAI for each plant. Distinct water-soaked lesions appeared 3 days after inoculation. When margins of the lesions extended, leaves wilted and gradually the lesions turned brown and there was production of sclerotia. As the disease advanced the plants wilted. Disease grading was performed as per Modified Petzoldt and Dickson scale of 1–9 on the French bean plants. Further, area under disease progress curve (AUDPC) for the necrotic lesions were calculated to measure the aggressiveness of the isolates.
Mycelial Compatibility Grouping (MCG).
All the isolates of S. sclerotiorum were paired in all possible combinations on PDA medium amended with red colouring agent (Tolaram and Deochand) at 1 g/l. Mycelial discs (5 mm in diameter) were cut with sterile cork borer from actively growing margin of four-day-old culture. For each pair of the isolate, one actively growing mycelial disc from each isolate was aseptically placed on Petri plates (90 mm diameter) with PDA at 30 mm distance and incubated in dark at 23-25ºC. Reactions of the pairing were scored as compatible or incompatible after 10 days of incubation. Incompatible reactions were scored with formation of a barrage zone identified by an apparent thin to wide red line on the colony or by formation of aerial mycelia along the barrage zone between the paired isolates. Compatible reactions were identified when mycelia of the paired isolates fused to form a single colony without the barrage line (Kohn et al. 1990; Schafer and Kohn 2006; Yanar and Onaran 2011). Intensity of antagonism was rated for each pairing as follows: 0 = no antagonism, mycelia and sclerotia of the two isolates intermingling; 1 = aversion zone of various wide present. All combinations were inoculated in triplicate and binary matrix was generated.
Statistical analysis.
Grouping of the isolates based on individual or combined morphological and cultural parameters was performed by K-mean clustering technique implemented in SPSS version 24. The same package was used to classify the isolates using UPGMA cluster analysis algorithm.
Results and Discussion
In the present study, 37 Sclerotinia sclerotiorum were isolated from the infected plants and sclerotia samples of different hosts collected from different agro-ecological regions of the states of Assam, Mizoram, Nagaland, Sikkim and West Bengal (Table 1). Pure characterized culture of each isolate (in duplicate) was deposited in National Agriculturally Important Microbial Culture Collection (NAIMCC) of ICAR National Bureau of Agriculturally Important Microorganisms, located at Mau NathBhanjan, Uttar Pradesh and Accession Numbers for all the 37 isolates were obtained authenticating the fungal isolates to be Sclerotinia sclerotiorum. Sequence of the ITS regions was submitted to NCBI GenBank database for all the isolates and accession numbers were obtained (Table 1). Out of the 37 isolates, 36 were studied further for phenotypic, genotypic and pathogenic variability.
Sclerotinia rot was recorded on maximum number of crops during the winter months of 2015–16 in the states surveyed. A total of nine host plants were found infected in Darrang and Kamrup districts of Assam during winter months of 2015–16 with maximum disease incidence of 18% on mustard and pea (Table 1). Minimum incidence (3.33%) of the disease was recorded on periwinkle (Catharanthus roseus) and gerbera. Sclerotinia disease on chilli and urdbean with incidence of 10% and 6.66%, respectively, was recorded from Kolasib, Mizoram during January, 2016. During winter of 2015–16, the pathogen was observed on 9 different hosts of different locations in Dimapur district of Nagaland causing incidence in the range of 3.33–20%. Only one host crop of the pathogen, chayote (Sechium edule) was recorded from Sikkim during December, 2016. Highest number of hosts (16) was recorded from four districts of West Bengal during the study period. Among the four districts (North 24-Parganas, Hoogly, Nadia and Purulia), 13 hosts were recorded from Nadia district alone. Sclerotinia disease incidence ranged from 4–16.66% for different hosts during winter of 2016 and 2017 in the state of West Bengal. During cold months of 2017–18, Sclerotinia incidence was recorded from only one host (carrot) at Kanchiara village of Nadia district.
