Abstract
Because of its known anti-bacterial properties, we explored the potential of Xanthium strumarium, an invasive, enormous mass-producing weed, for the control of Ralstonia solanacearum which causes bacterial wilt (BW) of tomato. Both in-vitro and in-planta experiments were conducted, using different concentrations of the dried powders of the plant parts applied to infested soil at different times. Addition of a 20% (w/v) aqueous extract of leaf powder or succulent shoot powder to wells cut in nutrient agar inhibited growth of R. solanacearum. In in-planta experiments, 4.5% (w/w) leaf powder applied to artificially infested soil 10 days before transplant (DBT), produced the best effect and enhanced root length, shoot length, and plant fresh bio-mass by 64%, 37%, and 42%, respectively, as compared to inoculated control. Leaf powder also lowered the area under disease progress curve (AUDPC) by 38%, and the pathogen counts (g−1 dry soil) by 1.202 log10 units. Succulent shoot powder (4.5% w/w) applied 20 DBT proved to be better than other application times and increased root length, shoot length, and plant fresh bio-mass by 55%, 42%, and 57%, respectively, as compared to inoculated control. Succulent shoot powder also decreased AUDPC by 35%, and the pathogen counts (g−1 dry soil) by 1.294 log10 units. Our data strongly suggest that 4.5% (w/w) of leaf or succulent shoot powder, applied 20 DBT, can be an effective component of the integrated disease management (IDM) against BW.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tomato (Solanum lycopersicum L.) is one of the most valuable vegetable crops. Besides being a major source of vitamins, minerals and other nutrients, it is considered as a cash crop for medium and small-scale land holders of Pakistan. It has diverse uses including raw, cooked or processed. Originally being a tropical plant, it is now grown worldwide. China, USA, India and Turkey are the top producers of tomato. In Pakistan, tomato consumption frequently exceeds its production forcing the country to import the commodity from India and Afghanistan. Pakistan’s average tomato yield is much lower as compared to the world’s, i.e., 10.7 tons/ha vs. 36 tons/ha (MINFAL 2009). Among many other factors responsible for low yield, the occurrence of bacterial diseases is one major cause (Laterrot 1998). Bacterial wilt (BW) of tomato, caused by Ralstonia solanacearum, is one of the most destructive diseases of the crop. The initial symptoms of this disease include wilting of leaves during the hot part of the day and recovering during night. Later, the whole plant permanently wilts and dies. The presence of long, dark brown streaks in vascular bundles of young infected stem is another prominent symptom. The distinctive sign of the disease, however, is the release of white milky exudates by freshly cut infected stem. The disease quickly progresses after infection, especially if the temperature ranges between 29 °C and 35 °C (Champoiseau et al. 2009).
The pathogen is highly diversified and is considered as a species complex. It consists of a large number of strains which differ from each other in terms of biochemical properties, pathogenicity and geographical distribution (Hayward 1994). Race 3, biovar 2 of this species complex is the most important causing huge annual losses in as many as 80 countries of the world, including Pakistan (Floyd 2007). Yield losses range from 10 to 30% in tobacco, 0–90% in tomato, 80–100% in banana, 20% in groundnut and up to 90% in potato (Elphinstone 2005). In Pakistan, Punjab and Sindh provinces are significantly affected by this disease. The frequency of the disease in these provinces ranges from 5 to 25%, indicating a potential threat to the cultivation of many solanaceous vegetables (Burney et al. 1999). Multiple vegetable crops have been shown to be seriously vulnerable to this disease. The incidence of the disease was found to be 22% in sweet peppers, 17% in hot peppers, 13% in tomatoes, 10% in potatoes and 5% in brinjals (Begum et al. 2012). Furthermore, biovar 3 is the most dominant strain of BW pathogen found in Pakistan (Begum et al. 2012; Shahbaz et al. 2015).
Control of plant bacterial diseases in general and BW in particular, is not easy. The main hurdles include the ability of the pathogen to survive in weeds, in multiple hosts, deeper in soil, in water, and its high variability (Wang and Lin 2005; Nguyen and Ranamukhaarachchi 2010). Chemical control is not feasible because of the non-availability of efficient commercial antibacterial chemicals, the ability of the pathogen to develop resistance against them and environmental hazards caused by them (Oguwike et al. 2013). The efficacy of biological control is limited by the unreliable colonization, narrow range and the high inoculum requirement of the bio-control agents (Whipps and Gerhardson 2007). The use of resistant varieties to control the disease is rendered ineffective because of the high variability of the pathogen and the breakdown of resistance under conditions favorable for disease development. This situation necessitates the use of integrated disease management (IDM) which is considered as the best measure for the control of diseases like BW. IDM approach has the potential to reduce BW up to 100% under in-vitro or under field conditions (Anith et al. 2004).
One effective component of IDM against plant diseases including BW could be the use of medicinal plants having antibacterial properties. Most plant products are reportedly (Tripathi and Dubey 2004) safe, non-phytotoxic, biodegradable and could be used in different formulations such as green manure, dried powder organic amendments (OAs) (Naz et al. 2015a, b) or aqueous extract treatments (Askarne et al. 2012) for the control of plant diseases caused by different pathogens. Many plants have been found to have strong anti-bacterial properties (Lo Cantore et al. 2004). The use of such plants could provide an effective component of IDM against different bacterial plant diseases including BW. One-month-old potted tomato plants (cv. Pullrex) sprayed with aqueous extracts of fresh Allium sativum (1%, w/v) and Ficus carica (30%, w/v) were protected against Pseudomonas syringae pv. syringae (bacterial speck of tomato), Xanthomonas vesicatoria (bacterial spot of tomato), and Clavibacter michiganensis subsp. michiganensis (bacterial canker of tomato) (Balestra et al. 2009). The extracts controlled the diseases up to 65% (A. sativum) and 38% (F. carica) of that of the standard copper treatment (Balestra et al. 2009). Similarly, when cakes of Brassica juncea L. or neem ( at 4 q ha−1) were incorporated into soil, a 50% reduction in the incidence of soft rot disease (caused by Pectobacterium chrysanthemi Burkholder) of Aloe barbadensis Miller and Aloe vera (L.) Tourn and a four-fold increase in rhizome yield was achieved (Sharma et al. 2010). Aqueous extracts and dried powders of the medicinal plant weed, Adhatoda vasica were found to restrict the in-vitro growth of R. solanacearum as well as control BW under screen-house conditions (Din et al. 2016). It was hypothesized that the residues of plants suppress pathogen population either by improving chemical, physical and biological properties of soil or by releasing anti-bacterial compounds (Cardoso et al. 2006).
