Abstract
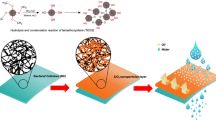
Inspired by the hydrophobic waxy layer structure on the back of the desert beetle, a super-hydrophobic material was prepared via the sol–gel silanisation of porous cellulose membrane (CM) using triethoxysilanes with different carbon chain lengths (R-siloxane) and (CH3CH2O)4Ti as a catalyst in 3 min. The R-siloxane CM was both super-hydrophobic and oleophilic (water contact angle (CA) > 150°, dodecane CA ≈ 0°) and resisted liquids with surface tensions as low as 15 mN·m−1, correlated with the length of the R-siloxane and roughness of the CM. Instructed by the water collection behavior of the desert beetle, a pristine filter CM was added on the R-siloxane filter CM to accelerate separation of Span 80–stabilised water-in-oil emulsions by generating a surface energy gradient (SEG). The composite membrane with SEG separated the emulsions with higher surfactant concentrations and provided a higher efficiency up to 99.98% compared with single-layer R-siloxane membrane. This bio-inspired catalytic one-step approach for preparing R-siloxane cellulose composite membranes can potentially replace petroleum-based products for oil purification.
Graphical Abstract
Bio-inspired preparation of R-siloxane composite cellulose membrane with surface energy gradient for highly efficient water-in-oil emulsion separation.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
To achieve carbon neutrality, degradable materials which can reduce use of petroleum products or increase their combustion efficiency through oil purification are highly desired [1,2,3,4,5,6]. However, several materials with oil–water mixture separation ability are petroleum-based and expensive [7]. Therefore, developing a device with resisting water penetration and selective separation ability is required. Traditionally, porous micro- or nano-structured materials (such as melamine sponge and fabric) based on water-repellent polymers (polyvinylidene fluoride, polydivinylbenzene, polysulphone, polyethersulphone, and sulphonated polyethersulphone) have been widely applied in high-performance filters to fuel cells [2, 8, 9] and lung assist devices [10]. However, the high cost and unsustainability of these materials have limited their applications. Cellulose membrane (CM), with a rough texture and porous structure, is a useful substrate owing to its easy fabrication, flexibility, recyclability, scalability, and low cost [11,12,13,14,15]. Owing to these advantages, CM has been widely applied as packages, filters, cell-based assays, and antibacterial materials [16,17,18,19]. However, the hydrophilicity of cellulose fibers limits its applicability in emulsion separation since moisture and humidity can cause undesirable effects [8, 20]. For separation applications, CM should be modified to resist wetting by liquids (especially water).
Research has proven that the rough back surface of the desert beetle contains many micron-sized block bump structure, and the block bump surface has no hydrophobic waxy components, whereas the other rough area is covered with hydrophobic wax. This unique hydrophilic block bump structure is used to collect and store water droplets and the other hydrophobic waxy parts are used to transfer droplets to its mouth. The desert beetle gathers tiny water droplets from hot desert air via micron-scale hydrophilic bumps. Surface energy gradient (SEG) generated between high-surface-energy hydrophilic bumps and the super-hydrophobic region (with low surface energy) could help water droplets grow to an appropriate size [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]. Consequently, SEG controls the size of droplets and gravity force water droplets to slide towards the beetle’s mouth. Inspired by this phenomenon, the Zeng group prepared a stainless mesh with super-hydrophilic micro-scale SiO2 bumps on super-hydrophobic fluorosilane-modified nano-scale SiO2 coating, which could separate the surfactant-stabilized water-in-oil emulsions, providing oil purity > 99.9% [38].
According to the hydrophobic waxy layer structure on the back of desert beetle, R-siloxane super-hydrophobic CM was prepared by sol–gel silanisation using RSi[OCH2CH3]3, [CH3CH2O]4Ti, and CH3CH2OH [39,40,41,42,43,44]. Furthermore, instructed by the water collection behavior of the desert beetle, a composite membrane with SEG was formed by placing a pristine filter CM on the R-siloxane filter CM to separate Span 80–stabilised water-in-dodecane emulsions (SSWIDEs) [45, 46]. Systematic studies on the super-hydrophobic behavior of R-siloxane CM based on surface free energy and surface roughness were conducted [47,48,49]. A super-hydrophilic pristine filter CM was added on the super-hydrophobic R-siloxane filter CM to perform SSWIDE separation experiments inspired by SEG providing the water collection mechanism of the desert beetle [21,22,23,24, 50,51,52,53]. A double-layer composite membrane with SEG could separate the emulsions with higher surfactant concentrations and have higher efficiency than that of a single-layer R-siloxane membrane.