In the present investigation, Rumex scutatus was recorded for the first time in the World as a host of S. sclerotiorum by fulfilling the Koch’s postulates, and 5 hosts (Catharanthus roseus, Cardamine sp., Galinsoga parviflora, Sechium edule and Vigna mungo) of S. sclerotiorum were recorded for the first time from India (Table 1). Mimosa pudica has also been reported as a new host of S. sclerotiorum during the course of the study (Borah et al. 2018).
S. sclerotiorum isolates exhibited significant variation in terms of the seven cultural and morphological parameters studied in the current experiment (Table 2). Colour of the colony for most isolates was creamy white while growth type was categorized as sparse irregular, fluffy irregular, fluffy regular and circular irregular. Upon full growth of the colony, pigmentation intensity varied among the isolates as per visual estimation and grading. It was observed that 83.3% of the isolates were light brown to whitish in colour, the remaining brown to black coloured isolates belonged to Assam and Nagaland. Growth rate of the 36 S. sclerotiorum isolates varied from 0.8 to 1.9 mm/h of which 5.6% belonged to fast growth rate (1.88–1.59 mm/h; French bean isolate AS4 from Assam and Capsicum isolate WB1 from West Bengal), 58.33% exhibited medium growth rate (1.37–1.11 mm/h) and 36.1% showed slow growth (0.99–0.80 mm/h). Considering biomass production, 33.33% of the total isolates were grouped in high biomass (2.21–1.78 g) producing category, 41.67% were medium (1.65–1.27 g) and 33.33% were of low (0.86 – 1.24 g) biomass producers. S. sclerotiorum isolates were grouped into three clusters (low, moderate and high) on the basis of number of sclerotia produced. An equal number of isolates (16 or 44.44% each) of different hosts and geographical locations belonged to moderate and low sclerotia forming groups i.e., group II and group III. Isolates categorized under group I (11.11% of isolates) produced high number of sclerotia and comprised of 4 members (AS4, AS6, WB2, WB14), two each from Assam and West Bengal. WB2 isolate produced more than 40 sclerotia per Petri plate while Nagaland isolate NG1 produced minimum number of 12 sclerotia per plate on an average. Outer diameter of sclerotia of the different isolates ranged from 0.14 to 6.90 mm. The 36 isolates were categorized into three clusters viz., large (6.90–5.87 mm; 8.33%), medium (4.42–2.77 mm; 66.67%) and small (2.57–0.14 mm; 25%) based on size of sclerotia. Fresh weight of sclerotia varied within and among the isolates from different states and were categorized into three groups, viz., high (0.29–0.23 g; 25%), medium (0.22–0.15 g; 47.22%) and low (0.14–0.07 g; 27.78%). Likewise, significant difference was observed in dry weight of sclerotia for all the isolates. Three groups of the isolates formed based on the sclerotial dry weight were high (0.21–0.16 g; 22.22%), medium (0.14–0.09 g; 55.56%) and low (0.08–0.03 g; 22.22%).
On the basis of the K-mean clustering of all the cultural and morphological parameters taken together, 36 S. sclerotiorum isolates from different hosts collected from Assam, Mizoram, Nagaland, Sikkim and West Bengal were classified into three clusters (Table 3). However, morphological variability did not support the geographical origin and hosts of isolates. Among the 7 morphological parameters analyzed, growth rate and number of sclerotia had significant influence on grouping of S. sclerotiorum isolates (Table 3). Hence, growth rate and number of sclerotia can be used as morphological markers in study of variability among S. sclerotiorum isolates. Significant influence of growth rate and number of sclerotia might be attributed to the pathogenic as well as survivability of this pathogen. Box-Whisker plot (Fig. 1) indicated that faster growth rate and higher number of sclerotia producing S. sclerotiorum isolates were grouped in cluster-I while comparatively slower growth rate and lesser number of sclerotia producing S. sclerotiorum isolates were grouped in cluster-II. Similar level of variability of morphological parameters were also observed in the isolates from the respective states.