In order to make use of, on a large scale, an effective plant residue for the control of a plant bacterial disease, it is imperative that the plant in question has good anti-bacterial properties, produces a huge vegetative bio-mass in a short time, and is available at a low cost. Cocklebur, Xanthium strumarium L. (Asteraceae) is one such plant. It is an annual weed ranging in height from 20 to 150 cm. The stem is hairy and profusely branched giving the plant a bushy appearance (Saha et al. 2012). In Pakistan, the plant grows in waste places and along road sides producing huge biomass right after spring rains and is available free of cost. Because of its long vegetative period (March to September) and ability to quickly produce huge biomass, it is a good candidate for plant disease control through soil organic amendments. The plant possesses anti-bacterial properties and has many bio-active compounds such as alkaloids, flavonoids, quinone and others (Farooq et al. 2014). However, the ability of this plant to control plant diseases, particularly plant bacterial diseases, to the best of our knowledge, has not been reported so far. Therefore, we explored the possibility to use this plant as soil organic amendment to eliminate or reduce soil-borne phase of the BW pathogen. The aim of the study was to test several hypotheses such as (i) is there any difference between the disease-suppressing ability of the residues from different parts (stems, leaves, or succulent shoot) of the plant?; (ii) is there any influence of the application time of the residue in terms of disease control?; (iii) is there a dose effect of the residue in controlling bacterial wilt?
Material and methods
Procurement of plants, preparation of plant extracts and inoculum
Plants of X. strumarium were collected from waste places, roadsides, fields in the outskirts of Peshawar, and authenticated by a weed scientist of the department of Weed Science, The University of Agriculture, Peshawar, Pakistan. Plant parts such as leaves, succulent shoots, and stems were separated, rinsed with tap water and shade dried for several weeks. The brittle-dried plant parts were separately ground to fine powders. To prepare different concentrations (5%, 10% and 20% w/v) for in-vitro use, powders of different plant parts were separately soaked (using sterilized distilled water) in dark for 48 h. To obtain particle-free aqueous extracts, the soaked powder suspensions were filtered through clean muslin cloth, and the solid residues were discarded.
To prepare the bacterial inoculum to be used in various experiments, a pre-identified, characterized and −80 °C-preserved pure culture of the pathogen (Din et al. 2016; Khan et al. 2019; Najeeb et al. 2019), obtained from the pathogenic plant bacterial culture collection of the department of Plant Pathology, The University of Agriculture, Peshawar, was grown on nutrient agar (NA) plates, at 27 °C for 48 h. The surfaces of the NA plates were then flooded with 0.85% sterilized saline solution, scraped with rubber spatula and the resulting suspension was adjusted to 108 cfu/ml (OD600 = 0.3; Lin et al. 2014). This suspension was used as inoculum for subsequent experiments.
In-vitro studies
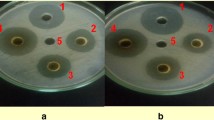
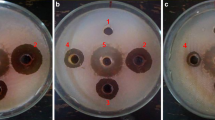
To test the extent of the in-vitro growth inhibition of the bacterial pathogen by different concentrations of the water extracts of the dried powders of leaves, succulent shoots and stem of X. strumarium, agar well diffusion method (Perez et al. 1990) was used. To prepare bacterial lawns for the in-vitro experiment, 100 μl of the bacterial suspension (108 cfu/ml, i.e.OD600 = 0.3; Lin et al. 2014) were placed per NA plate and spread uniformly using sterilized bent glass rod. Using sterilized cork borer (9 mm diameter), four wells along the circumference and one in the center were punched in the NA medium. Four of the five wells of each plate were filled with water extracts of three different concentrations (100 μl each), one (positive control) with 100 μl (100 ppm) streptomycin and one (negative control) with 100 μl sterilized distilled water (SDW). The plates were incubated at 27 °C overnight. The antibacterial activity was assessed by measuring the diameter of the zone of inhibition (mm). The relative antibacterial potency of the given preparation was calculated by comparing its zone of inhibition with that of the SDW and the antibiotic. Completely randomized design with factorial arrangement having four replications per treatment was used.
Screen house studies
Growing tomato plants, inoculation of the potted soil and transplantation
To grow pathogen-free tomato plants, large earthen pots were filled with pasteurized (100 °C for 6 h; Naz et al. 2015a) field soil (sand: clay: silt; 30%:23%:47%) and tomato seeds (cv. Rio Grande) were directly sown in. Irrigation and fertilizer requirements were fulfilled as per horticultural recommendations. Small plastic pots (15 cm-diameters; each having I kg soil) were filled with field soil (Aysan et al. 2003; Khan et al. 2019; Najeeb et al. 2019). The soil of each pot was mixed with the respective treatment powder, pre-moistened and poured with 35 ml of the bacterial suspension (as described before). One-month-old, vigorous and healthy tomato seedlings were then transplanted to these plastic pots. Each pot received one transplant.