2 Experimental section
2.1 Materials
Blot, lace, and transfer CMs with different roughness values were purchased from Asia Symbol Shandong (China) Pulp & Paper Co. Ltd. Seven commercially available triethoxysilane reagents (RSi[OCH2CH3]3) with different carbon chain lengths were obtained from Aladdin (Shanghai, China): (i) methyltriethoxysilane (CH3Si[OCH2CH3]3, C1-siloxane); (ii) propyltriethoxysilane (CH3[CH2]2Si[OCH2CH3]3, C3-siloxane); (iii) hexyltriethoxysilane (CH3[CH2]5Si[OCH2CH3]3, C6-siloxane); (iv) octyltriethoxysilane (CH3[CH2]7Si[OCH2CH3]3, C8-siloxane); (v) dodecyltriethoxysilane (CH3[CH2]11Si[OCH2CH3]3, C12-siloxane); (vi) cetyltriethoxysilane (CH3[CH2]15Si[OCH2CH3]3, C16-siloxane); (vii) octadecyltriethoxysilane (CH3[CH2]17Si[OCH2CH3]3, C18-siloxane). Tetraethoxy titanate (TT) and ethanol were obtained from Macklin (Shanghai, China), and were used as the catalyst and solvent, respectively. Pentane, methanol, n-decylalchol, dipropylene glycol (DG), benzyl alcohol (BA), ethylene glycol (EG), diiodo dethane (DD), glycerol, Span 80 solution, and dodecane were all obtained from Aladdin (Shanghai, China).
2.2 Formation of R-siloxane super-hydrophobic and separation membrane materials
Blot, lace, and transfer CMs were dipped into a mixture agent (RSi[OCH2CH3]3:CH3CH2OH:TT = 10 mL:4.5 mL:0.5 mL) for 0.5–1 min in the liquor phase and then dried for 0.5–10 min at 150 °C. RSi(OCH2CH3)3 was allowed to hydrolyse and condensate with itself and the hydroxyl-rich cellulose surface. During the separation of the water-in-oil emulsion, a pristine filter CM was placed on the R-siloxane filter CM (R-SM) to fabricate the separation membrane with double-layer CMs (R-DMs).
2.3 Preparation of surfactant-stabilized water-in-oil emulsion
Various weights (0.03, 0.06, 0.09, and 0.12 g) of Span 80 were dissolved accordingly in dodecane (99.47, 99.44, 99.41, and 99.38 mL) respectively; then, deionized water (0.5 mL) was poured into each solution [54, 55]. After stirring at 10,000 r/min for 2 h by an emulsifying machine, SSWIDEs with Span 80 concentrations of 0.3, 0.6, 0.9, and 1.2 mg/mL were formed. The prepared SSWIDEs were used instantly.
2.4 Characterization
Scanning electron microscope (SEM) images were obtained using a field emission SEM (Zeiss Ultra Plus, Germany). Prior to measurement, the samples were sputtered with a gold layer for 10 s using a Hitachi E-1010 ion sputter (Hitachi, Ltd., Japan). The X-ray photoelectron spectroscopy (XPS) spectra of the CM surface before and after treatment were obtained using a scanning XPS microprobe instrument (PHI-5000 Versa Probe, Ulva-Phi, Japan, monochromatic Al Kα source, hv = 1486.6 eV). The attenuated total reflectance Fourier transform infrared (ATR-FTIR) measurements were recorded using a Thermo Scientific Nicolet iS50 FTIR spectrometer (Thermo Fisher, USA). The CA measurement and advancing and receding CA measurements were performed using a CA measurement system (Ramé-Hart model 500-F1, Ramé-Hart Instrument Co.) at room temperature (20–25 °C) with ~ 20% relative humidity. The three-dimensional (3D) profiles and root mean square roughness values were obtained using a Taylor-Hobson CCI HD optical profiler according to the ISO25178 norm. The area of separation membrane was 12.6 cm2 and 100 mL of SSWIDEs was separated each time. According to the separation time of 20 s, the oil flux was about 14,286 L.m−2.h−1. Oil purity of the emulsion filtrates separated by separation membranes was measured by the Karl Fischer moisture metre (DK-8A, China). The grain diameters of water-in-oil emulsions were obtained from the Malvern Zeta sizer (Nano ZS 90, England). A polarizing microscope (OLYMPUS BX51, Japan) was used to analyze the feed and filtrate of SSWIDEs before and after separation. The photos and videos were recorded using a digital camera (Canon, Japan).
3 Results and discussion
3.1 Bio-inspired design and catalytic one-step preparation of R-siloxane super-hydrophobic membrane
Schematics of the preparation process of R-siloxane CM are shown in Fig. S1. RSi(OCH2CH3)3 quickly reacted with the water inside the CM to produce RSi(OH)3 and then underwent dehydration condensation reaction with the hydroxyl groups on itself and cellulose, introducing siloxane groups of different carbon chain lengths into the CM surface (Scheme 1) [56].
