Abstract
Symptoms of a vascular wilt were observed on many plants of Lavandula × allardii (hybrid of Lavandula dentata and L. latifolia) cultivated in a nursery near Albenga, Liguria region, Northern Italy. After identification based on morphological characteristic and ITS sequence analysis, the pathogen was identified as Fusarium oxysporum. Eight single-spore isolates obtained from infected tissues were used for a phylogenetic analysis in order to identify the forma specialis. Translation elongation factor 1-α (EF-1α), intergenic spacer (IGS) and three genes encoding for polygalacturonase genes (pg1, pg5 and pgx1) were amplified by PCR. Gene sequences were aligned with other formae speciales of Fusarium oxysporum obtained from GenBank databases in order to build phylogenetic trees. Results obtained for each genomic region showed a unique group with isolates derived from L. × allardii well separated from the other formae speciales. Results obtained, together with pathogenicity tests, allowed us to introduce a new forma specialis named F. oxysporum f. sp. lavandulae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Lavandula (Lamiaceae) includes at least 34 different species, self-sown in the Mediterranean basin (Miller 1985). Among these, the most used in traditional medicine (Gámez et al. 1987) and/or in pharmaceutical and cosmetic industries (Cavanagh and Wilkinson 2002) are: Lavandula angustifolia Mill. (Lavender), L. latifolia Medik., L. fragrans L. (L. angustifolia × L. latifolia), L. stoechas L. and L. multifida L. Lavandula × allardii is a hybrid of L. dentata and L. latifolia, commonly used as ornamental plant. This species presents long light purple flowers separated with small bracts on stems 35–45 cm long. The toothed leaf color could be considered a soft grey green and the blooms appear in early summer.
The main diseases of the Lavandula genus are caused by different Phytophthora (P. nicotianae, P. pelgrandis, P. palmivora and P. parassitica) and Pythium species (Faedda et al. 2013; Davino et al. 2002; Putnam 1991). Additionally, infections by Fusarium sporotrichioides and Fusarium solani were found in different species of Lavandula (Cosic et al. 2012; Ren et al. 2008), while Fusarium oxysporum was described on L. pubescens (Perveen and Bokhari 2010).
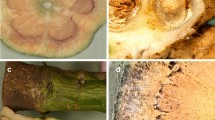
During December 2014, 14-month-old plants of tree-shaped L. × allardii grown in pots in a nursery located in Regione Carenda near Albenga (Northern Italy) at 0 m below sea level (GPS coordinates: 44.074558 North, 8.219336 East) showed symptoms of a previously unknown wilt. The disease affected 70% of 4000 plants. Symptoms consisted of chlorosis and yellowing of leaves, followed by wilting of leaves and branches (Fig. 1a). Brown discoloration was observed in the vascular stem system (Fig. 1b). The fungal causal agent of the disease was isolated from symptomatic vascular tissues. The pathogen was identified as F. oxysporum by morphological and molecular assays and the Koch’s postulates were fulfilled (Garibaldi et al. 2015).
Chlorosis, yellowing and wilts caused by Fusarium oxysporum on naturally affected plants of Lavandula × allardii (a). Stem tissues of Lavandula × allardii with vascular browning caused by Fusarium oxysporum (b). Three-septate, slightly falcate macroconidia (c). Short monophialides with unicellular, ovoid-elliptical microconidia (d) and chlamydospores (e) of Fusarium oxysporum isolated from Lavandula × allardii
F. oxysporum is one of the most important plant pathogens, causing wilt on economically important vegetable and ornamental crops (Gullino et al. 2012). About 130 different formae speciales of this pathogen have been described (Armstrong and Armstrong 1981; O’Donnell and Cigelnik 1999; Baayen et al. 2000; O’Donnell et al. 2009; Leslie 2012). The identification of formae speciales is commonly based on pathogenicity assays (Recorbet et al. 2003) and supported by molecular identification tools (Lievens et al. 2012).
In this work, a phylogenetic study was performed in order to identify the forma specialis of F. oxysporum able to infect L. × allardii.
