Abstract
Monosporascus root rot and vine decline of melon (MRRVD) is a destructive disease complex mainly occurring in semiarid cultivation areas. In the last decade, in the melon producing area of Sardinia (Italy), yield reductions up to 100% were recorded due to the occurrence of MRRVD. The present study aimed to undertake a two-year survey of fungal pathogens associated with MRRVD grown as a monocrop in two locations in Central Sardinia, Sinis, and Sassu, and to investigate the possible role of soil fungal diversity in the disease development. Melon plants were affected by similar symptoms but colonized by a set of soil-borne fungal pathogens different between surveyed sites and cropping seasons, including Plectosphaerella melonis, P. cucumerina, Fusarium solani, Macrophomina phaseolina and Monosporascus cannonballus. Olpidium bornovanus and O. virulentus were isolated using bait plants and detected by NGS analysis. Bait plants had a general decrease in biomass and yellowing of foliage. The presence of most of the isolated pathogens was also confirmed by the NGS analysis of the soil microbiome. Our results confirm that among the fungal complex implicated in the occurrence of RRVD in Sardinia, O. bornovanus along with O. virulentus likely assume a key role in the development of the disease alone and possibly in association with other pathogens. Differences in the pathogenic fungal spectrum here recorded in symptomatic roots may be associated with agricultural practices, soil physicochemical characteristics, and fungal community composition and function profile.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Root rot and vine decline of cucurbits (RRVD, also referred to as “collapse”) is a destructive soil-borne disease complex of melon (Cucumis melo L.) and watermelon (Citrullus lanatus (Thunb.) Matsum. & Nakai) mainly occurring in arid and semiarid cultivation areas of these crops (Cohen et al. 2012). Symptoms on roots are characterized by brown lesions, corky areas, and rots resulting in rapid wilt of plants followed by partial or total fruit loss. Symptomatic roots are often colonized by different fungal pathogens, although a single pathogenic organism may predominate (Infantino et al. 2004; Chilosi et al. 2008). The soilborne ascomycete Monosporascus cannonballus Pollack & Uecker has been reported as causal agent of the Monosporascus root rot and vine decline of melon (MRRVD) in different Mediterranean areas including Italy (Chilosi et al. 2008), Israel (Cohen et al. 2000), Egypt (El-Desouky et al. 2003) and Greece (Markakis et al. 2017). In Tunisia, M. cannonballus and the related species M. eutypoides (Petrak) von Arx, have been associated with root rot and vine decline also of watermelon (Ben Salem et al. 2013). In Apulia (South Italy), where melon is mainly cultivated in open field, Plectosphaerella melonis (T. Watanabe & M. Sato) A.J.L. Phillips, A. Carlucci & M.L. Raimondo (= Acremonium cucurbitacearum Alfaro-García, W. Gams & García-Jim.), Rhizopycnis vagum D.F. Farr, Nodulisporium melonis T. Watanabe & M. Sato, and Plectosphaerella cucumerina (Lindfors) W. Gams (= Plectosporium tabacinum (J.F.H. Beyma) M.E. Palm, W. Gams & Nirenberg), have been frequently isolated from symptomatic roots and appeared to be the main cause of the disease (Infantino et al. 2004; Chilosi et al. 2008; Carlucci et al. 2012). Acremonium cucurbitacearum was also reported as the predominant causal agent of collapse in Spain (García‐Jiménez et al. 1994, 2000), along with M. cannonballus (Beltrán et al. 2008).
Monosporascus cannonballus is considered to be the main responsible for MRRVD alone (Bruton et al. 2000; Chilosi et al. 2008), and in association with the zoosporic fungal parasite of root epidermal cells Olpidium bornovanus (Sahtiyanci) Karling (= Leiolpidium bornovanum Doweld) (Pivonia et al. 1997; Stanghellini and Misaghi, 2011; Stanghellini et al. 2010, 2014; Aleandri et al. 2017). Olpidium bornovanus is also considered as a direct virulent pathogen of melon, causing extensive browning of the roots and growth reductions (Stanghellini et al. 2010, 2014) as well as a vector of several viruses of cucurbits, including melon necrotic spot virus (MNSV). This virus has been reported as a probable cause of melon vine decline in Guatemala (De Cara et al. 2008). In Central Italy, O. bornovanus was isolated from soil with a history of MRRVD (Aleandri et al. 2014) and confirmed to be a melon root pathogen and implicated in MRRVD occurrence likely in association with O. virulentus (Sahtiyanci) Karling (= Olpidiaster virulentus Doweld) (Aleandri et al. 2017). Similarly, both Olpidium species were recently associated with RRVD of watermelon and cucumber in Sardinia (Schianchi et al. 2019; 2020). Olpidium virulentus is a vector of viruses of tomato and lettuce, but not of cucurbits and generally is not considered to be a plant pathogen (Maccarone 2013). Nevertheless, in Ontario, O. virulentus along with the fungal pathogen Verticillium dahliae Kleb., was associated with vine decline of tomato (Johnston-Monje et al. 2017).
Melon is one of the most economically important vegetable crops in Sardinia, covering in 2019 approximately 850 ha, with a yield of 25,000 t (ISTAT 2020). The commonest cultivated melon varieties belong to C. melovar. inodorus, mainly concentrated in the Province of Oristano (West Sardinia). In this cultivation area, melon growers have recorded in the last decade important yield losses due to the occurrence of root rot and vine decline symptoms, which resulted in yield reductions of up to 100% in affected sectors of fields (Balmas et al. 2016). In the same area, diseased melon plants showing necrotic symptoms on leaves and branches, initially referred to as collapse, were caused by MNSV (Tomassoli and Barba 2000). In general, the onset and development of diseases caused by soil-borne pathogens depend on the interaction of several ecological factors, such as the soil microbial community structure and its interactions with pathogens which may play a central role in suppressing the development of the diseases. In fact, the overall soil microbiota may exert beneficial effects, such as stimulating plant growth, plant defences and antagonising plant pathogens, especially in the rhizosphere (Crecchio et al. 2018; Frąc et al. 2018). In this view, the aim of the present study was to assess the occurrence of root-infecting fungi in two melon fields in Central Sardinia with a history of RRVD during 2013 and 2014, and their possible role on the development of the disease. This was accomplished through: i) the isolation and identification of the putative causative fungal agents from roots of symptomatic plants; ii) verify that Olpidium species and MNSV are involved in the onset and development of the disease; iii) the assessment of the soil fungal community structure.