In this work, TT was used as the catalyst. Consequently, the catalytic hydrolysis and condensation reaction time of RSi(OCH2CH3)3 was reduced from 180 to 3 min (Scheme 2) [57]. During the modification, the intermediate formed by the reaction of TT and H2O promotes the hydrolysis of RSi(OCH2CH3)3 and regenerates TT by itself, which is the key to catalysis. TT as a catalyst accelerates the hydrolysis of RSi(OCH2CH3)3 and the condensation reaction, forming Si–O-Si (hydrophobic network) and Si–O-C (connection between the hydrophobic layer and the CM surface) bonds rapidly and stably. The water that comes from the condensation reaction re-enters the reaction system to react with TT. Hence, there is no need to add acid or alkali and additional water to this reaction. The film produced by the reaction is elastic and adheres strongly to the base CM. The coated CM is chemically stable, is harmless to the human body, and can be decomposed in the environment [58]. Also, the modified CM retained its roughness, porosity, and mechanical properties (Tables S1 and S2). Scheme 3 presents an overview of a schematic illustration of the preparation of composite SSWIDE separation membranes with SEG inspired by the water catchment and transportation of the desert beetle. The rough back surface of the beetle contains many micron-sized block bump structure that has no hydrophobic waxy components, whereas the other rough area is covered with a hydrophobic waxy layer. The hydrophilic block bump structure is used to collect and store water droplets and the other hydrophobic parts are used to transfer droplets. Inspired by these structures and their functions, a composite membrane with SEG was further prepared by putting a pristine super-hydrophilic filter CM on the R-siloxane filter CM to separate SSWIDEs (Scheme 3b).
3.2 Liquid repellency mechanism of R-siloxane CM based on surface free energy and roughness
The Young equation reveals the relationship between the solid–liquid-gas three-phase interfacial tension and the static contact angle (CA) on a chemically homogeneous, smooth solid surface. The Young equation is as follows [59]:
where θY, ϒSV, ϒSL, and ϒLV are the CA, solid–gas, solid–liquid, and liquid–gas surface free energies per unit area of the droplet on the ideal solid surface, respectively. From Eq. (1), it is apparent that water forms a higher θY on surfaces with lower ϒSV, even on rough hydrophobic surfaces. Thus, the bionic architecture can be realized, assuming that the graft density values of triethoxysilanes with different carbon chain lengths (R-siloxane) on the CM are equal, by noting that (i) CnH2n+1 and –Si–O–Si– groups have lower energies than –OH groups, and (ii) longer R chains result in more efficient grafting and expose a higher ratio of –CnH2n+1 groups on the surface of the CM. The surface of the CM composed of a web of partially oriented cellulose fibers is incompletely smooth; therefore, the liquid wetting on its surface depends on the surface free energy and the surface topography. Wenzel proposed the wetting theory of rough surfaces based on Young’s equation, known as the Wenzel formula [60]:
where CA and ϒ are the CA of the droplet on the rough surface and the roughness factor of the solid surface, respectively. The roughness factor is the ratio of the real area of the solid–liquid contact to its projection in the horizontal direction: ϒ = 1 indicates a smooth surface, and ϒ > 1 indicates a rough surface. Therefore, for hydrophobic materials, the rougher the solid surface is, the greater the CA, and the more hydrophobic the surface is. The opposite trend holds for hydrophilic materials. Therefore, the CAs of super-hydrophobic materials will enhance with the increase of sample’s surface roughness. However, the Wenzel state—a theory derived when the liquid is completely immersed in the rough structure of the solid—is generally used to represent a highly adherent super-hydrophobic surface. Also, cases exist where the liquid droplet exhibits a composite wetting area consisting of liquid–solid and liquid–air contacts (the air being trapped between the grooves of the rough surface). Cassie and Baxter further supplemented the wetting theory in the case of incomplete infiltration and proposed the Cassie-Baxter equation [61].
In the Cassie-Baxter model, the surface CA that a liquid droplet makes on the rough surface is given by Eq. (3), where ƒ1 is the area fraction of the droplet in contact with the solid, and ƒ2 = 1 − ƒ1 is the area fraction of the liquid interface in contact with the air trapped between grooves.
In both models, increasing ϒ leads to a higher apparent CA for hydrophobic (θY > 90) surfaces. The profile roughness was used as a substitute for ϒ. Thus, Young, Wenzel, and Cassie-Baxter equations help in constructing a rough structure and simultaneously reduce the surface energy of the surface chemical composition to prepare super-hydrophobic materials [61].