Manjunatha et al. (2014) reported variability in colony appearance of 9 S. sclerotiorum isolates on PDA as white fluffy for 4 isolates, white suppressed for 3 isolates and dull white suppressed for rest 3 isolates. Similarly, Upadhayay et al. (2015) found difference in colony colour and growth pattern for 12 isolates from different hosts. Garg et al. (2009) for first time in Australia, reported occurrence of 3 darkly pigmented isolates among 8 isolates of S. sclerotiorum from oilseeds. Results of the present investigation corroborates with the findings of Abreu and Souza (2015), who characterized 50 S. sclerotiorum isolates from Brazil into 3 categories of predominant colony colour developed on PDA medium as white, beige and from brown to black. Significant differences in colony growth rate of S. sclerotiorum isolates were also observed by several researchers (Goswami et al. 2008; Garg et al. 2009; Abreu and Souza 2015; Sushree et al. 2017; Sharma et al. 2018). The present results validate with the findings of Manjunatha et al. (2014), who categorized 9 S. sclerotiorum isolates collected from different hosts and different locations of India, into three groups viz., fast, medium and slow growing. Growth rate of the isolates varied from 0.24 mm to 1.25 mm/h. Sharma et al. (2013) observed considerable variability in fresh (7.87–3.03 g) and dry weight of mycelia (0.12 g) among 17 geographical isolates from oilseed crop. Variability in S. sclerotiorum isolates were also reported by several researchers based on sclerotial characteristics. Clustering of the isolates into groups based on number of sclerotia produced was in accordance with the findings of Ghasolia and Shivpuri (2007) and Vakilizarj et al. (2013). Ghasolia and Shivpuri (2007) categorized 38 isolates of S. sclerotiorum in small, medium and large with sclerotia size of less than 3 mm, 3.1–5.0 mm and more than 5 mm, respectively. In another study, Ahmadi et al. (2012) reported that Artemisia population isolates had the biggest sclerotial diameter (31.49 mm). Populations of tobacco, soybean, cabbage and rapeseed were intermediate while lettuce and sunflower showed least sclerotia size of 24.27 and 22.97 mm, respectively. Akram et al. (2008) considered weight of sclerotia as one of the most important parameters along with other morphological characters to study variability among 16 isolates of the chickpea stem rot pathogen. Irani et al. (2011) found variability amongst MCGs of 186 isolates from sunflower in dry weight of sclerotia.
All the isolates of S. sclerotiorum exhibited positive activity and significant variations for pectinase. Based on qualitative assay, highest and lowest level of pectinase activities (1.042 and 1.252) were observed in West Bengal isolates WB13 and WB7, respectively. Cellulase activity for the isolates ranged between 0.061 – 1.048. However, data revealed no significant differences among the isolates for cellulase activity. There were significant variations among isolates in their ability to produce oxalic acid. Oxalic acid was produced by the isolates in the range of 1.001 – 1.126, minimum and maximum amount being produced by the isolates NG8 from Nagaland and WB7 from West Bengal, respectively.
Significant variations in virulence of the 36 S. sclerotiorum isolates were observed. Isolates were categorized into 3 virulence groups on the basis of AUDPC and the categorized groups were designated as low virulent (LV), moderately virulent (MV) and highly virulent (HV). According to AUDPC, 16.7, 38.9 and 44.4% of the isolates encompassing 6, 14 and 16 numbers of the isolates were categorised as high, moderate and low aggressive, respectively. Figure 2 indicates the grouping of the S. sclerotiorum isolates based on hierarchical clustering. Present investigation was in line with that of Zancan et al. (2015) who obtained three groups for 25 isolates based on their aggressiveness as was evident from straw test rating (Modified Petzoldt and Dickson scale) and t grouping. Irani et al. (2011) also categorized S. sclerotiorum isolates into most aggressive, moderately aggressive and least aggressive based on significant differences in pathogenicity of isolates regardless of isolate origin. Karimi et al. (2012) adopted lesion length scoring system to categorize 64 isolates of S. sclerotiorum into five groups based on the virulence pattern of the isolates. Four Brassica sp. isolates were classified into three groups, viz., highly virulent, virulent and moderately virulent (Sharma et al. 2018).