Materials and methods
Plant cultivation
Two farms in the melon-producing area of Central Sardinia in the Province of Oristano, with a history of RRVD were surveyed at Sassu, Arborea, (39°49′06.4″N 8°35′05.38″E) and Sinis, Cabras (39°54′34.40″N 8°26′22.85″E) in 2013 and 2014. Melon (“Valiente” F1, HM.Clause Italia) was cultivated as a monocrop in fields of about 1 ha in size and surveyed between late August and the first ten days of September, at the end of the cropping season, when plants approached maturity and symptoms of RRVD were present. The planting period was in May with the harvest in September.
Soil sampling and M. cannonballus ascospores determination
Soil samples were taken at 10- to 20-cm depth according to a “W” sampling pattern in 2014. Soil samples were then air-dried at room temperature for three weeks and sifted through a 2 mm sieve to remove soil clods before processing. The textural class of the surface horizon (0–20 cm depth) of soils from both sites fell within the clay and alkaline classification based on the particle size distribution and an average pH of 8.2 (Table 1).
The ascospore population of M. cannonballus density in each soil sample was determined in 2014 as described by Stanghellini and Rasmussen (1992).
Isolation of fungal community from diseased roots
For isolation of fungal pathogens, ten melon plants showing RRVD symptoms were sampled in Sassu and Sinis sites according to a “W” sampling pattern. Plants were carefully washed under current water and examined for disease symptoms. Isolations were performed from tap and secondary roots on Potato Dextrose Agar (PDA) (Oxoid Unipath) and Malt Agar (MA) (Oxoid Unipath) amended with streptomycin sulfate (200 mg L−1) and neomycin (100 mg L−1) as described by Chilosi et al. (2008). Monosporic or single-hyphal tip isolates were produced for each colony. Following sporulation, colonies were identified based on morphological criteria. Fusarium spp. were identified based on morphological characteristics of the structures on carnation leaf agar (CLA) and the morphology of the colonies on PDA. The fungal species obtained from the diseased roots were counted based on their frequency of occurrence. The percentage of occurrence was expressed using the formula: Frequency (%) = Number of isolates of a genus × 100/Total number of isolates.
Isolation and identification of Olpidium spp. by bait plants and plant biomass determination
Olpidium spp. were isolated from infested soil in 2014 as described by Herrera-Vásquez et al. (2009) with some modifications (Aleandri et al. 2017). The soil was sampled in both Sassu and Sinis sites at 0-30 cm depth according to a “W” sampling pattern. For each site, collected individual samples were mixed in composite samples. Melon hybrid “Dinero” (Syngenta Seeds, Milano, Italy) which was previously reported to be susceptible (Aleandri et al. 2017), was used as a bait plant. Plants grown in sterilized soil mix, as reported by Stanghellini et al. (2010), were used as a negative control. Seeds were surface sterilized with 3% sodium hypochlorite and sown in sterilized vermiculite for pre-germination. After 10 days, 10 plantlets, with three replicates, were transplanted into pots (0.5 L) containing a mixture of field soil, sterilized quartz sand, sterilized vermiculite (2:1:1 v/v). Negative controls (10 seedlings with three replicates) consisted of plants grown in sterilized potting mix. Plants were left in a growth chamber with 12 h photoperiod, at 26 °C day / 18–20 °C night for forty-five days. After this period, the seedlings were carefully removed, washed with distilled water. The roots were taken from each bait plant for the multiplex PCR assay and for microscopy analyses in detecting and identifying Olpidium spp.
For the estimation of the level of Olpidium spp. colonization, rootlets were prepared as described by Aleandri et al. (2017) and rated using the method described for mycorrhizal colonization (Trouvelot et al. 1986) with some modifications (Aleandri et al. 2017) using MYCOCALC (http://www.dijon.inra.fr/mychintec/Mycocalc-prg/%20download.html). Percentage of colonization by Olpidium spp. was rated on a scale with five classes describing increasing root infection percentages: 0, no infection; 1: 1%; 2: 5%; 3: 30%; 4: 70%; 5: 95% of infection. Colonization by Olpidium spp. was expressed as intensity of the Olpidium spp. colonization in the root system sampled, intensity = (95n5 + 70n4 + 30n3 + 5n2 + n1)/(total n. fragments observed), where n5 = number of fragments rated 5; n4 = number of fragments 4 etc.
To confirm the morphological identification of Olpidium spp., symptomatic roots were analyzed by the multiplex PCR assay as described by Aleandri et al. (2017). Genomic DNA was extracted from root tissue using Nucleospin plant II kit (Macherey–Nagel, Düren, Germany), according to the manufacturer’s protocols. The PCR reactions and products resolution were performed using Olpidium primers mix (Herrera-Vasquez et al. 2009).
For biomass determination, twenty plants per treatment were separated into the aerial part (stems and leaves), and roots and their tissues were dried in a forced-air oven at 70 °C to constant weight.
Melon necrotic spot virus detection
For MNSV detection, total RNA was extracted from aliquots of 10 mg from dried roots pooled sample using RNeasy Mini Kit (Qiagen, Germany) following the manufacturer's instructions. Further, total RNA extracts were obtained from fresh leaves and roots of healthy melon grown in protected greenhouses at CREA-DC and from a MNSV infected sample of CREA-DC virus collection (Tomassoli and Barba 2000; Herrera-Vasquez et al. 2010). Reverse transcriptase (RT) PCR was carried out in a single step using MNSV‐specific primers MNSV1 (5′-GGAGGCAACATTTCGTACA-3′) MNSV2 (5′-AGAGACCAAGCGATCAAAC-3′) (Herrera-Vasquez et al. 2010) designed to amplify a 651 bp fragment of the coat protein gene. One step RT-PCR protocol was performed in a total volume of 25 µl containing 2 µl of total RNA extract, 1X GoTaq® Reaction Buffer (Promega), 1.5 mM MgCl2, 0.2 mM of each dNTP, 0.4 μM of each primer, 0.75U GoTaq®G2 DNA Polymerase (Promega), 1.2U AMV Reverse Transcriptase (Promega) and 20U RNaseOUTTM Recombinant Ribonuclease Inhibitor (Invitrogen, ThermoFisher Scientific). Amplification was performed according to the following conditions: reverse transcription at 46 °C for 30 min, followed by denaturation at 95 °C for 5 min, and by 35 cycles of the following steps: 15 s at 94 °C, 30 s at 55ºC annealing and 45 s at 72 °C with final extension for 10 min at 72 °C. RT‐PCR‐amplified products were separated by electrophoresis on 1.2% agarose gel in 1 × TBE buffer and stained with ethidium bromide. Fragment sizes were determined by comparison with a 100 bp DNA Ladder Plus (MBI Fermentas).