3.3 Characterization of R-siloxane super-hydrophobic CM
3.3.1 Morphology, chemical structure, and wettability of R-siloxane super-hydrophobic CM
Figures 1a–c and S2 show that silanisation-coated CM is highly hydrophobic; water no longer seeps into the CM but instead form droplets on the CM surface with CA between 130 and 160°, and CA hysteresis from 4 to 8°. For the same CM substrate (same surface roughness), the liquid repellency of the R-siloxane CM initially increased and then decreased as the carbon chain length of the surface siloxane increased. Under the same carbon chain length, the lyophobic capacities of CMs with different roughness degrees are ordered as blot > lace > transfer. However, the hysteresis showed no noticeable trends. In practical applications of anti-wetting surfaces, water should be rapidly shed from the surfaces to avoid negative effects. Therefore, to test the water-shielding ability, the hybrid CM with R-siloxane CM upper part and pristine CM at the end was fixed at an inclination of 15° to facilitate the gravity driving water removal (Fig. S3, Videos S1 and S2). A water droplet was vertically released to impact the test surfaces. The upper surface could rapidly shed the water, whereas the pristine CM surface tended to capture the impacted droplet immediately. From the proposed RSi(OCH2CH3)3 modification mechanism, the reaction would lead to a mono-coating layer on cellulose fibers.
a SEM and water CA images of three pristine CMs with different surface topographies. b SEM and water CA images of C8-siloxane-coated CM. Blue, red, and green boxes correspond to magnified images of selected areas in each image. c Static CA and CA hysteresis of water on R-siloxane CM for three types of CM. d FTIR analysis of pristine and C8-siloxane blot CM. e XPS spectra of pristine and C8-siloxane blot CM. f Detailed scan on (i) C1s, (ii) O1s, and (iii) Si2p peaks
As displayed in Fig. S4, a film was attached to the cellulose fiber surface, and the number of holes with varying sizes remained unchanged following R-siloxane immersion [62]. Figure 1a and b reflect the R-siloxane-functionalized CM textured structure: mixture fibers (10–50-μm diameter, via SEM) and voids (10–100-μm diameter, via SEM). This textured structure enables it to form pediocratic composite solid–liquid-air interfaces. This architectural structure comprising voids and solid is the basis of other hydrophobic surfaces [50, 52, 63, 64]. Obtaining a super-hydrophobic surface requires both low surface energy and a certain roughness degree [63]. Under the hydrolysis and condensation of R-siloxane, adjacent silicon atoms can be chemically bonded to form nano-scale silicon bridges and be fixed on the micron-scale cellulose surface, forming a partial micro-nano composite structure (Fig. 1b).
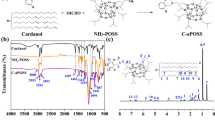
Figure 1d shows pristine and C8-siloxane blot CM spectra in the range of 500–4000 cm−1. The C8-siloxane blot CM has several additional absorption peaks compared with the blot CM. Among them, the peaks at 1081 cm−1 in the C8-siloxane blot correspond to the Si–O-C stretching, demonstrating the chemical bonding between the R-siloxane coating and CM. Simultaneously, the weakened broad peak of the -OH stretching vibrations of cellulose around 3200 cm−1 compared to that of the blot CM shows that most hydroxyl groups were condensed by dehydration to form Si–O-Si and Si–O-C bonds. The peaks at 1465, 2850, 2917, and 796 cm−1 correspond to the vibrational modes of Si–C and Si–O-Si, which proves the presence of “R” groups in the voids of the Si–O-Si network. XPS measurements on the C8-siloxane blot CM confirmed the attachment of the R-siloxane layer based on the peaks at binding energies of 101 and 152 eV (Si 2p and Si 2 s, respectively) and the change in C 1 s and O 1 s peaks (Fig. 1e and f), as well as the changes in the C 1 s, O 1 s, and Si 2p content (Table S3). SEM, FTIR, and XPS results proved that the surface of the CM was successfully coated with a R-siloxane film.
3.3.2 Formation kinetics of R-siloxane super-hydrophobic systems
Compared with hydroxyl-rich and micro-scale structure hydrophilic cellulose surface, the introduction of micro- and nano-scale R-siloxane protruding structures could improve the liquid repellency of the cellulose surface (Fig. 2a) [63]. The water CAs of the C8-siloxane blot CM were measured at different treatment times to monitor the kinetics of the modification process (Fig. 2c). The results showed that the modification was almost completed in 1 min under TT action, whereas the reaction without catalyst lasted more than 180 min. Figure 2b shows that a hydrophobic film was successfully coated on the surface of cellulose at the beginning of the modification reaction. After 3 min, many micro- and nano-scale protruding structures were formed on the surface of the cellulose. The constructed nano-scale convex structure further increased the surface roughness, thereby achieving super-hydrophobic properties [64]. The C8-siloxane hydrophobic layer formation on CM was confirmed by the increase in the Si/O ratio along with C8-siloxane treatment. The Si/O ratio of the C8-siloxane blot CM processed for more than 3 min remained almost the same, which indicates that C8-siloxane modification on cellulose was completed after 3 min (Fig. 2d). Figure 2e shows the C1s peaks of C8-siloxane blot CMs treated for different periods. Evidently, as the functionalization proceeded, the peak at 285.5–286 (C–OH peak) and 287.2–287.5 eV (C = O peak) originating from cellulose molecules significantly weakened after 3 min, which shows that the C8-siloxane coating was almost complete, and the thickness of the layer should be > the detection depth of XPS (> 20 nm). These results confirmed that C8-siloxane modification leads to a thin hydrophobic layer on cellulose fibers, and the reaction can be completed in 3 min.