Materials and methods
Single-spore isolates
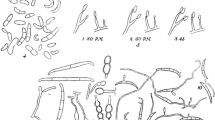
F. oxysporum isolates were obtained from affected plants of L. × allardii collected in the nursery reported above, by plating little pieces of affected vascular tissues onto potato dextrose agar (PDA) medium amended with streptomycin sulphate (0.025 mg ml−1). The isolates produced a white to pale violet mycelium with pale purple pigments in the agar medium and short monophialides with unicellular, ovoid-elliptical microconidia. Successively, each isolate was grown on potato dextrose broth (PDB) (Liofilchem) to distribute the serial dilution of conidial suspensions onto PDA medium. Using a stereomicroscope, single germinated microconidia were selected and transferred onto PDA plates to obtain single-spore cultures. The single-spore isolates of F. oxysporum from L. × allardii used in this work are listed in Table 1. On PDA, single-isolates of F. oxysporum from L. × allardii produced microconidia measuring 4.8–13.1 × 2.3–4.5 (mean 7.4 × 3.3) μm (Fig. 1d). On carnation leaf agar (CLA) (Fisher et al. 1982), slightly falcate macroconidia were produced in pale orange sporodochia. Macroconidia had 3-septa, a foot-shaped basal cell, a short apical cell, and measured 19.6–39.2 × 2.7–4.6 (mean 27.0 × 3.6) μm (Fig. 1c). On the same medium, terminal and intercalary chlamydospores were observed that appeared rough walled, mostly singles, 6.2–9.8 (mean 7.9) μm in diam (Fig. 1e). (Garibaldi et al. 2015).
Pathogenicity assays
Pathogenicity assays were performed with all the isolates listed in Table 1. The inoculum of each isolate was obtained in PDB, shaking cultures (90 rpm) for 10 days at 25 °C ± 1, with 12 h of fluorescent light and 12 h of dark per day. Successively, all the suspensions were filtered and the conidial concentrations were determined with a haemocytometer. Then, conidial suspensions were adjusted with deionized water to obtain the final concentration of 1 × 107 conidia/ml used in the assays. Six 11-month-old healthy plants of L. × allardii were inoculated for each isolate by dipping roots in the suspension without injuring their tissues. Six plants were dipped only in sterilized water and used as controls. Successively, plants were transplanted in 2-l pots containing a substrate (sphagnum peat:perlite:pine and bark:clay; 50:20:20:10) previously steam-sterilized and transferred to a greenhouse, at temperatures ranging from 25 to 31 °C.
DNA extraction
Genomic DNA extraction was carried out by using a commercial kit (Omega Bio-Tek), according to the manufacturer’s instructions. For each isolate, fresh mycelium was obtained by 50 ml culture on PDB incubated at 25 °C. After 6 days, the cultures were filtered and approximately 50 mg of mycelium was transferred into a 2 ml tube containing 400 μl of lysis buffer and two tungsten beads (Stainless Steel Beads, 5 mm, Qiagen, Hilden, Germany). Mycelium was homogenized using Qiagen Tissue Lyser for 3 min, at 28 repetitions per minute and the lysate obtained was used for DNA extraction. DNA concentration was measured using a (Thermo USA) spectrophotometer, and the extracted DNA was stored at −20 °C until further use.
PCR amplification
Five different regions were used for the phylogenetic analysis. In particular the elongation factor 1 α (EF-1α), three polygalacturonase genes (pg1, pg5 and pgx1) and finally the intergenic spacer (IGS), were amplified. PCR reactions were performed with the primers reported on Table 2. The same amplification conditions were used for EF-1α, pg1, pg5 and and pgx1 using a Thermal cycler (Biorad) in a 20 μl reaction mixture containing: 10 ng of gDNA, 0.5 μM of each primer, 1 U of Taq DNA polymerase (Qiagen), 2 μL of PCR buffer 10×, 1 μl of dNTPs stock (final concentration 0.25 mM), and 0.8 μl of MgCl2 (final concentration 1 mM). The cycling conditions included an initial denaturing step at 94 °C for 5 min, followed by 50 cycles of denaturation at 94 °C for 1 min, annealing at 52 °C for 1 min, extension at 72 °C for 2 min, and final extension at 72 °C for 7 min. For IGS amplification a PCR reaction was performed in a 50 μl reaction mixture containing: 30 ng of gDNA, 5 μl of 10 μM stock (final concentration 1 μM) of each primer, 3 unit of Taq DNA polymerase (Qiagen), 5 μl of PCR buffer 10×, 5 μl of dNTPs stock (final concentration 0.25 mM), and 10 μl of 5X Q solution (for amplified a G-C rich regions). A negative control (no template DNA) was included in all experiments. PCR profile were analyzed via 1% agarose gel (Agarose D-1 LOW EEO, Eppendorf). After purification with QIAquick PCR purification kit (Qiagen, Hilden, Germany), purified PCR bands were sequenced via BigDye Direct Sanger Sequencing Kit (Thermo Fisher, Waltham, Massachusetts, USA). EF-1α, pg1, pg5 and and pgx1 regions were sequenced in both directions while for the IGS region we used also two internal primers: CNS12 (GCACGCCAGGACTGCCTCGT) and RU46.67 (GTGTCGGCGTGCTTGTATT) (Mbofung et al. 2007). Sequences were deposited at GenBank and accession numbers are given in Table 3.