Soil fungal community analysis
For DNA extraction, an aliquot of soil samples collected in the 2014 cropping season was kept at 5 °C until processing. Three biological replicates of each soil sample were processed independently. Five grams per sample were then extracted for total genomic DNA extraction using the ZR Soil Microbe DNA MidiPrep (Zymo Research, USA) following the manufacturer’s instructions. Amplifiability of DNA samples was checked by PCR with ITS5 x ITS4 primers (White et al. 1990). Twenty microliters of genomic DNA samples (10 ng/µl) were sent to Macrogen Inc. (Korea) for amplicon sequencing with 454 GS FLX technology. For amplicon formation, the fungal ITS1 region was chosen (Schoch et al. 2012) and amplified with the ITS1F (CTTGGTCATTTAGAGGAAGTAA) (Gardes and Bruns 1993) and ITS2 (GCTGCGTTCTTCATCGATGC) (White et al. 1990) primers. Data from pyrosequencing were analysed according to Chiellini et al. (2019) and significant changes in the relative abundance of microbial taxa were detected by linear discriminant analysis effect size (LEfSe) method (Segata et al. 2011).
Results
Symptoms observation, M. cannonballus ascospores quantification and culturable fungal species isolation.
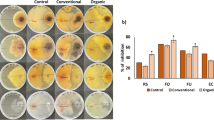
Foliar symptoms of diseased field-grown plants from both farm locations were similar and characterized by yellowing and gradual desiccation of the vines during fruit maturation (Fig. 1). Roots and rootlets of the affected plants were generally characterized by reddish-brown lesions of the tap and secondary roots and necrosis of secondary and tertiary roots. Several fungal pathogens were isolated from symptomatic plants sampled in the sites of Sassu and Sinis, with differences in species pattern and isolation frequency between locations and cropping seasons (Table 2). In 2013, Plectosphaerella melonis was the most frequent fungal species isolated from roots and rootlets from symptomatic melon plants in both surveyed sites, with a higher frequency of isolation from Sassu. In both sites, Macrophomina phaseolina (Tassi) Goidanich were also isolated. Fusarium solani (Mart.) Sacc. was commonly isolated from symptomatic roots collected in Sassu but not in those from Sinis. By contrast, isolates (or strains) belonging to Fusarium incarnatum-equiseti species complex and M. cannonballus were recovered only from plants from Sinis.
A different picture emerged from the etiological analysis performed in 2014. Fusarium solani appeared as the most frequent pathogen in both locations. In Sassu, P. cucumerina, Rhizoctonia solani Kuhn and Fusarium oxysporum Schltdl. were also isolated, whereas in Sinis M. phaseolina and Cylindrocarpon sp. were recovered.
Ascospores of M. cannonballus were recovered from both surveyed melon fields in 2014. The average numbers of ascospores were 0.8 ± 0.25 g−1 soil from Sassu and 1.8 ± 0.25 g−1 soil from Sinis.
Olpidium spp. characterization from bait plants and biometric parameters analysis.
In 2013, Olpidium spores were observed microscopically in rootlets from symptomatic roots sampled in both surveyed sites. This result prompted us to undertake a deeper investigation in the 2014 cropping season on the Olpidium species potentially involved in the disease using bait plants grown on the naturally infested soil showed symptoms of yellowing. Control plants grown in sterilized soil did not show apparent symptoms. Based on the morphology of the resting spores, O. bornovanus was detected in roots of bait plants from Sassu alone and together with O. virulentus in that from Sinis. The root colonization by Olpidium spp. rated using MYCOCALC software reached an intensity level in the root system of bait plants of 41.4% and 31.2% respectively from Sinis and Sassu. The identification of both O. bornovanus and O. virulentus was confirmed by the multiplex PCR assay.
None of the root extracts from the root pooled sample were positive for MNSV by RT‐PCR. The expected DNA fragment of 651 bp was amplified from the extract of the positive control sample, but not from healthy leaves and root extracts.
Results of dry biomass analysis of aerial part and root system from bait plants are shown in Table 3. Plants grown in sterilized control soils from Sinis had significantly higher aerial part biomass compared to that naturally infested, whereas those from Sassu were statistically similar. Root biomass from plants grown in sterilized soil from Sinis was similar to that from the naturally infested soil, whereas those grown in the sterilized one from Sassu resulted statistically higher than that from naturally infested sampled in Sinis.
Analysis of soil fungal community composition
The analysis of the soil fungal microbiota in both sites was carried out on samples collected in 2014. The results revealed the predominance of fungi belonging to the phylum Ascomycota (about 60% of the total), followed by Zygomycota and Basidiomycota (Fig. 2). The overall fungal community structure analysis showed significant differences between the two sites. More specifically, the relative abundance of Zygomycota and Glomeromycota was much higher in Sinis than in Sassu (+ 98.7% and 217.9%, respectively, p < 0.05). On the other hand, significant differences in Ascomycota and Basidiomycota frequency did not occur.