a Schematic illustration of super-hydrophobic systems. (I) Super-hydrophilic blot CM surface, and (II) surface covered by hydrophilic hydroxyl groups and H2O molecules. (III) Super-hydrophobic blot CM surface, and (IV) surface covered by hydrophobic R-siloxane layer and H2O molecules. b SEM images of C8-siloxane blot CM fiber with different treatment times. c Kinetics test of hydrophobic C8-siloxane modification process. d Si/O ratio of C8-siloxane blot CM with different treatment times. e C1s peak spectra of C8-siloxane blot CM with different treatment times
3.3.3 A wider variety of liquid repellency of R-siloxane CM
Many practical applications involve CAs of liquids that have lower surface tension than water. To determine the hydrophobicity of the R-siloxane CM with organic liquids, the CAs of 10 liquids with surface tensions ranging from 15 and 83 mN.m−1 were measured on the surface of R-siloxane CM (Fig. 3a) [65]. The R-siloxane CM resisted wetting by liquids with surface tensions > 29 mN.m−1 (decyl alcohol). Test liquids with lower surface tensions ≤ 29 mN.m−1 wicked into the CM. For the same CM substrate (same surface roughness), the liquid repellency of the R-siloxane CM initially increased and then decreased as the chain length of the surface R-siloxane increased. One possible explanation for this behavior is the formation of nano-structures (bumps and depressions) on the surface of the cellulose fibers coated with the R-siloxanes (from C1-siloxane to C8-siloxane). In contrast, the carbon chain length was greater than C8, no obvious nano-scale bumps and depressions were found on the formed micro- and nano-scale protrusions (Fig. 3c). Under the same carbon chain length, the lyophobic capacity order was blot > lace > transfer (Fig. 3b). Therefore, the high roughness structure and low surface energy of the super-hydrophobic CM followed the characteristics of the desert beetle [50, 66].
a Dependence of CAs on surface tension for 5-μL droplets of liquid. Liquid surface tensions (ϒLV) at 20 °C in mN.m−.1:pentane (15.5), methanol (22.3), n-decylalchol (29.4), DG (34.1), BA (39.3), EG (46.1), DD (51.7), glycerol (64.1), water (72.8), and 6 M aqueous solution of NaCl (82.6). b 3D profiles of 0.23 mm × 0.17 mm areas of blot, lace, and transfer CMs. c SEM images of micro-nano protrusions for R-siloxane layer with different carbon chain lengths (C1, C3, C6, C8, C12, C14, and C18). Blue and red circles correspond to nano-bumps and depressions
3.3.4 ƒ2 value and surface free energy of R-siloxane CM
To interpret the relationship between ϒLV Cos CA ϒLV of the test liquids t, Eq. (3) in terms of surface tensions expressed in Equation was further expressed [62].
To illustrate the effect of R-siloxane with different carbon chain lengths on the wettability of the CMs, Fig. 4c shows CA data plotted for three types of silanisation, C3, C8, and C16, on the blot CMs. For ϒLV Cos CA plotted versus ϒLV, the data for R-siloxane blot CM collapse onto straight lines with negative slopes, a behavior qualitatively consistent with Eq. (4). The measured data for the three R-siloxane blot CMs fell within straight lines with slopes of −0.89 and −0.97 (ƒ2 between 0.89 and 0.97). This behavior suggests that the liquid interface primarily contacts the air pockets trapped between the grooves on the CM surface (Fig. 4a) [67]. A similar behavior was observed for the lace and transfer CMs (data not shown). Figure 4b and d show the CA data plotted for C8-siloxane CMs with different surface roughness values (6.27, 3.59, and 2.06 μm) to illustrate the topography effect on the wettability of the CMs. Within the range of the surface roughness tested, the liquids followed the Cassie-Baxter model. The data for C8-siloxane blot, lace, and transfer tested fell within straight lines with slopes (ƒ2) of −0.90, −0.84, and −0.74, showing that high CM roughness leads to better liquid repellency. However, the linear trend of the CA data has obvious and systematic deviations when the surface tension of the test liquid is close to the wicking transition of the R-siloxane CM. The deviation is present for all R-siloxanes with different carbon chain lengths and all CMs with different surface roughness degrees (Fig. 4c and d). This deviation implies a different wetting method from the classic Cassie-Baxter model, which would be an interesting phenomenon for future research.