Among the morphological parameters, growth rate, dry weight of sclerotia and biomass production exhibited positive correlation with pathogenicity parameters (Table 4). Growth rate and biomass were positively correlated with AUDPC at 1% significance level. Dry weight of sclerotia was positively correlated with AUDPC at 5% level of significance. Stepwise linear regression analysis considering virulence (AUDPC) as dependent and all morphological parameters as independent variables have identified two models (Model 1 and 2). It was observed that only growth rate of the fungus was able to describe 32.8% variation in AUDPC whereas growth rate and biomass conjointly could able to explain 42.0% of variation in virulence of the pathogen. Therefore, growth rate and biomass production might be considered as two most significant predictors for predicting virulence of S. sclerotiorum isolates. Validation of models showed the relationship of observed and estimated AUDPC according to model 2 (Fig. 3). Thus, fast growth rate and high biomass production by the pathogen might allow faster and better colonization of hosts and simultaneously successful pathogenesis in Sclerotinia–French bean host-pathogenic system according to the present findings.
There was positive correlation between pectinase activity and oxalic acid production with virulence level of the isolates at 1% level of significance. Stepwise multiple regression analysis of biochemical parameters with virulence of S. sclerotiorum has generated two models (Model 3 and 4; Table 4). In model 3, oxalic acid production was able to explain 53.4% of variation in virulence of S. sclerotiorum isolates whereas pectinase and oxalic acid production could explain 54.9% variation in virulence in model 4. Thus, production of oxalic acid and pectinase were identified as two most important predictors for analyzing virulence of S. sclerotiorum. Validation of model 4, i.e., relationship of AUDPC with production of pectinase and oxalic acid is shown in Fig. 4.
Biochemical variability did not support geographical origin and hosts of the isolates. Pectinase was known to assist in penetration of host tissues as reported by Bateman and Beer (1965). The present findings were in accordance with findings of Asoufi et al. (2007) who reported that higher pectinase and cellulase activities were associated with virulence of indigenous S. sclerotiorum isolates in Jordan valley. S. sclerotiorum was known to produce and secrete oxalic acid into their surrounding media as reported by Cessna et al. (2000). Production of oxalic acid was considered as a pathogenicity determinant in S. sclerotiorum by several workers (Godoy et al. 1990; Li et al. 2008; Williams et al. 2011). Several researchers opined that in S. sclerotiorum infection, oxalic acid was essential for disease development because it degrades the structure of plant cell walls, although its precise role was not well understood (Maxwell and Lumsden 1970; Godoy et al. 1990; Zhou and Boland 1999). Oxalate, as a signaling molecule, acts in relation to suppression of host defense and provides a more conducive environment by hijacking the host cell by altering an array of plant expressions during early biotrophic stage of infection by S. sclerotiorum (McCaghey et al. 2019). Thus, the results of the present investigation further confirmed the inference drawn by all such previous studies and established the relationship between oxalic acid production with virulence of S. sclerotiorum isolates of West Bengal and North Eastern states.