In Fig. 3 are graphically represented the results of the LEfSe analysis, showing statistically and biologically consistent differences between Sassu and Sinis sites. Differences are represented in the colour of the most abundant class (red indicating Sassu, green indicating Sinis, and yellow non-significant). As expected, at the phylum level Ascomycota generally displayed a higher fungal abundance in Sassu site than in that of Sinis. Specifically, most of such differences occurred within fungi belonging to Venturiales, Eurotiomycetes, Pezizomycetes, and Saccharomycetes taxa (b, d, i and k in Fig. 3) which abundance values in Sassu resulted almost 2–3 times than in Sinis, ranging from 3.2–4.3% to 0.5–1.9%, respectively. Consistent differences in the compositions and structures among the beneficial fungal communities of the surveyed fields were found by NGS analysis (Table 4). Most of them were detected from the soil of Sassu with a higher relative abundance of beneficial and antagonistic fungi such as Cladosporium cladosporioides (Fresen.) G.A. de Vries, Sordaria araneosa Cain. Moreover, to a lesser extent, also genera Cladosporium, Chrysosporium, Aspergillus, and Penicillium were more abundant in Sassu than in Sinis. Despite Sordariomycetes showed higher values in Sinis than in Sassu, the relative abundance of some of its genera belonging to Sordariales such as Acremonium, Fusarium, and Cercophora spp. displayed significantly higher values in Sassu than Sinis. Among Ascomycota, Spegazzinia (Incertae sedis), Pleospora (Dothideomycetes), and Lecythophora (Sordariomycetes) were the main genera which were more abundant in Sinis than in Sassu.
Cladogram generated by LEfSe analysis showing enriched taxa in soils from Sassu (red) and Sinis sites (green). The central points represents the root of the tree (Fungi), and each ring represents the next lower taxonomic level (phylum through genus). The diameter of each circle represents the relative abundance of the taxon
Conversely, in Sinis site, taxa belonging to Zygomycota revealed to be about 100% more abundant than in Sassu, and most of which corresponded to Mortierella genus (about 14% in Sassu and 30.2% in Sinis). Moreover, also Glomeromycota and Chytridiomycota were more abundant in Sinis than in Sassu. Specifically, Glomeraceae and the genus Olpidium showed significantly higher values in Sinis than in Sassu.
Different cucurbit pathogens were detected in both surveyed sites (Table 5). Among them, O. bornovanus, M. cannonballus, P. melonis and F. solani detection from soil paralleled their occurrence in the cropping season in 2013 on symptomatic plants. Conversely, M. phaseolina isolated from roots sampled from both sites, F. equiseti isolated roots from Sinis and O. virulentus baited from soil sampled in Sinis were not detected by NGS analysis. In 2014, fungal pathogens isolated by plants corresponding to that found by NGS analysis were F. solani from both sites, and P. cucumerina, from Sassu.
Discussion
In this study, we characterized the culturable potential pathogens colonizing roots of plants with symptoms of MRRVD and soil fungal composition in two fields located in the same climatic region with soil characterized by limited differences.
The results presented in this work indicate that the MRRVD symptoms observed in two consecutive years in two different locations in Central Sardinia on melon grown as a monocrop can be associated with a different pattern of potential pathogens. MNSV which was previously found to be associated with the disease (De Cara et al. 2008) was not detected. In 2013, P. melonis, which is considered as one of the main causal agents of the disease in the Mediterranean basin (Alfaro-Garcìa et al. 1996; Chilosi et al. 2008; Carlucci et al. 2012), appeared to be the most frequently isolated species in both sites. Fusarium solani, a species complex containing isolates that cause diseases in many horticultural crops including melon (Chilosi et al. 2008), was the preponderant pathogen isolated from symptomatic roots sampled in 2014. From symptomatic plants sampled in Sassu, M. phaseolina and P. cucumerina have been also isolated respectively in 2013 and 2014. The former is a polyphagous pathogen occurring in both temperate and tropical regions of the world (Manici et al. 1995) and is often referred to as the causative agent of charcoal rot because of the dark coloration of the colonized host tissues. The latter has been reported as one of the fungi associated with cucurbit collapse by Carlucci et al. (2006). Interestingly, M. phaseolina infection of melon was found to be often accompanied by various Fusarium species, mostly F. solani (Chilosi et al. 2008; Cohen et al. 2012). Fusarium equiseti was frequently isolated from symptomatic plants collected in Sinis in 2013, however, to our knowledge, it has never been associated with RRVD. Monosporascus cannonballus has been isolated from 20% of symptomatic plants from Sinis in the cropping season 2013, whereas was not detected in Sassu and in both localities during 2014. This result might be due to differences in inoculum potential and environmental conditions such as the microbial community and edaphic characteristics. Ascospores density was consistently higher in soil from Sinis (1.8 ascospores g−1 soil), than that found from the soil in Sassu (0.8 ascospores g−1 soil). The value from Sinis is close to that indicated by Waugh et al. (2003) as a minimum threshold of inoculum for this disease, but still within the range reported in other melon and watermelon producing areas in the Mediterranean area where the disease occurs (Beltrán et al. 2007; 2008; Rhouma et al. 2019). It has been shown that M. cannonballus is adapted to hot, semiarid climates with saline and alkaline soils (Martyn and Miller 1996). Moreover, it has been shown by Boughalleb et al. (2010) in Tunisia that vertisol vs. other soils, disease incidence, the percentage of clay, and pH had a significant correlation with ascospores density at the end of the growing season. In a study aimed to assess the relationship between physicochemical soils properties and spatial distribution of M. cannonballus ascospores densities in Tunisia, a positive correlation was found between ascospores density and organic matter, organic carbon, the mass of nitrogen, and electrical conductivity with an ascospores highest level at 10–20 cm depth (Rhouma et al. 2019). In this respect, there are no apparent chemical-physical differences in the soils of the two monitored locations that can be associated with the differences in inoculum density recorded. The soil from Sinis had higher levels of organic matter and organic carbon, while Sassu had a higher level of total nitrogen and salinity. The only difference between soil was the highest level of the organic matter recorded in Sinis soil. This might have favored the disease by increasing the soil moisture level (Libohova et al. 2018), which is a factor that was found to directly affect colonization of root epidermal cells by zoospores of O. bornovanus and indirectly affect ascospore germination and root colonization by M. cannonballus (Stanghellini et al. 2014).