Influence of dried powders of leaves, stems, succulent shoots and their doses
Dried powders (0 g, 15 g, 30 g, and 45 g/kg potted soil) of leaves, succulent shoots and stem of X. strumarium were thoroughly mixed with the field soil of each pot before inoculation and transplantation. Total of 12 treatments (4 × 3 = 12) were used and each treatment was replicated eight times using factorial Completely Randomized Design. Total of 96 pots were divided into three sets of 32 pots each. One set (32 pots) received leaves powder, the second set (32 pots) received succulent shoot powder and third set (32 pots) received stem powder. Among the set of 32 pots, eight pots were amended with 0 g, eight with 15 g, eight with 30 g and eight with 45 g plant powder. The weather was rainy (frequent spring rains) during the whole duration of the experiment, so, potted plants were watered (irregularly) as needed. Plants were fertilized with 100 ml (per pot) 0.1%, 20-5-32 + micronutrients hydro-sol fertilizer (Engro Crop Ltd) only once at the beginning of the experiment (Kokalis-Burelle et al. 2005). The experiment was terminated 60 days after transplanting. Data were recorded on plant shoot and root length (by measuring the length of the main shoot and root using transparent plastic ruler), plant fresh bio-mass (taking the weight of the entire plant using electronic balance), and disease severity. The experiment was repeated concurrently in another location a few km away in March–April; 28 ± 5 °C (maximum temperature) with no modifications.
Influence of time of application and different doses of dried powder of succulent shoot
To test the influence of time of application of the dried powder of succulent shoots of X. strumarium on the control of BW, four doses (as in experiment 1) of dried powder of succulent shoots were applied to potted soil 0 days, 10 days and 20 days before transplanting (DBT). Mixing of the doses (powder) with potted soil was done before pre-moisting and artificially inoculating the soil of each pot. There were total of twelve (3 × 4 = 12) treatments and each treatment was replicated eight times using factorial CRD. The 96 pots of the experiment were divided into three sets of 32 pots each. One set (32 pots) received succulent shoot powder 20 DBT, second set (32 pots) received succulent shoot powder 10 DBT and the third set (32 pots) received succulent shoot powder 0 DBT. Among the set of 32 pots, eight pots were amended with 0 g, eight with 15 g, eight with 30 g and eight with 45 g plant powder. The timing and incubation of the experiment were the same as described in experiment 1.
Assessment of disease severity
To assess disease severity over the growing season, data were recorded four times (at 15 days interval) using 1–5 disease rating scale (Wai et al. 2013). Values for each treatment were converted to disease index (%) as per Abdel-Monaaim et al. (2011)
Where ∑n = summation of all categorized values (each value = category number x number of plants present in that category) for each treatment. N = number of plants per treatment; 5 = highest category of rating scale. For each treatment, area under disease progress curve (AUDPC) was calculated as per Madden et al. (2007):
n = total number of observations; Ti = time at ith observation; Xi= quantity of infection at ith observation.
Monitoring changes in population of R. solanacearum in artificially inoculated soil
Soil samples were taken from all treatments and analyzed for the presences of the culturable bacteria, using 10-fold serial dilution pour-plate method. Two soil cores (12 × 1.1 cm) per pot were taken (using sterilized cork borer) from the vicinity of roots of tomato plants. The 16 soil cores of each treatment were thoroughly mixed together to make a composite sample (Schonfeld et al. 2003; Gruter et al. 2006). Three sub-samples were then taken from each composite sample. Each sub-sample was serially (10-fold) diluted up to 10−7. To do this, 5 g soil was added to 45 ml of SDW, stirred and thoroughly mixed for a few minutes, using a magnetic stirrer. Using the 10−7 dilution, 100 μl per plate were spread on TZCNA selective medium followed by an overnight incubation at 27 °C. Four plates per sub-sample were used. To prepare TZCNA, tetrazolium chloride or TZC (0.5% w/v) was added (1 ml/100 ml) to the molten autoclaved NA medium (Goszczynska et al. 2000) before pouring into Petri plates. Bacterial colonies recovered on all four 10−7 plates for each sub-sample were counted, averaged and cfu g−1 dry soil calculated (0 days and 60 days after soil inoculation). Bacterial counts g−1 soil, were converted to log10. Decrease in the soil population of the bacterium was calculated by subtracting the final log10 values from the initial log10 values.
Statistical analysis
Treatments such as various concentrations of aqueous extracts prepared from finely ground powders of the plant parts and different doses of the ground powders applied to soil at different intervals of time were considered as independent variables. Plant yield contributors such as shoot and root length, plant fresh biomass and other parameters such as inhibition zones, disease severity and cfu g−1 dry soil were regarded as dependent variables. The data of the two experiments were pooled together. Pooling of data was validated by pre-t-test (showing no statistical difference) performed for the growth rate of tomato plants grown in two nearby locations with similar environment. To find out the influence of the different treatments on the control of BW, data recorded on disease severity, AUDPC (area under disease progress curve) and other parameters were subjected to analysis of variance using Statistix (Campbell and Madden 1990). Treatment means were compared using Fisher’s protected least significance difference (LSD) test at p = 0.05 (Gomez and Gomez 1984).
Results
In-vitro studies
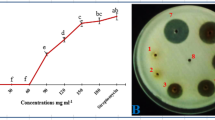
The results (in-vitro bacterial growth inhibition) of aqueous extracts prepared from the dried powders of plant parts (leaves, succulent shoots and stem) as well as those of different concentrations of each plant part differed significantly (P ≤ 0.05) from each other (Fig. 1). Water extracts prepared from dried powder of leaves inhibited the in-vitro bacterial growth more than the extracts of the other parts. The least growth inhibition was obtained by extract prepared from dried powder of stem. In case of comparison of concentrations of each plant part, it was found that 20% (w/v) concentration achieved the biggest zone of inhibition, followed by 10% and 5%. Among parts-concentration combinations, the largest zone of inhibition was recorded for extract prepared from leaf powder at 20% concentration followed by 20% concentration of extract prepared from succulent shoot powder. Extract prepared from stem powder at 5% concentration gave smallest zone of inhibition.
Inhibition zones of in-vitro bacterial growth: Different concentrations (5%, 10%, 20% w/v) of finely ground dried powders of leaves, succulent shoots and stems of X. strumarium were used to prepare aqueous extracts. One hundred μl of each extract/SDW/streptomycin were placed per well, and the inoculated NA plates were incubated overnight at 27 °C. The experiment was repeated once and data were pooled
Screen house studies
Influence of finely ground dried powders of plant parts and their doses of on plant growth parameters
The results of the dried powders of plant parts as well as their doses on all yield-contributing factors were found to be significantly (p ≤ 0.05) different from each other. Leaf powder at the rate of 45 g/kg potted soil gave the best results followed by succulent shoot powder at the same rate. The least influence was observed on growth parameters when soil was amended with 15 g powdered stems (Table 1). Soil amended with 45 g of leaf powder improved root length (cm) by 64%, shoot length (cm) by 37%, and fresh biomass (g) of tomato plants by 42% as compared with the values of inoculated control. The lower dose (30 g/kg soil) of leaf powder also improved root and shoot lengths and plant fresh bio-mass. The corresponding percent increases for these parameters were 54%, 27%, and 31%, respectively.