Analysis of liquid CA data on R-siloxane CM. a Schematic illustration of f2 effect on the wettability of the CMs. b 3D profiles of 0.23 × 0.17 mm C8-siloxane blot, lace, and transfer CMs. c Plot of ϒLV cos CA versus ϒLV for three R-siloxanes of different chain lengths on blot CM. d Plot of ϒLV cos CA versus ϒLV for three different types of CM treated indistinguishably with C8-siloxane. e Dispersive and polar components of apparent surface free energy for blot, lace, and transfer CMs functionalized with R-siloxanes (C1, C3, C6, C8, C12, C14, and C18). f C1s peak spectra of blot CM functionalized with R-siloxanes (C1, C3, C6, C8, C12, C14, and C18)
The estimation of the surface energies of the functionalized CMs developed by the Owens–Wendt-Rebel-Kaelble method allows us to calculate the dispersed and polar components of the surface energy. The SEG energies of CMs coated with different carbon chain lengths of R-siloxanes were estimated using the geometric mean equation [68]:
where θapp is the static CA, and ϒSA and ϒLA are the interfacial free energies of solid-air and liquid–air interfaces. The superscripts d and p refer to the dispersive and polar components of the surface energy, respectively. By solving Eq. (5) for EG and pure water as test liquids, surface free energies of C8-siloxane blot were obtained between 18 and 22 mN.m−1, close to the reported value of polytetrafluoroethylene (18–20 mN.m−1) in Fig. 4e [59, 69]. The C1s peaks of CMs coated by different carbon chain length R-siloxanes are shown in Fig. 4f. The peaks at 285.5–286 (C–OH peak) and 287.2–287.5 eV (C = O peak) originating from cellulose molecules significantly weakened as the carbon chain length exceeded C6, showing that the thickness of the R-siloxane layer increases as the carbon chain length grows. The C8-siloxane blot CM showed a higher ratio of –Si–O–Si– groups to –CnH2n−1 groups exposed on the surface than that of the other R-siloxane CMs (Table S3). The calculated values for the surface free energies of the C8-siloxane blot CM benefit from better film wrapping properties and a higher ratio of low-surface-energy groups exposed on the surface.
3.4 Applications and chemical stability of R-siloxane CM
The method can be simply applied on different substrates to form various functional materials. For example, after silanisation, the R-siloxane CM with super-hydrophobic properties can be used as packing bags and straws to replace plastic (Fig. 5a–c, g, and h, and Video S3). Many characteristics of the substrates were retained after the super-hydrophobic modification, such as the colour of the CM (Fig. 5d) and the conductivity of the electrode sheet (Fig. 5f). Moreover, owing to the porous structure and flexibility of the CM, the writing and printing ability of these super-hydrophobic CM can still be performed (Fig. 5e), extending the applications of super-hydrophobic CM to information storage materials [58]. Super-hydrophobic wood and fabric can also be produced in the same manner (Fig. 5i–g).
Application of the method on formation of super-hydrophobic CM, fabrics, and wood and chemical stability tests of the C8-siloxane blot surfaces. a Copy CM, b kraft CM, c wrapping CM, i hard wood chip, and j fabric can be made highly hydrophobic by this method. Characteristic feature of CM, such as d colour and e writing, can be retained after modification. f Electrode sheet material was endowed with good hydrophobic ability while retaining original conductivity. g–h Straw made of super-hydrophobic CM maintained its shape for more than 12 h in water. Liquid repelling effects of (k) HCl (pH = 1) and NaOH (pH = 14) immersion, (l) anhydrous alcohol immersion, m placements, and n UV irradiation
The stability of super-hydrophobic materials has attracted much attention, and a series of liquid repellency tests on the C8-siloxane blot CM surface have been conducted by simulating different environments. As shown in Fig. 5k, The C8-SP was placed in hydrochloric acid solution with pH = 1 or sodium hydroxide solution with pH = 14 for up to 120 h, and the super-hydrophobic properties were still maintained, which demonstrated that the C8-SP has good corrosion resistance to strong acids and alkalis. There is no significant change in the elemental composition of the membrane surface after immersion in HCl and NaOH solutions (Table S4). After immersing the C8-siloxane CM in ethanol solvent for 120 h, being irradiated with a 254-nm wavelength ultraviolet (UV) lamp for 25 h and placed in a laboratory environment for up to 8 months, respectively, the water (water CA remained > 150°) and glycerol repellency decreased (Fig. 5l–n). The experimental results showed that the surface of the CM modified by RSi(OCH2CH3)3 had good chemical stability because R-siloxane polymers have no active groups that could react with the acid or alkali.