Olpidium bornovanus was isolated in bait plants from both sites and detected by NGS analysis, while O. virulentus was isolated only by bait plants from Sinis. The potential role of these pathogens in the disease occurrence can be suggested since M. cannonballus was not detected in the rootlets. Bait plants from both sites had a general decrease in biomass and showed yellowing of foliage, thus indicating that Olpidium infection had a negative effect on plant health. Notably, among Chytridiomycetes, Olpidium spp. was more abundant in Sinis (0.97%) than Sassu (0.64%). Previous work has shown that O. bornovanus, in combination with O. virulentus and M. cannonballus, was capable to induce root rot and vine decline in bait plants from soil sampled under greenhouse in a farm with a known RRVD history (Aleandri et al. 2017). Similarly, O. virulentus was suggested as one of the causative agents of vine decline of tomato in Ontario, Canada (Johnston-Monje et al. 2017). Therefore, our results suggest that, in agreement with previous findings, MRRVD, which was previously attributed in Italy mainly to M. cannonballus, P. melonis and P. cucumerina can be associated in Central Sardinia also to O. bornovanus and O. virulentus.
Soil microbial diversity and community structure are known to be shaped by both spatial and temporal variability, especially in the rhizosphere where the continuous flow of organic compounds derived from roots is available (Smalla et al. 2001). Fungal community composition was reported as most closely associated with changes in soil nutrient status, whereas soil pH was shown to be the most influential factor for the bacterial community (Lauber et al. 2008). According to this finding, we found in the two sites a variation in carbon, total nitrogen, and P2O5 content which, although slight, may have influenced the fungal community composition, while this appeared not structured by pH, since an identical result in the two sites was detected.
Among soil microbial communities, beneficial microorganisms not only serve plants in acquiring water and nutrients but also promote plant growth and stimulate plant health (Berendsen et al. 2012). A group of beneficial fungi implicated in promoting plant health, such as the mycorrhizal fungus Rhizophagus irregularis and the antagonistic Trichoderma atroviride Bissett and T. aureoviride Rifai from both fields. Other species, such as C. cladosporioides, Sordaria araneosa Cain, were mainly present in soils from Sassu, whereas the relative abundance of beneficial fungi was consistently lower in Sinis. Aspergillus spp. and Penicillium spp., which include saprophytic and antagonistic species were poorly represented in both Sassu (0.66% and 0.29%, respectively) and Sinis (0.28% and 0.94%, respectively). The different composition of the population of pathogenic fungi detected on symptomatic plants in the two surveyed locations might be related also to the different composition of the beneficial microbiological community along with difference in some soil chemical characteristics, such as organic carbon and nitrogen.
The predominant species of Ascomycota detected in Sassu have been described as belonging to Pezizomycetes, Saccharomycetes, and Sordariales. Among Pezizomycetes, the dominant genus was Scutellinia, which showed the highest percentages in Sassu compared to Sinis (1.96% vs 0.02%, respectively) and whose species are generally considered saprophytic fungi well adapted to bare ground and scattered vegetation, with relatively high pH and low organic matter (Schumacher 1993). The presence of Saccharomycetes, mainly represented by saprophytic species of the family Dipodascaceae (2.6%), have a widespread distribution and are commonly found in decaying plant tissues. Their higher presence in Sassu might be related to the different N source availability in such soils (De Hoog et al. 1986). However, the main genus of this family is Geotrichum candidum Link, which is known to cause disease sour rot in several plants (Thornton et al. 2010), including melon (Kim et al. 2011).
The increase of the relative abundance of soil Sordariales in Sassu compared to Sinis (1.2% vs 0.2%, respectively) might be due to the higher sand values occurring in Sassu. It was recently reported that the presence of soil macro-aggregates (> 2 mm) might enhance the abundance of soil Sordariales (Tian et al. 2019). Interestingly, Sordariomycetes also include Xylariales which dominate Sinis soils compared to Sassu (11.9% vs 6.3%, respectively), which includes Monosporascus spp. and M. cannonballus. However, the occurrence of M. cannonballus detected by NGS analyses was 0.02% in Sinis soils whereas it was not detected in Sassu. Nevertheless, most of the species belonging to Xylariales detected were “unidentified”, both in Sassu (5.9%) and Sinis (11.2%).
On the other hand, Sinis soils were dominated by the fungal genus Mortierella, belonging to Zygomycota and accounting for 30.2% of the total fungal sequences (only 14.3% in Sassu soil). This might explain the low abundance of Fusarium spp. which were more abundant in Sassu (1.7%) than in Sinis (0.3%). Previous studies have shown that some species of Mortierella are enriched in suppressive soils, and several isolates have been revealed as potential antagonistic agents against various plant pathogens such as Fusarium spp. (Xiong et al. 2017).
Conclusions
Our overall results confirm that among the fungal complex implicated in the occurrence of RRVD, O. bornovanus along with O. virulentus, probably in association not only with M. cannonballus as previously found but also with other pathogenic fungi (P. melonis, M. phaseolina and F. solani), likely are implicated in the development of the disease. Differences in the pathogenic fungal spectrum here recorded in symptomatic roots may be associated with agricultural practices, soil physicochemical characteristics, microclimatic conditions, and fungal community composition and function profile. Further research should focus on getting information on direct disease determinants such as the composition of roots exudates produced by melon grown in different agronomical conditions and their role in providing the basis for the establishment of plant–microorganism interactions in soil. The rapid and continued developments of NGS techniques, along with a sharp decrease of the prices of the analyses, will allow in the next future to better understand and decipher the role of the forces shaping the structure of microbial populations of natural and agricultural soils (Srivathsan et al. 2018; Nilsson et al. 2019).
Data Availability Statement
The datasets sequenced and analyzed in this study can be found in the NCBI repositories as BioProject PRJNA673326 and BioSamples as SAMN16604350 (Sassu location) and SAMN16604351 (Cabras location).