Influence of times of application and doses of finely ground dried powder of succulent shoot on plant growth parameters
Both the times of application of the dried powder of succulent shoot of X. strumarium as well as its different doses significantly (p ≤ 0.05) affected the yield-contributing plant growth parameters such as plant fresh biomass, shoot length and root length (Table 2). It was found that generally, the increase in doses and time of application increased the plant growth parameters. As compared to control, root length, shoot length, plant fresh biomass of the plants were enhanced by 55%, 42%, 57%, respectively, when treated with 45 g/kg soil applied 20 days before transplanting. The lowest values for plant growth parameters were recorded when 15 g/kg soil of succulent shoot powder was applied to potted soil 0 days before transplanting.
Influence of application times, plant parts and finely ground dried powder doses of X. strumarium on population dynamics (cfu g−1 dry soil) of R. solanacearum
The higher dose (45 g kg−1 soil) applied 20 DBT, significantly (p ≤ 0.05) reduced the pathogen population (a reduction of 1.29 log 10 units) in artificially infested soil as compared to 15 g kg−1 soil treatment and the control (Table 3). The same dose applied 10 DBT, also achieved significant reduction in pathogen population in comparison to other doses and the control. However, the results displayed by all lower doses, applied 10 DBT and all doses applied 0 DBT were non-significant. Regarding the influence of plant parts in reducing the pathogen population in infested soil, the higher dose (45 g kg−1 soil) of leaf powder (applied 10 DBT) significantly (p ≤ 0.05) reduced the pathogen population (a reduction of 1.20 log 10 units) as compared to lower doses and control. Likewise, the higher dose (45 g kg−1 soil) of succulent shoots powder (applied 10 DBT) also differed significantly (p ≤ 0.05) from lower doses and the control. The results of the stem powder doses were non-significant.
Influence of application times, plant parts and finely ground dried powder doses of X. strumarium on AUDPC (area under disease progress curve)
It was found that, in general, higher doses and prolonged times of application decreased the AUDPC more than the lower doses and shortened application times. Treatment of 45 g/kg soil applied 20 DBT gave the lowest AUDPC values and reduced the AUDPC values by 35% when compared to those of control (0 g/kg soil) (Table 4). This treatment was followed by 45 g/kg soil applied 10 DBT which reduced the AUDPC value by 33%. The treatment 15 g/kg soil applied 0 DBT proved to be the least effective. It allowed the highest AUDPC value. Moreover, AUDPC values decreased with increase in doses. The effects of the dried powders of plant parts and those of the different doses differed significantly (p ≤ 0.05) from each other. The treatment combination of dried powder of leaves powder at 45 g/kg soil gave the lowest (1432.5) AUDPC value (Table 4). In comparison to control, it was 38% lower. The treatment combination of dried powder of succulent shoot at 45 g/kg soil reduced AUDPC value by 28% as compared to control. Stem powder at 15 g/kg soil proved to be the least effective and gave the highest AUDPC value.
Discussion
The ability of bacterial wilt (BW) pathogen to use multiple survival mechanisms in the absence of its live host makes its management elusive. The pathogen can survive in soil, water, seeds (Huet 2014), as well as in xylem vessels of weeds (Wenneker et al. 1999). Lack of the effective disease control chemicals (Saddler 2005; Denny 2007) and the instability of BW-resistant varieties (Hayward 1991) make the management of this disease even harder. Therefore, the use of multi-component-based IDM is the only solution to this problem. Besides other components, dried powders and green manures of different plants could be an effective (Bonanomi et al. 2007; Naz et al. 2015a, b; Din et al. 2016), environment-friendly (Qasem and Abu-Blan 1996) and affordable component of IDM against different diseases including BW. Using Brassica juncea L. or neem cakes as soil organic amendments (OAs), Sharma et al. (2010) increased rhizome yield of Aloe by four fold and reduced soft rot (Pectobacterium chrysanthemi) of Aloe barbadensis Miller and Aloe vera (L.) Tourn by 50%. Brassica species, if mulched into soil at flowering time, release anti-microbial substances such as isothiocynates, nitriles and thiocynates. These chemicals significantly reduce soil populations of R. solanacearum (Arthy et al. 2005). Likewise, the addition of Thymus spp. to soil and its decomposition released the volatile compound thymol which effectively controlled BW of tomato (Pradhanang et al. 2003; Ji et al. 2005; Ji et al. 2007).
The results of our studies indicated that the application of dried powder of X. strumarium to soil at different times and rates significantly improved plant growth characters, reduced bacterial counts g−1 soil and decreased AUDPC values. Higher doses of finely ground dried powders of leaves, when applied 20 days before transplanting (20 DBT), were found to be superior to the dried powders of other plant parts used at lower rates and applied fewer days before transplanting. Naz et al. (2015a, b) obtained similar results when green manures or dried powders of Fumaria parviflora were applied to soil. The organic amendment suppressed root-knot nematode (Meloidogyne incognita) populations in tomato crop as well as improved plant growth parameters. The most obvious reason for the ability of X. strumarium to control BW would be the presence of anti-microbial, bio-active secondary metabolites. Common bio-active compounds present in this plant include alkaloids, flavonoids, terpenoids, saponins, tannins (Devkota and Das 2015), phenolics like chologenic and ferulic acids, thiazinediones (Han et al. 2006), triterpenoid saponin (Yadava and Jharbade 2007), and xanthanolide sesquiterpene lactones (Kim et al. 2003). These anti-microbial substances, released on the decomposition of organic matter, kill pathogens (Philogène et al. 2005) by different mechanisms such as rupture of cell membrane, coagulation of cell proteins, and interference with other vital functions (Sikkema et al. 1994). Flavonoids usually affect proteins such as extracellular proteins, cytoplasmic proteins or enzymes (Al-Obaidi 2014). Alkaloids damage DNA or inhibit enzymes or both (Tanaka et al. 2006). Similarly, saponins have been reported to react with the sterol component of cell membrane (Wang et al. 2000).