MCGs of the 36 isolates of S. sclerotiorum were determined in pairwise combination of within and among the isolates. All pairings within the isolates itself were compatible. Eleven MCGs were identified, the largest MCG was VII with 8 isolates in total, 7 isolates (WB6, WB7, WB8, WB9, WB10, WB14 and WB15) were mostly from different flower hosts of a single field in West Bengal and the other isolate (AS9) was also from gerbera from Assam. MCG-VIII comprised of five isolates NG5, NG6, NG7, NG8 and NG9, from different hosts of the same field from Nagaland. This MCG did not give any compatible reaction with isolates from any other location. MCG-I and IV comprised of 4 isolates each respectively. The MCG-II and V included 3 isolates in each group. MCGs III, IX and X consisted of 2 isolates each. Mizoram urdbean isolate (MZ2) produced no compatible reaction in any pairing with other 35 isolates and made up the singleton MCG-XI (Table 5). The 36 field isolates of S. sclerotiorum used in this study represented a heterogeneous collection from different hosts and geographical areas. Moreover, MCG-IV has been found to be most virulent followed by MCG-II and MCG-IX has been least virulent (Fig. 5).
Genetic or cytoplasmic dissimilarities between members of different MCG of S. sclerotiorum might have resulted from isolation and adaptation of different isolates of the pathogen to different ecological niches or variable selection pressures. From Table 5 it could be observed that northeastern isolates were under MCG I-V, VIII and XI whereas the West Bengal isolates were mostly under MCG VI, VII, IX and X with few exceptions. In our findings, MCGs revealed some degree of collinearity with the virulence and geographical location as evidenced in Table 5. Leslie (1993) considered isolates as clones which were having mycelial compatibility because of genetic connection to vegetative compatibility. The findings of Hambleton et al. (2002) indicated that high frequency of S. sclerotiorum clones at local scale could sometimes be sampled repeatedly over several years in the same locality and in some cases over a wider geographic area. Clarkson et al. (2013) reported temporal and spatial divergence in S. sclerotiorum MCGs, however, divergence was originated from the same ancestry. Aban et al. (2018) reported location specificity of the S. sclerotiorum MCGs from Australia. Upadhyay et al. (2015) categorized 12 S. sclerotiorum isolates from various hosts into four MCGs with similar cultural and morphological characteristics and plant hosts family. However, in most of the MCGs isolates combined irrespective of hosts and geographic locations representing a spatial structure of S. sclerotiorum population in these regions. Kohn et al. (1991) as well as Carbone and Kohn (2001) indicated that transposition leading to new fingerprints occurred more frequently than mutational events resulting in new MCGs thereby supporting the hypothesis that S. sclerotiorum populations were spatially structured. In our case, MCGs of S. sclerotiorum depicted divergence from each other at 5% level of significance in respect of AUDPC. Figure 5 revealed that isolates with similar aggressiveness mostly were categorized under same group. MCG IV had highly virulent isolates AS4, AS6, WB3 and SK1 while MCG IX had low virulent isolates WB2 and WB4.
Conclusion
Thirty-seven numbers of S. sclerotiorum were isolated from the infected samples belonging to various crops and weed families obtained from different agro-ecological zones of the 4 north eastern states and the eastern state of West Bengal and their identities were confirmed based on morphological characteristics, pathogenicity test and sequence analysis of the amplified rDNA-ITS regions. Cultural and morphological characteristics revealed considerable diversity among the isolates based on mycelial growth pattern, growth rate, colony colour, biomass production, numbers, size, weight and pattern of sclerotia formation. The isolates were virulent with varied level of aggressiveness. Overall 16.7% of the isolates were highly virulent which comprised of 13.3%, 22.2% and 11.1% of the isolates from West Bengal (n = 15), Assam (n = 9) and Nagaland (n = 9), respectively. Other 44.4% of the total isolates were less virulent. Among the morphological parameters considered, growth rate and biomass were the two most significant indicators for predicting the virulence of S. sclerotiorum isolates contributing upto 42% of total variability. Among the various biochemical parameters, pectinase activity and oxalic acid production ability of the fungal isolates were found to be the most important predictors for virulence contributing upto 55% of the total variability. The isolates obtained from one geographical location and from the same fields were mostly grouped in the same MCG of the eleven MCGs identified among the 36 isolates. This research work tries to take into account the variability of S. sclerotiorum, a highly devastating pathogen with wide host range and high degree of survivability in soil. Being considered as an emerging pathogen in the eastern and north-eastern Indian subcontinent due to recent climatic shift with increasing number of new host reports and aggravated disease scenario, an overall understanding of the pathogenic variability and host-pathogenic interaction will help manage the pathogen in a holistic way.