References
Aleandri MP, Martignoni D, Reda R, Alfaro-Fernandez A, Font MI, Armengol J, Chilosi G (2014) First report of Olpidium bornovanus and O. virulentus on melon in Italy. Plant Dis 98:997. https://doi.org/10.1094/PDIS-10-13-1041-PDN
Aleandri MP, Martignoni D, Reda R, Chilosi G (2015a) Effects of preconditioning through mycorrhizal inoculation on the control of melon root rot and vine decline caused by Monosporascus cannonballus. J Phytopathol 163:898–907. https://doi.org/10.1111/jph.12389
Aleandri MP, Chilosi G, Bruni N, Vettraino TA, AM, Vannini A, (2015b) Use of nursery potting mixes amended with local Trichoderma strains with multiple complementary mechanisms to control soil-borne diseases. Crop Prot 67:269–278. https://doi.org/10.1016/j.cropro.2014.10.023
Aleandri MP, Martignoni D, Reda R, Alfaro-Fernandez A, Font MI, Armengol J, Chilosi G (2017) Involvement of Olpidium bornovanus and O. virulentus in the occurrence of melon root rot and vine decline caused by Monosporascus cannonballus in Central Italy. J Plant Pathol 99:169–176. https://doi.org/10.4454/jpp.v99i1.3787
Alfaro-Garcìa A, Armengol J, Bruton BD, Gams W, Garcìa-Jimenez J, Martinez-Ferrer G (1996) The taxonomic position of the causal agent of Acremonium collapse of muskmelon. Mycologia 88:804–808. https://doi.org/10.1080/00275514.1996.12026718
Balmas V, Infantino A, Chilosi G, Siddu G, Murru S (2016) Collasso delle cucurbitacee, diffusione e difesa in Sardegna. L’Informatore Agrario 18:53–56
Beale RE, Pitt D (1995) The antifungal properties of Minimedusa polyspora. Mycol Res 99:337–342. https://doi.org/10.1016/S0953-7562(09)80910-6
Beltrán R, Vicent A, Garcia-Jiménez J, Armengol J (2007) Quantification of Monosporascus cannonballus ascospores in muskmelon fields in Eastern Spain. J Phytopathol 155:248–250. https://doi.org/10.1111/j.1439-0434.2007.01215.x
Beltrán R, Vicent A, García-Jiménez J, Armengol J (2008) Comparative epidemiology of Monosporascus root rot and vine decline in muskmelon, watermelon, and grafted watermelon crops. Plant Dis 92:158–163. https://doi.org/10.1094/PDIS-92-1-0158
Ben Salem I, Correia KC, Boughalleb N, Michereff SJ, León M, Abad-Campos P, García-Jiménez J, Armengol J (2013) Monosporascus eutypoides, a cause of root rot and vine decline in Tunisia, and evidence that M. cannonballus and M. eutypoides are distinct species. Plant Dis 97:737–743. https://doi.org/10.1094/PDIS-05-12-0464-RE
Berendsen RL, Pieterse CM, Bakker PA (2012) The rhizosphere microbiome and plant health. Trends in Plant Sci 17:478–486. https://doi.org/10.1016/j.tplants.2012.04.001
Boughalleb N, Salem IB, Beltrán R, Vicent A, Sierra AP, Abad-Campos P, Garcìa-Imenez J, Armengol J (2010) Occurrence of Monosporascus cannonballus in watermelon fields in Tunisia and factors associated with ascospore density in soil. J Phytopathol 158:137–142. https://doi.org/10.1111/j.1439-0434.2009.01591.x
Bruton BD, Garcia-Jimenez J, Armengol J, Popham TW (2000) Assessment of virulence of Acremonium cucurbitacearum and Monosporascus cannonballus on Cucumis melo. Plant Dis 84:907–913. https://doi.org/10.1094/PDIS.2000.84.8.907
Carlucci A, Raimondo ML, Frisullo S (2006) Different morphotypes of Plectosporium tabacinum associated with collapse of cucurbit in Apulia. J Plant Pathol 88, S36
Carlucci A, Raimondo ML, Santos J, Phillips AJL (2012) Plectosphaerella species associated with root and collar rots of horticultural crops in southern Italy. Persoonia: Molecular Phylogeny and Evolution of Fungi 28:34. https://doi.org/10.3767/003158512X638251
Chiellini C, Cardelli V, De Feudis M, Corti G, Cocco S, Agnelli A, Massaccesi L, Donato Alessi G, Mengoni A, Mocali S (2019) Exploring the links between bacterial communities and magnetic susceptibility in bulk soil and rhizosphere of beech (Fagus sylvatica L.). App Soil Ecol 138:69–79. https://doi.org/10.1016/j.apsoil.2019.02.008
Chilosi G, Reda R, Aleandri MP, Camele I, Altieri L, Montuschi C, Languisco L, Rossi V, Agosteo GE, Macrì C, Carlucci A, Lops F, Mucci M, Raimondo ML, Frisullo S (2008) Fungi associated with root rot and collapse of melon in Italy. EPPO Bull 38:147–154. https://doi.org/10.1111/j.1365-2338.2008.01200.x
Clement JA, Dombrowski AD, Sigmund JM, Goetz MA (2016) Occurrence of Podosporin B In Podospora decipiens and Sordaria araneosa. Planta Med 82:PC25. https://doi.org/10.1055/s-0036-1578727
Cohen R, Pivonia S, Burger Y, Edelstein M, Gamliel A, Katan J (2000) Toward integrated management of Monosporascus wilt of melons in Israel. Plant Dis 84:496–505. https://doi.org/10.1094/PDIS.2000.84.5.496
Cohen R, Pivonia S, Crosby KM, Martyn RD (2012) Advances in the biology and management of Monosporascus vine decline and wilt of melons and other cucurbits. Hortic Rev 39:77–120. https://doi.org/10.1002/9781118100592.ch2
Crecchio C, Mimmo T, Bulgarelli D, Pertot I, Pii Y, Perazzolli M, Scagliola M, Cesco S (2018) Beneficial Soil Microbiome for Sustainable Agriculture Production. In: Lichtfouse E (ed) Sustainable Agriculture Reviews 31. Springer, Cham, pp 443–481. https://doi.org/10.1007/978-3-319-94232-2_9
De Cara M, López V, Córdoba MC, Santo M, Jordá C, Tello JC (2008) Association of Olpidium bornovanus and melon necrotic spot virus with vine decline of melon in Guatemala. Plant Dis 92:709–713. https://doi.org/10.1094/PDIS-92-5-0709
de Hoog GS, Smith MT, Guého E (1986) A revision of the genus Geotrichum and its teleomorphs. Stud Mycol 29:1–131