Dried powders of plants, in addition to having antimicrobial substances, could contain natural elicitor compounds which act as SAR activators to trigger the inactive defense systems of plants (Kagale et al. 2004; Walters et al. 2005; Hassan et al. 2009; Mitra and Paul 2017). For example, aqueous extracts of Hibiscus sabdariffa, Punica granatum and Eucalyptus globulus were found to have both the anti-microbial compounds which inhibited the in-vitro growth of the BW pathogen as well as SAR-eliciting compounds, which elicited strong systemic resistance in potato plants against bacterial wilt (Hassan et al. 2009). Aqueous leaf extract of Datura metel was also reported (Kagale et al. 2004) to have both anti-bacterial compounds and SAR-activating compounds. It inhibited the in-vitro growth of Xanthomonas oryzae pv. oryzae (Xoo) and activated SAR against bacterial leaf blight of rice. Moreover, the addition of finely ground dried powders of plants, when added to soil, improve chemical and physical attributes of soil including water holding capacity, compactness, ion adsorption and soil pH buffering (Brady and Weil 1999; Mazola 2002). These factors influence pathogen’s viability and distribution in soil, nutrient availability and the release of bio-active substances from crop residues as well as soil microbes (Huang et al. 2006). More importantly, the addition of organic amendments to soil enhances the activities and abundance of decomposers. Obviously, an OA left in soil for longer time would result in enhanced activities and numbers of soil microbes. This might explain why our results of 20 DBT were significantly better than other application times. The effects of the amendments are more pronounced in the top 0–5 cm soil but can be extended to deeper layers via mixing (Treonis et al. 2010). The soil microbes whose activities and numbers increase as a result of addition of organic amendments to soil may have a general mechanism of action against the pathogen or be pathogen-specific, having a particular mechanism of action. The enhancement of a select group of microbes is usually because of the host or the pathogen (Mazola 2002; Huang et al. 2006). For example, certain wheat genotypes selectively enhance the populations of specific 2,4-DAPG (antibiotic)-producing pseudomonads, resulting in the control of take-all disease of wheat (Mazola, 2002). Similarly, in comparison to control treatment, there was a significant increase in the number of rhizosphere microorganisms in amended soils particularly antagonistic to Verticillium dahlia (Huang et al. 2006).
Our results indicated that the effect of the finely ground dried powders of X. strumarium was dose-dependent; greater doses produced better effect against BW of tomato than smaller doses. These results are in agreement with those of other researchers. Naz et al. (2015a), for example, proved that increasing doses of Fumaria parviflora accordingly decreased nematode galls, GI, egg masses and females present in the roots of infected tomato plants. The higher dose of 30 g/kg potted soil controlled root-knot nematodes and increased plant growth parameters more than the lower doses under both green house and natural field conditions. Our results also showed that the application time of 20 DBT was better than 10 DBT. We speculate that in case of 20 DBT, plant powders decomposed for relatively longer time, thus releasing more bio-active secondary metabolites. Moreover, the pathogen got exposed for relatively longer time to these metabolites, resulting in better disease control. Also, the activities and numbers of antagonistic microbes were more pronounced in this treatment combination than others, resulting in lower AUDPC and enhanced yield-contributing parameters. Aliyu et al. (2011) reported similar results. When they amended soil with a relatively bigger dose (12.5 g kg−1 soil) of neem leaf powder, they achieved more reduction in disease severity of cowpeas and more increase in plant growth parameters.
The ability of this weed to produce a very large bio-mass in a short time which is available cost-free, its long vegetative phase (March–September), the long shelf-life of its finely ground powder at room temperature (unpublished data) and its effectiveness against BW of tomato and other economic crops, makes it a low cost disease management tool for our poor local farmers. Several additional steps could be taken to further enhance the disease management efficacy of this tool. For example, the soil-borne inoculum of the pathogen could be effectively reduced by plastic mulching of the dried powder-mixed moistened soil during hot summer days before tomato transplantation. The pathogen is heat-sensitive and is killed at soil temperature of 45 °C or above for 2 days (Kang et al. 2007). The temperature of many tomato-growing areas of Pakistan is quite high and this target temperature of 45 °C could be easily achieved through plastic mulching. The extra heat produced by plastic mulching will also enhance the decomposition of organic matter and trap the volatile compounds released (Bonanomi et al. 2007). To reduce the in-put cost, the powder could be target-applied to individual tomato plants as cheap local labor is available. Our data suggested that the disease suppressiveness of the dried powder was dose-dependent. The maximum safe dose that we used was 45 g/kg soil. However, after doing phytotoxicity studies, this dose could be further increased to achieve a better disease control. Powder particle size seems to be important for its bio-activity. Our preliminary results indicated (unpublished data) that the in-vitro bacterial growth inhibition zones produced by aqueous extracts of very fine powders were significantly bigger than those produced by aqueous extracts of relatively coarse powders. This suggests that more complete mechanical disruption of the plant material probably releases more anti-bacterial substances resulting in bigger inhibition zones. So, use of very fine powder could further enhance its disease-control ability. Additionally, the incorporation of this medicinal weed as a dried powder or green manure in seed-bed soils could produce disease-free tomato transplants. And pathogen-free transplants would ensure a healthy crop resulting in higher yield. To control many rice diseases, rice farmers in Pakistan routinely incorporate neem (Azadirachta indica) leaves in seed-bed soils to produce disease-free seedlings. To explore the possibility of synergistic effect, the finely ground powders of X. strumarium could be combined with the powders of other medicinal plants or with small amounts of commercial bactericides such as copper fungicides. This strategy would also discourage the development of chemical resistance in the pathogen. Thus, our findings suggest that X. strumarium as an OA used alone or in combinations with other treatments could prove to be an effective, low-cost disease management tool for BW in tomato and possibly other crops.