References
Aban CL, Taboada G, Spedaletti Y, Aparicioab M, Curti AC, Casalderrey NB, Maggio ME, Chocobar MO, Salgado M, Galvan MZ (2018) Molecular, morphological and pathogenic diversity of Sclerotinia sclerotiorum isolates from common bean (Phaseolus vulgaris) fields in Argentina. Plant Pathol 67:1740–1748
Abreu MJ, Souza EA (2015) Investigation of Sclerotinia sclerotiorum strains variability in Brazil. Gen Mol Res 14(2):6879–6896
Ahmadi MR, Nik-khah MJ, Aghajani MA, Ghobakhloo M (2012) Morphological variability among Sclerotinia sclerotiorum populations associated with stem rot of important crops and weeds. World Appl Sci J 20(11):1561–1564
Akram A, Iqbal SM, Ahmed N, Iqbal U et al (2008) Morphological variability and mycelial compatibility among the isolates of Sclerotinia sclerotiorum associated with stem rot of chickpea. Pak J Bot 40:2663–2668
Asoufi H, Hameed KM, Mahasnch A (2007) The cellulose and pectinase activities associated with virulence of indigenous Sclerotinia sclerotiourum isolates in Jordon valley. Plant Pathol J 23(4):233–238
Atallah ZK, Larget B, Chen X, Johnson DA (2004) High genetic diversity, phenotypic uniformity, and evidence of outcrossing in Sclerotinia sclerotiorum in the Columbia Basin of Washington State. Phytopathol 94:737–742
Bateman DF, Beer SV (1965) Simultaneous production and synergistic action of oxalic acid and polygalacturonase during pathogenesis of Sclerotium rolfsii. Phytopathology 55:204–211
Boland GJ, Hall R (1994) Index of plant hosts of Sclerotinia sclerotiorum. Can J Pl Pathol 16:93–108
Borah TR, Dutta S, Roy Barman A (2018) First report of Sclerotinia sclerotiorum on Mimosa pudica in India. New Dis Rep 38:14. https://doi.org/10.5197/j.2044-0588.2018.038.014
Carbone I, Kohn LM (2001) Multilocus nested haplotype networks extended with DNA fingerprints show common origin and fine-scale, ongoing genetic divergence in a wild microbial metapopulation. Mol Ecol 10:2409–2422
Cessna SG, Sears VE, Dickman MB, Low PS (2000) Oxalic acid, a pathogenicity factor for Sclerotinia sclerotiorum, suppresses the oxidative brust of the host plant. Plant Cell 12:2191–2199
Chowdhury S (1946) Effect of hydrogen-ion concentration on the growth and parasitism of Sclerotium rolfsii. Sacc, Indian J Agric Sci 16:290–293
Clarkson JP, Coventry E, Kitchen J, Carter HE, Whipps JM (2013) Population structure of Sclerotinia sclerotiorum in crop and wild hosts in the UK. Plant Pathol 62:309–324
Dutta S, Borah Tasvina R, Roy Barman A, Hansda S, Ghosh PP (2016) Overview of Sclerotinia sclerotiorum in India with special reference to its emerging severity in Eastern Region. Indian Phytopath 69(4s):260–265
Garg H, Kohn LM, Andrew M, Li H, Sivasithamparam K, Barbetti MJ (2009) Pathogenicity of morphologically different isolates of Sclerotinia sclerotiorum with Brassica napus and B. juncea genotypes. Eur J Plant Pathol 126:305–315
Garg H, Li H, Sivasithamparam K, Kuo J, Barbetti MJ (2010) The infection processes of Sclerotinia sclerotiorum in cotyledon tissue of a resistant and a susceptible genotype of Brassica napus. Ann Bot 106(6):897–908
Ghasolia RP, Shivpuri A (2007) Morphological and pathogenic variability in rapeseed and mustard isolates of Sclerotinia sclerotiorum. Indian Phytopathol 60:76–81