El-Desouky SM, El-Wakil AA (2003) Occurrence of Monosporascus root rot and vine decline of cantaloupe and watermelon in Egypt. Egypt J Phytopathol 31:141–150
Falk SP, Gadoury DM, Seem PRC, RC, (1995) Partial control of grape powdery mildew by the mycoparasite Ampelomyces quisqualis. Plant Dis 79:483–490. https://doi.org/10.1094/PD-79-0483
Frąc M, Hannula SE, Bełka M, Jędryczka M (2018) Fungal biodiversity and their role in soil health. Front Microbiol. https://doi.org/10.1007/s42161-019-00314-6
García-Jiménez J, Velázquez MT, Jorda C, Alfaro-Garcia A (1994) Acremonium species as the causal agent of muskmelon collapse in Spain. Plant Dis 78:416–419
García-Jiménez J, Armengol J, Sales R, Jordá C, Bruton BD (2000) Fungal pathogens associated with melon collapse in Spain. EPPO Bull 30:169–173. https://doi.org/10.1111/j.1365-2338.2000.tb00873.x
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118. https://doi.org/10.1111/j.1365-294X.1993.tb00005.x
Herrera-Vásquez JÁ, del Carmen CM, Alfaro-Fernández A, del Carmen C-S, Jordá C (2009) Multiplex PCR assay for the simultaneous detection and differentiation of Olpidium bornovanus, O. brassicae, and O. virulentus. Mycol Res 113:602–610. https://doi.org/10.1016/j.mycres.2009.01.007
Herrera-Vásquez JÁ, Córdoba-Sellés MC, Cebrián M, Rosselló JA, Alfaro-Fernández A, Jordá C (2010) Genetic diversity of Melon necrotic spot virus and Olpidium isolates from different origins. Plant Pathol 59:240–251. https://doi.org/10.1111/j.1365-3059.2009.02208.x
Infantino A, Carlucci A, Pucci N, Ciuffreda G, Montuschi C, Lops F, Uccelletti A, Mucci M, Frisullo S (2004) Funghi che causano marciumi radicali e il collasso delle cucurbitacee in Italia. Petria 14:77–89
ISTAT (Istituto Nazionale di Statistica, Italy) 2020. http://dati.istat.it/Index.aspx?QueryId=33703#
Jager G, Velvis H (1984) Biological control of Rhizoctonia solani on potatoes by antagonists. 2. Sprout protection against soil-borne R. solani through seed inoculation with Verticilium biguttatum. Neth J Plant Pathol 90:29–33. https://doi.org/10.1007/BF02014180
Johnston-Monje D, Loewen S, Lazarovits G (2017) Mycobiomes of tomato plants with vine decline. Can J Plant Pathol 39:184–200. https://doi.org/10.1080/07060661.2017.1325938
Lauber CL, Strickland MS, Bradford MA, Fierer N (2008) The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol Biochem 40:2407–2415. https://doi.org/10.1016/j.soilbio.2008.05.021
Lewis JA, Papavizas GC (1993) Stilbella aciculosa: a potential biocontrol fungus against Rhizoctonia solani. Biocontrol Sci Techn 3:3–11. https://doi.org/10.1080/09583159309355253
Libohova Z, Seybold C, Wysocki D, Wills S, Schoeneberger P, Williams C, Lindbo D, Stott D, Owens PR (2018) Reevaluating the effects of soil organic matter and other properties on available water-holding capacity using the National Cooperative Soil Survey Characterization Database. J Soil Water Conserv 73:411–421. https://doi.org/10.2489/jswc.73.4.411
Lieckfeldt E, Kullnig CM, Kubicek CP, Samuels GJ, Börner T (2001) Trichoderma aureoviride: phylogenetic position and characterization. Mycol Res 105:313–322. https://doi.org/10.1017/S0953756201003616
Lo Piccolo S, Alfonzo A, Giambra S, Conigliaro G, Lopez-Llorca LV, Burruano S (2015) Identification of Acremonium isolates from grapevines and evaluation of their antagonism towards Plasmopara viticola. Ann Microbiol 65:2393–2403. https://doi.org/10.1007/s13213-015-1082-5
Kim YK, Kim TS, Shim HS, Park KS, Yeh WH, Hong SJ, Shim C, Kim J, Jong-Ho Park J, Han E, Lee MH, Hyeong-Jin J (2011) First report of sour rot on post-harvest oriental melon, tomato, cucumber, potato, pumpkin and carrot caused by Geotrichum candidum. Res Plant Dis 17:232–234. https://doi.org/10.5423/RPD.2011.17.2.232
Maccarone LD (2013) Relationships between the pathogen Olpidium virulentus and viruses associated with lettuce Big-Vein Disease. Plant Dis 97:700–707. https://doi.org/10.1094/PDIS-10-12-0979-FE
Manici LM, Caputo F, Cerato C (1995) Temperature responses of isolates of Macrophomina phaseolina from different climatic regions of sunflower production in Italy. Plant Dis 79:934–938
Markakis EA, Trantas EA, Lagogianni CS, Mpalantinaki E, Pagoulatou M, Ververidis FN, Goumas DE (2017) First report of root rot and vine decline of melon caused by Monosporascus cannonballus in Greece. Plant Dis 102:1036. https://doi.org/10.1094/PDIS-10-17-1568-PDN
Martyn RD, Miller ME (1996) Monosporascus root rot and vine decline: An emerging disease of melons worldwide. Plant Dis 80:716–725. https://doi.org/10.1094/PD-80-0716
Nilsson RH, Anslan S, Bahram M, Wurzbacher C, Baldrian P, Tedersoo L (2019) Mycobiome diversity: high-throughput sequencing and identification of fungi. Nat Rev Microbiol 17:95–109. https://doi.org/10.1038/s41579-018-0116-y
Pivonia S, Cohen R, Kafkafi U, Ze’ev IB, Katan J (1997) Sudden wilt of melons in southern Israel: fungal agents and relationship with plant development. Plant Dis 81:1264–1268. https://doi.org/10.1094/PDIS.1997.81.11.1264
Rhouma A, Salem IB, M’hamdi M, Boughalleb-M’Hamdi N, (2019) Relationship study among soils physico-chemical properties and Dawn Brooks ascospores densities for cucurbit fields in Tunisia. Eur J Plant Pathol 153:65–78. https://doi.org/10.1007/s10658-018-1541-5