References
Abdel-Monaaim MF, Abo-Elyousr KAM, Morsy KM (2011) Effectiveness of plant extracts on suppression of damping-off and wilts diseases of lupine (Lupinustermis forsik). Crop Prot 30:185–191. https://doi.org/10.1016/j.cropro.2010.09.016
Aliyu TH, Balogun OS, Adeoti OM (2011) Pathogenic responses of cowpea (vigna unguiculata) inoculated with cucumber mosaic virus to soil amendment with neem leaf powder. Agrosearch 11:99–110. https://doi.org/10.4314/agrosh.v11i1.10
Al-Obaidi O (2014) Studes on anti bacterial and anticancer activity of Nerium oleander extracts. Eur Chem Bull 3:259–262. https://doi.org/10.17628/ecb.2014.3.259-262
Anith KN, Momol MT, Kloepper JW, Marois JJ, Olson SM, Jones JB (2004) Efficacy of plant growth-promoting rhizobacteria, acibenzolar-s-methyl, and soil amendment for integrated management of bacterial wilt on tomato. Plant Dis 88:669–673. https://doi.org/10.1094/PDIS.2004.88.6.669
Arthy JR, Akiew EB, Kirkegaard JA, Trevorrow PR (2005) Using Brassica spp. as biofumigants to reduce the population of Ralstonia solanacearum. Bacterial wilt disease and the Ralstonia solanacearum species complex. American Phytopathological Society Press, St Paul, pp 159–165
Askarne L, Talibi I, Boubaker H, Boudyach EH, Msanda F, Saadi B, Aoumar AB (2012) Use of Moroccan medicinal plant extracts as botanical fungicide against citrus blue mould. Lett Appl Microbiol 56(1):37–43. https://doi.org/10.1111/lam.12012
Aysan Y, Karatas A, Cinar O (2003) Biological control of bacterial stem rot caused by Erwinia chrysanthemi on tomato. Crop Prot 22:807–811. https://doi.org/10.1016/S0261-2194(03)00030-9
Balestra GM, Heydari A, Ceccarelli D, Ovidi E, Quattrucci A (2009) Antibacterial effect of Allium sativum and Ficus carica extracts on tomato bacterial pathogens. Crop Prot 28(10):807–811. https://doi.org/10.1016/j.cropro.2009.06.004
Begum N, Haque MI, Mukhtar T, Naqvi SM, Wang JF (2012) Status of bacterial wilt caused by Ralstonia solanacearum in Pakistan. Pak J Phytopathol 24(1):11–20 http://pakps.com/pjp/files/11-20-irfan-paper.pdf
Bonanomi G, Antignani V, Pane C, Scala F (2007) Suppression of soilborne fungal disease with organic amendments. J Plant Pathol 89:311–324 https://www.jstor.org/stable/41998409
Brady N, Weil R (1999) Elements of the nature and properties of soil spp. Prentice-Hall, Upper Saddle River, p 559 https://trove.nla.gov.au/version/45005208
Burney K, Roshan Z, Iftikhar A (1999) Bacterial wilt caused by Ralstonia (Pseudomonas) solanacearum in Solanaceous crops of Pakistan. In: Proceeding 2nd national conference of plant pathology, pp 27–29
Campbell CL, Madden LV (1990) Introduction to plant disease epidemiology. Wiley, New York
Cardoso SC, Soares ACF, Brito ADS, Laranjeira FF, Ledo CAS, Santos AP (2006) Control of tomato bacterial wilt through the incorporation of aerial part of pigeon pea and crotalaria to soil. Summa Phytopathol 32:27–33. https://doi.org/10.1590/S0100-54052006000100004
Champoiseau PG, Jeffrey BJ, Timur MM (2009) Ralstonia solanacearum race 3 biovar 2; Detection, exclusion and analysis of select agent. Educational modules, Bacterial Wilt of Tomato, 1–17. https://www.ars.usda.gov/ARSUserFiles/00000000/opmp/RalstoniaR3b2May2010.pdf
Denny T (2007) Plant pathogenic Ralstonia species. In: Plant-associated bacteria. Springer, Dordrecht, pp 573–644
Devkota A, Das RK (2015) Antimicrobial activities of Xanthium strumarium L. J Nat Hist Mus 29:70–77. https://doi.org/10.3126/jnhm.v29i0.19039
Din N, Ahmad M, Siddique M, Ali A, Naz I, Ullah N, Ahmad F (2016) Phytobiocidal management of bacterial wilt of tomato caused by Ralstonia solanacearum (Smith) Yabuuchi. Span J Agric Res 14(3):1006. https://doi.org/10.5424/sjar/2016143-9012
Elphinstone JG (2005) The current bacterial wilt situation: a global overview. In: Allen C, Prior P, Hayward AC (eds) Bacterial wilt disease and the Ralstonia solanacearum species complex. American Phytopathological Society Press, St Paul, pp 9–28 https://ci.nii.ac.jp/naid/10029718988/#cit
Farooq U, Waseem B, Muzaffar R, Tripathi J, Tharani M, Sharma M (2014) A comparative study of phytochemical investigation of Xanthium strumarium medicinal plant. Int J Res Pharm Chem 4(1):96–100 http://www.ijrpc.com/files/15-426.pdf
Floyd J (2007) New pest response guidelines: Ralstonia solanacearum Race 3 biovar 2//USDA APHIS- PPQ, Emergency and Domestic Programs, 45 pp
Gomez KA, Gomez AA (1984) Statistical procedures for agricultural research. Wiley, New York
Goszczynska T, Serfontein JJ, Serfontein S (2000) Media and diagnostic tests, introduction to practical phytobacteriology. Bacterial Diseases Unit, ARC-Plant Protection Research Institute, Pretoria, pp 60–73
Gruter D, Schmid B, Brandl H (2006) Influence of plant diversity and elevated atmospheric carbon dioxide levels on below ground bacterial diversity. BMC Microbiol 6:1–8. https://doi.org/10.1186/1471-2180-6-68
Han T, Li H, Zhang Q, Zheng H, Qin L (2006) New thiazinediones and other components from Xanthium strumarium. Chem Nat Compd 42:567–570. https://doi.org/10.1007/s10600-006-0215-2