Alignment and phylogenetic analyses
Similarity searches (BLASTN, default parameters) were performed for all sequences. The sequences obtained were used for CLUSTALW multiple sequence alignments through MEGA6 software set to default parameters. Manual corrections were performed for each alignment in order to delete trimmer regions outside and discard incomplete sequences. Phylogenetic trees for each genomic region were constructed in MEGA6 (Tamura et al. 2013) using the Neighbor joining method with 1000 bootstrap repeats with pairwise deletion option. The evolutionary distances were computed using the Tajima-Nei method and are in the units of the number of base substitutions per site. In each analysis all samples were included together with sequences derived from different F. oxysporum formae speciales obtained from the GenBank database.
Results
Pathogenicity assay
About 40 days after the inoculation, first symptoms of wilting appeared on leaves of plants of L. × allardii inoculated with the isolates Folav-1, Folav-2, Folav-3, Folav-4. Successively, symptoms appeared also on plants inoculated with the isolates Folav-5, Folav-6, Folav-7 and Folav-8. When the disease progressed, twigs and stems wilted, vascular tissues were discoloured and plants died about 60 days after the artificial inoculation. F. oxysporum was reisolated from plants inoculated with all the isolates, while controls remained symptomless.
Molecular phylogenetic analysis of EF-1α region
Amplification of the EF-1α, gene resulted in 750 bp fragments of DNA. After multialignment with other formae speciales present on GenBank, a portion of 413 bp was used for the phylogenetic analyses. The results obtained showed that isolates obtained from L. × allardii were well distinct from other formae speciales supported by a 67 bootstrap value (Fig. 2). Sequence used sowed an 0.022 distance mean with 0.002 of standard error. Sequences used for the phylogenetic analysis were deposited on GenBank (Table 3).
Molecular phylogenetic analysis of IGS region
As observed on EF 1-α analysis, also in this case all isolates from L. × allardii grouped together separated from other formae speciales present on GenBank with a strong bootstrap value (100) (Fig. 2). Sequence used sowed an 0.032 distance mean with 0.002 of standard error. A 1994 bp sequence were obtained for each isolate and the sequences used for the phylogenetic analysis were deposited in GenBank (Table 3).
Molecular phylogenetic analysis of endo- and exopolygalacturonase
In order to confirm the previous results, three different genes encoding for two endopolygalacturonase (pg1 and pg5) and one exopolygalacturonase (pgx1) we used. Once again, the results confirmed what had been observed in the previous phylogenetic analysis (Fig. 3; Fig. 4). We used a portion of these gene and in particular 903 bp for pg1 gene, 1225 bp for pg5 and finally 1602 bp for pgx1. All results obtained from these sequences showed again a well separated group for the L. × allardii isolates with strong bootstrap value (99 for pgx1, 100 for pg1 and pg5). Phylogenetic tree was obtained with distance mean of 0.028 for pgx1 0.018 for pg1 and 0.07 for pg5 and respectively standard error of 0.002 for pgx1 and pg1 and 0.001 for pg5.