Godoy G, Steadman JR, Dickman MB, Dam R (1990) Use of mutants to demonstrate the role of oxalic acid in pathogenicity of Sclerotinia sclerotiorum on Phaseolus vulgaris. Physiol Mol Plant Pathol 37:179–191
Goswami K, Tewari AK, Awasthi RP (2008) Cultural, morphological and pathological variability in isolates of Sclerotinia sclerotiorum (Lib.) de Bary. J Mycol Plant Pathol 38:650–652
Hambleton S, Walker C, Kohn LM (2002) Clonal lineages of Sclerotinia sclerotiorum previously known from other crops predominate in 1999–2000 samples from Ontario and Quebec soybean. Can J Plant Pathol 24:309–315
Irani H, Heydari A, Javan-Nikkhah M, İbrahimov AŞ (2011) Pathogenicity variation and mycelial compatibility groups in Sclerotinia sclerotiorum. J Plant Prot Res 51:329–336
Kamvar ZN, Amaradasa BS, Jhala R, McCoy S, Steadman JR, Everhart SE (2017) Population structure and phenotypic variation of Sclerotinia sclerotiorum from dry bean (Phaseolus vulgaris) in the United States. Peer J 5:e4152; https://doi.org/10.7717/peerj.4152
Karimi E, Safaie N, Shams-bakhsh M (2012) Mycelial compatibility groups and pathogenic variation of Sclerotinia sclerotiorum populations from canola in the Golestan province of Iran. J Agric Sci Technol, 14421–14434
Kohn L, Stasovski E, Carbone I, Royer J, Anderson J (1991) Mycelial incompatibility and molecular markers identify genetic variability in field populations of Sclerotinia sclerotiorum. Phytopathology 81:480–485. https://doi.org/10.1094/Phyto-81-480
Kohn LM, Carbone I, Anderson JB (1990) Mycelial interactions in Sclerotinia sclerotiorum. Exper Mycol 14:255–267
Leslie J (1993) Fungal vegetative compatibility. Ann Rev Phytopathol 31(1):127–150. https://doi.org/10.1146/annurev.py.31.090193.001015
Li Z, Zhang M, Wang Y, Li R, Dilantha Frernando WG (2008) Mycelial compatibility group and pathogenicity variation of Sclerotinia sclerotiorum populations in sunflower from China. Canda and England Plant Pathol J 7(2):131–139
Malvarez G, Carbone I, Grunwald NJ, Subbarao KV (2007) New populations of Sclerotinia sclerotiorum from lettuce in California and peas and lentils in Washington. Phytopathol 97:470–483
Manjunatha N, Prameela Devi T, Prabhakaran N, Gurdevi Navali V, Swasthi Patil S (2014) Morphological and molecular diverisity of Sclerotinia sclerotiorum (Lib.) de Bary isolates of India. The Bioscan 9(4):1763–1767
Maxwell DP, Lumsden RD (1970) Oxalic acid production by Sclerotinia sclerotiorum in infected bean and in culture. Phytopathology 60:1395–1398
McCaghey M, Willbur J, Smith DL, Kabbage M (2019) The complexity of the Sclerotinia sclerotiorum pathosystem in soybean: virulence factors, resistance mechanisms, and their exploitation to control Sclerotinia stem rot. Trop Plant Pathol 44:12–22
Mehta N (2014) Epidemiology and forecasting for the management of rapeseed-mustard diseases. J Mycol Pl Pathol 44:131–147
Monga D (1988) Peduncle rot of Motihari tobacco in Cooch Behar district. Indian Phytopathol 41(3):504
Purdy LH (1979) Sclerotinia sclerotiorum: history, diseases and symptomatology, host range, geographic distribution and impact. Phytopathol 69:875–880
Saharan GS, Mehta N (2008) Sclerotinia diseases of crop plants: Biology, ecology and disease management. Springer, Netherlands: 481