Hassan MAE, Bereika MFF, Abo-Elnaga HIG, Sallam MA (2009) Direct antimicrobial activity and induction of systemic resistance in potato plants against bacterial wilt disease by plant extracts. Plant Pathol J 25(4):352–360. https://doi.org/10.5423/PPJ.2009.25.4.352
Hayward AC (1991) Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum. Annu Rev Phytopathol 29:65–87. https://doi.org/10.1146/annurev.py.29.090191.000433
Hayward AC (1994) The hosts of Pseudomonas solanacearum. In: Hayward AC, Hartman GL (eds) Bacterial wilt: the disease and its causative agent, Pseudomonas solanacearum. CAB International, Wallingford, pp 9–25 https://www.cabdirect.org/cabdirect/abstract/19952310916
Huang J, Li H, Yuan H (2006) Effect of organic amendments on Verticillium wilt of cotton. Crop Prot 25:1167–1173. https://doi.org/10.1016/j.cropro.2006.02.014
Huet G (2014) Breeding for resistance to Ralstonia solanacearum. Front Plant Sci 5:715–719. https://doi.org/10.3389/fpls.2014.00715
Ji P, Momol MT, Olson SM, Pradhanang PM, Jones JB (2005) Evolution of thymol as biofunmigant for control of bacterial wilt of tomato under field conditions. Plant Dis 89(5):497–500. https://doi.org/10.1094/PD-89-0497
Ji P, Momol MT, Olson SM, Rich JR, Jones JB (2007) Development of an integrated approach for managing bacterial wilt and root-knot on tomato under field conditions. Plant Dis 91(10):1321–1326. https://doi.org/10.1094/PDIS-91-10-1321
Kagale S, Marimuthu T, Thayumanavan B, Nandakumar R, Samiyappan R (2004) Antimicrobial activity and induction of systemic resistance in rice by leaf extract of Datura metel against Rhizoctonia solani and Xanthomonas oryzae pv. oryzae. Physiol Mol Plant Pathol 65(2):91–100. https://doi.org/10.1016/j.pmpp.2004.11.008
Kang JM, Lee MH, Shim JK, Seo ST, Sherestha R, Cho MS, Hahn JH, Park DS (2007) PCR-based specific detection of Ralstonia solanacearum by amplification of cytochrome c1 signal peptide sequences. J Microbiol Biotechnol 17(11):1765–1771 https://www.ncbi.nlm.nih.gov/pubmed/18092459
Khan RAA, Ahmad B, Ahmad M, Ali A, Naz I, Fahim M (2019) Management of Ralstonia solanacearum (Smith) Yabuuchi wilt in tomato (Solanum lycopersium L.) with dried powder of the medicinal plant Withania somnefera (L) Dunal. Pak J Bot 51(1):297–306. https://doi.org/10.30848/PJB2019-1(8)
Kim YS, Kim JS, Park S, Choi S, Lee CO, Kim S, Kim Y, Kim SH, Ryu SY (2003) Two cytotoxic Sesquiterpene lactones from the leaves of Xanthium strumarium and their in vitro inhibitory activity on Farnesyl transferase. Planta Med 69:375–377. https://doi.org/10.1055/s-2003-38879
Kokalis-Burelle N, Chellemi DO, Perie X (2005) Effect of soils from six management systems on root-knot nematodes and plant growth in greenhouse assays. J Nematol l37:467–472 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2620999/
Laterrot HE (1998) Resistance to bacteria in tomato. In: Scarasci Mugnozza GT, Porceddu E, Pagnotta MA (eds) Genetics and breeding for crop quality and resistance. Proceedings of the XV EUCARPIA Congress, Viterbo, Italy, September 20–25. Kluwer Academic Publishers, Dordrecht, pp 103–110
Lin CH, Tsai KC, Prior P, Wang JF (2014) Phylogenetic relationships and population structure of R alstonia solanacearum isolated from diverse origins in Taiwan. Plant Pathol 63(6):1395–1403. https://doi.org/10.1111/ppa.12209
Lo Cantore P, Iacobellis NS, De Marco A, Capasso F, Senatore F (2004) Antibacterial activity of Coriandrum sativum L. and Foeniculum vulgare Miller var Vulgare (miller) essential oils. L Agric Food Chem 52(26):7862–7866 https://www.ncbi.nlm.nih.gov/pubmed/15612768
Madden LV, Hughes G, Van den Bosch F (2007) The study of plant disease epidemics. APS Press, St. Paul
Mazola M (2002) Mechanisms of natural soil suppressiveness to soilborne diseases. Antonie Van Leeuwenhoek 81:557–564. https://doi.org/10.1023/A:1020557523557
MINFAL (2009) Agriculture statistic of Pakistan. Ministry Of Food, Agriculture and Livestock (Economic Wing), Islamabad, pp 71–72
Mitra J, Paul PK (2017) A potent biocide formulation inducing SAR in plants. J Plant Dis Prot 124(2):163–175. https://doi.org/10.1007/s41348-016-0067-3
Najeeb S, Ahmad M, Khan RAA, Naz I, Ali A, Alam SS (2019) Management of bacterial wilt in tomato using dried powder of Withania coagulan (L) Dunal. Aust Plant Pathol. https://doi.org/10.1007/s13313-019-0618-8
Naz I, Saifullah, Palomeres-Rius JE, Khan SM, Ali S, Ahmad M, Ali A, Khan A (2015a) Control of southern Root-knot nematode, Meloidogyne incognita (Kofoid and white) chitwood on tomato using green manure of Fumaria parviflora Lam (Fumariaceae). Crop Prot 67:121–129. https://doi.org/10.1016/j.cropro.2014.10.005
Naz I, Saifullah, Palomeres-Rius JE, Block V, Khan SM, Ali S, Baig A (2015b) Sustainable management of the southern Root-knot nematode, Meloidogyne incognita (Kofoid and white) chitwood, by means of amendments of Fumaria parviflora. Int J Agric Biol 17:289–296 http://www.fspublishers.org/published_papers/72259_..pdf