Discussion
In this work, a new F. oxysporum was isolated from L. × allardii plants showing severe wilt symptoms in a nursery located in North Italy. Virulence of all isolates was verified by pathogenicity assay preformed in greenhouse and Koch’s postulates were fulfilled. Specialization on different host plants is a well-known characteristic of F. oxysporum (Gordon and Martyn 1997). However, the high presence of different transposable elements (Daboussi and Capy 2003) allows F. oxysporum to rearrange his genome, in relation with the selection pressure and the intensive cultivations of host plants. The horizontal transfer of pathogenicity genes is well-known for F. oxysporum species complex (Van der Does and Rep 2007). Therefore, the intensive cultivation of a large number of ornamentals offers to F. oxysporum the possibility of new specializations. In the last years, three new formae speciales have been identified in Italy by phylogenetic analysis based on endopolygalacturonase and exopolygalacturonase genes: F. oxysporum f. sp. crassulae on Crassula ovata (Ortu et al. 2013), F. oxysporum f. sp. echeveriae on Echeveria agavoides (Ortu et al. 2015a) and F. oxysporum f. sp. papaveris on Papaver nudicaule (Ortu et al. 2015b). In this work, phylogenetic analyses based on five different genomic regions have identified a new forma specialis of F. oxysporum on L. × allardii never described before. We propose this to be named F. oxysporum Schlechtendal f. sp. lavandulae f. sp. nov.
More studies should be done about the epidemiology of F. oxysporum on L. × allardii, in particular about the effect of temperature on the disease development. Also, the susceptibility of species and cultivars of Lavandula to this new forma specialis of F. oxysporum should be investigated to provide useful information to the growers of these crop. In fact, the growing of L. × allardii is still limited, contrary to L. officinalis and L. stoechas that are largely cultivated in Liguria region where the disease appeared.
References
Appel DJ, Gordon TR (1995) Intraspecific variation within populations of Fusarium oxysporum based on RFLP analysis of the intergenic spacer region of the rDNA. Exp Mycol 19:120–128
Armstrong GM, Armstrong JK (1981) Formae speciales and races of Fusarium oxysporum causing wilt diseases. In: Nelson PE, Toussoun TA, Cook RJ (eds) Fusarium: diseases, biology and taxonomy. Penn State University Press, University Park, pp 391–399
Baayen RP, O’Donnell K, Bonants PJM, Cigelnik E, Kroon LPNM, Roebroeck JA, Waalwijk C (2000) Gene genealogies and AFLP analysis in the Fusarium oxysporum complex identify monophyletic and non-monophyletic formae speciales causing wilt and rot disease. Phytopathology 90:891–900
Cavanagh HMA, Wilkinson JM (2002) Biological activities of lavender essential oil. Phytother Res 16:301–308
Cosic J, Vrandecic K, Jurkovic D, Postic J (2012) First report of lavender wilt caused by Fusarium sporotrichioides in Croatia. Plant Dis 96:591
Daboussi MJ, Capy P (2003) Transposable elements in filamentous fungi. Annu Rev Microbiol 57:275–299
Davino S, Cacciola SO, Pennisi AM, Li Destri Nicosia MG (2002) Phytophthora palmivora a new pathogen of lavender in Italy. Plant Dis 86:561
Faedda R, Cacciola SO, Pane A, Szigethy A, Bakonyi J, Man in't Veld WA, Martini P, Schena L, Magnano di San Lio G (2013) Phytophthora × pelgrandis causes root and collar rot of Lavandula stoechas in Italy. Plant Dis 97:1091–1096
Fisher NL, Burgess LW, Toussoun TA, Nelson PE (1982) Carnation leaves as a substrate and for preserving cultures of Fusarium species. Phytopathology 72:151–153
Gámez MJ, Jiménez J, Risco S, Zarzuelo A (1987) Hypoglycemic activity in various species of the genus Lavandula. Part I: Lavandula stoechas L. and Lavandula multifida L. Pharmazie 42:706–707
Garibaldi A, Bertetti D, Pensa P, Ortu G, Gullino ML (2015) First report of Fusarium oxysporum causing wilt on Allard's lavender (Lavandula × allardii) in Italy. Plant Dis 99:1868