Schafer MR, Kohn LM (2006) An optimized method for mycelial compatibility testing in Sclerotinia sclerotiorum. Mycologia 98:593–597
Sharma P, Meena PD, Kumar S, Chauhan JS (2013) Genetic diversity and morphological variability of Scleortinia sclerotiorum isolates of oilseed Brassica in India. Afr J Microbiol Res 7(18):1827–1833
Sharma P, Meena PD, Singh S, Rai PK (2015) Efficacy of micro-nutrients, fungicides and bioagents against Sclerotinia stem rot (Sclerotinia sclerotiorum) of Indian mustard. Int J Curr Microbiol App Sci 6(10):620–626
Sharma P, Samkumar A, RaoM SVV, Prasad L, Mishra DC, Bhattacharya R, Gupta NC (2018) Genetic Diversity Studies Based on Morphological Variability, Pathogenicity and Molecular Phylogeny of the Sclerotinia sclerotiorum Population From Indian Mustard (Brassica juncea). Front Microbiol 9:1169
Sushree A, Prasad R, Biswas SK, Kumar S (2017) Cultural and morphological variability of Sclerotinia sclerotiorum in rapeseed-mustard. J Mycopathol Res 55(2):179–182
Upadhyay P, Tiwari AK, Bisht KS (2015) Cultural, morphological, pathogenic variability and mycelia compatibility among the isolates of Sclerotinia sclerotiorum (Lib.) de Bary cause of Sclerotinia rot. The Bioscan 10(4):1813–1817
Vakilizarj Z, Rahnama K, Rahnema M (2013) The comparative growth and determination of the isolates reaction of Sclerotinia sclerotiorum on oilseed rape cultivars. Intl J Agron Plant Prod 4(5):928–935
Willetts HJ, Wong JAL (1980) The biology of Sclerotinia sclerotiorum, S. trifoliorum, and S. minor with emphasis on specific nomenclature. Bot Rev 46:101–165
Williams B, Kabbage M, Kim HJ, Britt R, Dickman MB (2011) Tipping the balance: Sclerotinia sclerotiorum secreted oxalic acid suppresses host defenses by manipulating the host redox environment. PLoS Pathog 7:e1002107
Yanar Y, Onaran A (2011) Mycelial compatibility groups and pathogenicity of Sclerotinia sclerotiorum (Lib.) De Bary causal agent of white mold disease of greenhouse grown cucumber in Antalya-Turkey. Afr J Biotech 10:3739–3746
Zancan WLA, Steadman JR, Higgins R, Jhala R, da Machado J, C (2015) Genetic and aggressiveness variation among Sclerotinia sclerotiorum dry bean isolates from Brazil fields. Biosci J 31:1143–1151
Zhou T, Boland GJ (1999) Mycelial growth and production of oxalic acid by virulent and hypovirulent isolates of Sclerotinia sclerotiorum. Can J Plant Pathol 21:93–99
Acknowledgements
We acknowledge with appreciation for receiving the culture accession for the 37 S. sclerotiorum isolates from National Agriculturally Important Microbial Culture Collection (NAIMCC) of ICAR National Bureau of Agriculturally Important Microorganisms, Mau NathBhanjan, Uttar Pradesh.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consent
Informed consent was obtained from all individual participants included in the study.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Borah, T.R., Dutta, S., Barman, A.R. et al. Variability and host range of Sclerotinia sclerotiorum in Eastern and North Eastern India. J Plant Pathol 103, 809–822 (2021). https://doi.org/10.1007/s42161-021-00815-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42161-021-00815-3