Nguyen MT, Ranamukhaarachchi SL (2010) Soil-borne antagonists for biological control of bacterial wilt disease caused by Ralstonia solanacearum in tomato and pepper. J Plant Pathol 92(2):395–405 https://www.jstor.org/stable/41998815
Oguwike FN, Onubueze DPM, Ughachukwu P (2013) Evaluation of activities of marigold extract on wound healing of albino wister rat. IOSR J Dent Med Sci 8:67–70. https://doi.org/10.9790/0853-0856770
Perez C, Pauli M, Bazerque P (1990) An antibiotic assay by agar well diffusion method. Acta Biol Med Exp 15:113–115 https://ci.nii.ac.jp/naid/10024190094/
Philogène BJR, Regnault-Roger C, Vincent C (2005) Botanicals: yesterday’s and today’s promises. In: Biopesticides of plant origin. Intercept, Lavoisier, pp 1–15 https://pubs.acs.org/doi/abs/10.1021/np058244q
Pradhanang PM, Momol MT, Rich JR, Olson SM, Jones JB (2003) Effect of plant essential oils on Ralstonia solanacearum population density and bacterial wilt incidence in tomato. Plant Dis 87(4):423–427. https://doi.org/10.1094/PDIS.2003.87.4.423
Qasem JR, Abu-Blan HA (1996) Fungicidal activity of some common weed extracts against different plant pathogenic fungi. J Phytopathol 144(3):157–161. https://doi.org/10.1111/j.1439-0434.1996.tb01507.x
Saddler GS (2005) Management of bacterial wilt disease. In: Allen C, Prior P, Hayward AC (eds) Bacterial wilt disease and the Ralstonia solanacearum species complex. APS Press, St. Paul, pp 121–132
Saha D, Kumar R, Ghosh S, Kumari M, Saha A (2012) Control of foliar diseases of tea with Xanthium strumarium leaf extract. Ind Crop Prod 37:376–382. https://doi.org/10.1016/j.indcrop.2011.12.030
Schonfeld J, Gelsomino A, Van-Overbeek LS, Gorissen A, Smalla K, Van-Elas JD (2003) Effects of compost addition and simulated solarisation on the fate of Ralstonia solanacearum biovar 2and indigeneous bacteria in soil. FEMS Microbiol Ecol 43:63–74. https://doi.org/10.1111/j.1574-6941.2003.tb01046.x
Shahbaz MU, Mukhtar T, Ul-Haque MI, Begum N (2015) Biochemical and serological characterization of Ralstonia solanacearum associated with chilli seeds from Pakistan. Int J Agric Biol 17:31–40
Sharma JR, Cheema GS, Saini SS, Gill BS (2010) Soft rot disease of Aloebarbadensis and its management. J Res Punjab Agric Univ 47:18–19 https://www.cabdirect.org/cabdirect/abstract/20113213221
Sikkema J, dse Bont JAM, Poolman B (1994) Interactions of cyclic hydrocarbons with biological membranes. Chemical compounds from essential oils also act on cytoplasmic membrane proteins. J Biol Chem 269(11):8022–8028 http://www.jbc.org/content/269/11/8022.short
Tanaka JCA, da Silva CC, de Oliveira AJB, Nakamura CV, Dias BP (2006) Antimicrobial activity of indole alkaloids from Aspidosperma ramiflorum. Braz J Med Biol Res 39(3):387–391. https://doi.org/10.1590/S0100-879X2006000300009
Treonis AM, Austin EE, Buyer JS, Maul JE, Spicer L, Zasada IA (2010) Effects of organic amendment and tillage on soil microorganisms and microfauna. Appl Soil Ecol 46:103–110. https://doi.org/10.1016/j.apsoil.2010.06.017
Tripathi P, Dubey NK (2004) Exploitation of natural products as an alternative strategy to control post-harvest fungal rotting of fruits and vegetables. Postharvest Biol Technol 32:235–245. https://doi.org/10.1016/j.postharvbio.2003.11.005
Wai KPP, Lee J, Mo H, Kim B (2013) Sources of resistance to bacterial wilt and restorer-of-fertility genotype for cytoplasmic male sterility in Capsicum Pepper. Hortic Environ Biotechnol 54(3):266–271. https://doi.org/10.1007/s13580-013-0006-1
Walters D, Walsh D, Newton A, Lyon G (2005) Induced resistance for plant disease control: maximizing the efficacy of resistance elicitors. Phytopathology 95(12):1368–1373. https://doi.org/10.1094/PHYTO-95-1368
Wang JF, Lin CH (2005) Integrated management of tomato bacterial wilt. AVRDC-The World Vegetable Center, Taiwan
Wang Y, McAllister TA, Yanke LJ, Cheeke PR (2000) Effect of steroidal saponin from Yucca schidigera extract on ruminal mmicrobes. J Appl Microbiol 88(5):887–896. https://doi.org/10.1046/j.1365-2672.2000.01054.x
Wenneker M, Verdel M, Groaeneveld R, Kempennar C, van Beuningen A, Janse J (1999) Ralstonia (Pseudomonas) solanacearum race 3 (bio var 2) in surface water and natural weed hosts: first report on stinging nettle (Urtica dioica). Eur J Plant Pathol 105(3):307–315. https://doi.org/10.1023/A:1008795417575
Whipps JM, Gerhardson B (2007) Biological pesticides for control of seed- and soil-borne plant pathogens. A training course guide. In: Van Elsas JD, Jansson JD, Trevors JT (eds) Modern soil microbiology, 2nd edn. CRC Press, Boca Raton, pp 479–501 https://ci.nii.ac.jp/naid/10021000596
Yadava RN, Jharbade J (2007) Novel biologically active triterpenoid saponin from the leaves of Xanthium strumarium Linn. Asian J Chem 19:1224–1230
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khan, R.A.A., Ahmad, M., Naz, I. et al. Sustainable management of bacterial wilt of tomato using dried powder of Xanthium strumarium L.. J Plant Pathol 102, 421–431 (2020). https://doi.org/10.1007/s42161-019-00451-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42161-019-00451-y