Gordon TR, Martyn RD (1997) The evolutionary biology of Fusarium oxysporum. Annu Rev Phytopathol 35:111–128
Gullino ML, Katan J, Garibaldi A (2012) The genus Fusarium and the species that affect greenhouse vegetables and ornamentals. In: Gullino ML, Katan J, Garibaldi A (eds) Fusarium wilts of greenhouse vegetable and ornamental crops. APS Press, St. Paul, pp 5–9
Hirano Y, Arie T (2009) Variation and phylogeny of Fusarium oxysporum isolates based on nucleotide sequences of polygalacturonase genes. Microbes Environ 24:113–120
Leslie JF (2012) Genetics and Fusarium oxysporum. In: Gullino ML, Katan J, Garibaldi A (eds) Fusarium wilts of greenhouse vegetable and ornamental crops. APS Press, St. Paul, pp 39–47
Lievens B, Hanssen IM, Rep M (2012) Recent developments in the detection and identification of formae speciales and races of Fusarium oxysporum: from pathogenicity testing to molecular diagnostics. In: Gullino ML, Katan J, Garibaldi A (eds) Fusarium wilts of greenhouse vegetable and ornamental crops. APS Press, St. Paul, pp 47–55
Mbofung GY, Hong SG, Pryor BM (2007) Phylogeny of Fusarium oxysporum f. Sp. lactucae inferred from mitochondrial small subunit, elongation factor 1-α and nuclear ribosomal intergenic spacer sequence data. Phytopathology 97:87–98
Miller AG (1985) The genus Lavandula in Arabia and tropical NE Africa. Notes Roy Bot Gard Edinburgh 42:503–528
O’Donnell K, Cigelnik E (1999) A DNA sequence-based phylogenetic structure for the Fusarium oxysporum complex. Phytoparasitica 27:69–70
O’Donnell K, Kistler HC, Cigelnik E, Ploetz RC (1998) Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proc Natl Acad Sci U S A 95:2044–2049
O’Donnell K, Gueidan C, Sink S, Johnston PR, Crous PW, Glenn A, Riley R, Zitomer NC, Colyer P, Waalwijk C, van der Lee T, Moretti A, Kang S, Kim H-S, Geiser DM, Juba JH, Baayen RP, Cromey MG, Bithell S, Sutton DA, Skovgaard KR, Kistler P, Elliott HC, Davis M, Sarver MBAJ (2009) A two locus DNA sequence database for typing plant and human pathogens within the Fusarium oxysporum species complex. Fungal Genet Biol 46:936–948
Ortu G, Bertetti D, Gullino ML, Garibaldi A (2013) A new forma specialis of Fusarium oxysporum on Crassula ovata. J Plant Pathol 95:33–39
Ortu G, Bertetti D, Gullino ML, Garibaldi A (2015a) Fusarium oxysporum f. Sp. echeveriae, a novel forma specialis causing crown and stem rot of Echeveria agavoides. Phytopathol Mediterr 54:64–75
Ortu G, Bertetti D, Martini P, Gullino ML, Garibaldi A (2015b) Fusarium oxysporum f. Sp. papaveris: a new forma specialis isolated from Iceland poppy (Papaver nudicaule). Phytopathol Mediterr 54:76–85
Perveen K, Bokhari N (2010) First report of Fusarium wilt of Lavandula pubescens caused by Fusarium oxysporum in Saudi Arabia. Plant Dis 94:1163
Putnam M (1991) Root rot of lavender caused by Phytophthora nicotianae. Plant Pathol 40:480–482
Recorbet G, Steinberg C, Olivain C, Edel V, Trouvelot S, Dumas-Gaudot E, Gianinazzi S, Alabouvette C (2003) Wanted: pathogenesis-related marker molecules for Fusarium oxysporum. New Phytol 159:73–92
Ren YZ, Tan H, Li ZJ, Du J, Li H (2008) First report of lavender wilt caused by Fusarium solani in China. Plant Pathol 57:377–377
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Van der Does HC, Rep M (2007) Virulence genes and the evolution of host specificity in plant-pathogenic fungi. Mol Plant-Microbe Interact 20:1175–1182
Acknowledgements
This work was supported by the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement n° 261752, PLANTFOODSEC “Plant and Food Biosecurity, Network of Excellence”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ortu, G., Bertetti, D., Gullino, M.L. et al. Fusarium oxysporum f. sp. lavandulae, a novel forma specialis causing wilt on Lavandula × allardii. J Plant Pathol 100, 391–397 (2018). https://doi.org/10.1007/s42161-018-0084-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42161-018-0084-0