3.5 SSWIDE separation performances of R-siloxane composite membranes
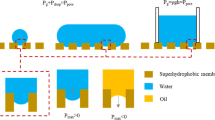
R-siloxane rendered the filter CM super-hydrophobic and lipophilic and thus could be used to separate water-in-oil emulsions. The super-hydrophilic pristine filter CM was covered on the super-hydrophobic R-siloxane filter CM to fabricate the composite separation membrane with SEG inspired by the water collection behavior of the desert beetle. To investigate the SEG effects on the separation performance of the membranes, SSWIDEs with different surfactant concentrations were prepared. The composite membrane area is 12.6 cm2 and 100 mL of SSWIDEs is separated each time. According to the separation time of 20 s, the oil flux is about 14,286 L.m−2.h−1. Optical microscope images, digital photos, and water-in-oil droplet sizes of SSWIDEs were used to record the state before and after separation. As recorded in Fig. 6a1–d1, there were many small water-in-oil liquid in the images for each emulsion with different surfactant concentrations before separation. The volume of the little fluid drop decreases, and the density increases while the transparency of the milk-like emulsions decreases as the surface content increases [70]. After being separated by R-SM, emulsions with Span 80 concentrations of 0.3 and 0.6 mg/mL turned into almost transparent, and few droplets were present in the corresponding optical microscope images (Fig. 6a2–b2). Based on the presence of droplets in the optical microscope image and the opacity of the emulsion in the optical image, the separation efficiency of R-SM seems poor when the surfactant concentration exceeds 0.6 mg/mL (Fig. 6c2–d2). However, almost no droplets appear, and the final liquids are transparent when the surfactant concentrations are in the range of 0.3–1.2 mg/mL (Fig. 6a3–d3) after the R-DM separation. Thus, R-DMs with SEG have a better separation ability than that of R-SM.
To further quantitatively measure the membrane’s separation efficiency, the water-in-oil droplet dimensions of the emulsion were measured; the diameter of the droplets was reduced along with the increased surfactant concentrations (Fig. 6a4–d4). When the surfactant concentrations were 0.3, 0.6, 0.9, and 1.2 mg/mL, the sizes of filtrates separated by R-SM were in the range of 10–60 nm, which indicates that SSWIDEs with high surfactant concentrations have low separation abilities. In contrast, all sizes of filtrates separated by R-DMs were < 20 nm (Fig. 6a5–d5). These results further reveal that R-DMs with SEG have a stronger separation ability for emulsions with high surfactant concentration than R-SM. When separated by R-SM, the water droplets stabilized by Span 80 in the emulsion were blocked by the super-hydrophobic surface while dodecane penetrated the lipophilic surface, and then, water–oil separation was achieved under gravity. For separation of R-DMs, the water droplets were collected and stored between the upper super-hydrophilic layer and the lower super-hydrophobic layer of the composite membrane; then, the oil could flow out. When SSWIDEs move from a super-hydrophilic surface with high surface energy to a super-hydrophobic surface with low surface energy, an unbalanced driving force is generated to collect water in the hydrophilic layer and prevent water droplets from passing through the hydrophobic R-SM membrane [22, 26]. This driving force based on the SEG is consistent with the water collection behavior of the desert beetle, and the equation of the driving force is described as follows [71]:
Here, ϒwater, θ super-hydrophilic surface, and θ super-hydrophobic surface are the surface tension of water, water CA of the super-hydrophilic original filter CM, and the super-hydrophobic R-siloxane filter CM, respectively. Based on the CAs of 0° and 155° of water on an original super-hydrophilic filter CM and C8-siloxane-coated super-hydrophobic filter CM, the driving force was calculated to be 108 mN.m−1. The additional driving force generated by the SEG was the main effect on the enhanced separation performance of R-DMs compared with R-SM.