Scervino JM, Papinutti VL, Godoy MS, Rodriguez MA, Della Monica I., Recchi M., Pettinari MJ, Godeas AM (2011) Medium pH, carbon and nitrogen concentrations modulate the phosphate solubilization efficiency of Penicillium purpurogenum through organic acid production. J Appl Microbiol 110:1215–1223
Schianchi N, Oggiano L, Chilosi G, Balmas V (2019) First report of Olpidium bornovanus and O. virulentus on watermelon in Sardinia. Italy J Plant Pathol 101:1253. https://doi.org/10.1007/s42161-019-00314-6
Schianchi N, Oufensou S, Porqueddu G, Chilosi G, Balmas V (2020) First report of Olpidium virulentus, O. bornovanus, O. brassicae on cucumber in Sardinia, Italy. J Plant Pathol 1–1 https://doi.org/10.1007/s42161-020-00605-3
Schumacher T (1993) Ecology and distribution of the genus Scutellinia in Norway. Arct Alp Mycol 3:215–233
Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA, Chen W (2012) Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc Natl Acad Sci 109:6241–6246. https://doi.org/10.1073/pnas.1117018109
Scholler M, Rubner A (1999) Arthrobotrys hertziana sp. nov. from the Canary Islands. Mycol Res 103:764–768. https://doi.org/10.1017/S095375629800759X
Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C (2011) Metagenomic biomarker discovery and explanation. Genome Biol 12:R60. https://doi.org/10.1186/gb-2011-12-6-r60
Smalla K, Wieland G, Buchner A, Zock A, Parzy J, Kaiser S, Roskot N, Heuer H, Berg G (2001) Bulk and rhizosphere soil bacterial communities studied by denaturing gradient gel electrophoresis: plant-dependent enrichment and seasonal shifts reveale. Appl Environ Microbiol 67:4742–4751. https://doi.org/10.1128/AEM.67.10.4742-4751.2001
Srivathsan A, Baloğlu Wang W, Tan WT, Bertrand D, Ng AHQ, Boey EJH, Koh JJY, Nagarajan N, Meier R (2018) A MinIONTM-based pipeline for fast and cost-effective DNA barcoding. Mol Eco Resour 18:1035–1049. https://doi.org/10.1111/1755-0998.12890
Stanghellini ME, Rasmussen SL (1992) A quantitative method for the recovery of ascospores of Monosporascus cannonballus from field soil. Phytopathology 82:1115
Stanghellini ME, Mathews DM, Misaghi IJ (2010) Pathogenicity and management of Olpidium bornovanus, a root pathogen of melons. Plant Dis 94:163–166. https://doi.org/10.1094/PDIS-94-2-0163
Stanghellini ME, Misaghi IJ (2011) Olpidium bornovanus-mediated germination of ascospores of Monosporascus cannonballus: a host-specific rhizosphere interaction. Phytopathology 101:794–796. https://doi.org/10.1094/PHYTO-11-10-0313
Stanghellini ME, Mohammadi M, Adaskaveg JE (2014) Effect of soil matric water potentials on germination of ascospores of Monosporascus cannonballus and colonization of melon roots by zoospores of Olpidium bornovanus. Eur J Plant Pathol 139:393–398. https://doi.org/10.1007/s10658-014-0395-8
Thornton CR, Slaughter CD, Davis RM (2010) Detection of the sour-rot pathogen Geotrichum candidum in tomatoes fruit and juice by using a highly specific monoclonal antibody-based ELISA. Int J Food Microbiol 43:166–172. https://doi.org/10.1016/j.ijfoodmicro.2010.08.012
Tian XL, Wang CB, Bao XG, Wang P, Li XF, Yang SC, Ding GC, Christie P, Li L (2019) Crop diversity facilitates soil aggregation in relation to soil microbial community composition driven by intercropping. Plant Soil 436:173–192. https://doi.org/10.1007/s11104-018-03924-8
Tomassoli L, Barba M (2000) Occurrence of Melon Necrotic Spot Carmovirus in Italy. EPPO Bull 30:279–280. https://doi.org/10.1111/j.1365-2338.2000.tb00895.x
Trouvelot A, Kough JL, Gianinazzi-Pearson V (1986) Mesure du taux de mycorhization VA d’un système radiculaire. Recherche de méthodes d’estimation ayant une signification fonctionnelle. In: Gianinazzi-Pearson V, Gianinazzi S (eds) Mycorrhizae: Physiology and Genetics. INRA, Paris, France , pp 217–221
Wang X, Radwan MM, Taráwneh A, Gao J, Wedge DE, Rosa LH, Cutler HG, Cutler SJ (2013) Antifungal activity against plant pathogens of metabolites from the endophytic fungus Cladosporium cladosporioides. J Agr Food Chem 61:4551–4555. https://doi.org/10.1021/jf400212y
White TJ, Brums T, Lee S, Taylor J (1990) Amplification phylogenetics. In: Innis N, Gelfand D, Sninsky J, White T (eds) PCR Protocols: a Guide to Methods and Applications. Accademic Press, San Diego, pp 315–322
Waugh M, Kim DH, Ferrin DM, Stanghellini ME (2003) Reproductive potential of Monosporascus cannonballus. Plant Dis 87:45–50. https://doi.org/10.1094/PDIS.2003.87.1.45
Xiong W, Li R, Ren Y, Liu C, Zhao Q, Wu H, Jousset A, Shen Q (2017) Distinct roles for soil fungal and bacterial communities associated with the suppression of vanilla Fusarium wilt disease. Soil Biol Biochem 107:198–207. https://doi.org/10.1016/j.soilbio.2017.01.010
Acknowledgements
This work was supported by a project grant from Regione Sardegna (delibera della Giunta Regionale n. 52/101). Authors thank Gianfranco Siddu (Op-Ortofrutta cooperativa produttori di Arborea) and Sandro Murru (Agenzia Laore Sardegna) for technical assistance in the on-field trials.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All other authors herewith declare that they have no conflict of interest. This study does not contain studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Infantino, A., Balmas, V., Schianchi, N. et al. Diversity of soil-borne fungal species associated to root rot and vine decline of melon in Sardinia (Italy). J Plant Pathol 103, 421–432 (2021). https://doi.org/10.1007/s42161-021-00774-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42161-021-00774-9