The oil purity of the emulsion filtrates separated by the single pristine filter CM (S), double pristine filter CMs (D), R-SM, and R-DMs was further quantitatively detected to compare the separation effect (Fig. S5). For different separation membranes, the oil purity of the filtrate after separation decreased as the surfactant amount increased (filtrate volume 100 mL). This trend occurs because as the surfactant amount increases, the droplets become smaller, and SSWIDEs are more stable and difficult to separate. When the emulsifier concentration is > 0.6 mg/mL, the oil purity of the filtrate separated by R-DMs exceeds 99.98%, which has a better separation effect than the S membranes (Video S4). Therefore, in terms of the separation efficiency, the conclusion showed that R-DMs > R-SM > D > S. Not only the separation efficiency, but also the recyclability of R-DMs is better than those of D (Fig. S6). SSWIE (1.2 g/mL) was used to check the recyclability. The separation efficiency was higher than 99.98% after being separated for 20 cycles. However, the oil purity of D began to below 99.8% after 10 cycles of separation. The upper super-hydrophilic filter CM of composite membrane would be replaced for each 20 separation cycles, and the upper one was attached on lower super-hydrophobic filter CM due to the wetting of emulsion. This trend can be attributed to two reasons. First, the stacking of double layers of filter CM with opposite super-wettability can generate a driving force from the SEG and improve the separation efficiency. Second, the super-position of the double layers of the filter CM will produce relatively smaller holes conducive to the separation of the emulsion (Fig. S7). Water-in-oil separation efficiency comparisons of this work and previous works are summarized in Table S5. The emulsion separation efficiency of this work is highly efficient compared to previous works.
4 Conclusions
First, a novel catalytic one-step preparation method (within 3 min) of an R-siloxane super-hydrophobic material was presented via the sol–gel silanisation of CM using RSiO(CH2CH3)3 with different carbon chain lengths, (CH3CH2O)4Ti, and CH3CH2OH inspired by the hydrophobic waxy layer structure on the back of desert beetle. Systematic studies of the super-hydrophobic behavior of the R-siloxane CM based on surface free energy (correlated with the length of the R-siloxane) and surface roughness (correlated with ƒ2) were conducted. The R-siloxane CM was both super-hydrophobic and oleophilic (water CA > 150°, dodecane CA ≈ 0°). The R-siloxane CM resisted wetting by liquids with surface tensions as low as 15 mN.m−1. This method offers good chemical stability and can be applied to different substrates, including wood, fabric, and electrode sheet materials. Second, based on the SEG found on the water collection behavior of the desert beetle, a super-hydrophilic pristine filter CM was added to the R-siloxane filter CM to perform SSWIDE separation. R-DMs with an SEG could separate emulsions with high surfactant concentrations with higher efficiency than that obtained with R-SM. The oil purity of the filtrate separated by R-DMs exceeds 99.98%. This bio-inspired catalytic one-step approach for preparing R-siloxane cellulose composite membranes has great potential in replacing petroleum-based products and oil purification applications.
References
Feig VR, Tran H, Bao Z (2018) Biodegradable polymeric materials in degradable electronic devices. ACS Cent Sci 4:337–348. https://doi.org/10.1021/acscentsci.7b00595
Xu T, Liu K, Sheng N, Zhang M, Liu W, Liu H, Dai L, Zhang X, Si C, Du H, Zhang K (2022) Biopolymer-based hydrogel electrolytes for advanced energy storage/conversion devices: properties, applications, and perspectives. Energy Storage Mater 48:244–262. https://doi.org/10.1016/j.ensm.2022.03.013
Du H, Liu W, Zhang M, Si C, Zhang X, Li B (2019) Cellulose nanocrystals and cellulose nanofibrils based hydrogels for bio-medical applications. Carbohyd Polym 209:130–144. https://doi.org/10.1016/j.carbpol.2019.01.020
Liu K, Du H, Zheng T, Liu W, Zhang M, Liu H, Zhang X, Si C (2021) Lignin-containing cellulose nanomaterials: preparation and applications. Green Chem. https://doi.org/10.1039/D1GC02841C
An L, Si C, Wang G, Sui W, Tao Z (2019) Enhancing the solubility and antioxidant activity of high-molecular-weight lignin by moderate depolymerization via in situ ethanol/acid catalysis. Ind Crops Prod 128:177–185. https://doi.org/10.1016/j.indcrop.2018.11.009
Xu R, Du H, Liu C, Liu H, Wu M, Zhang X, Si C, Li B (2021) An efficient and magnetic adsorbent prepared in a dry process with enzymatic hydrolysis residues for wastewater treatment. J Clean Prod 313:127834. https://doi.org/10.1016/j.jclepro.2021.127834
Chen C, Chen L, Chen S, Yu Y, Weng D, Mahmood A, Wang G, Wang J (2020) Preparation of underwater superoleophobic membranes via TiO2 electrostatic self-assembly for separation of stratified oil/water mixtures and emulsions. J Membr Sci 602:117976. https://doi.org/10.1016/j.memsci.2020.117976
Liu K, Liu W, Li W, Duan Y, Zhou K, Zhang S, Ni S, Xu T, Du H, Si C (2022) Strong and highly conductive cellulose nanofibril/silver nanowires nanopaper for high performance electromagnetic interference shielding. Advanced Composites and Hybrid Materials. https://doi.org/10.1007/s42114-022-00425-2
Salamatov V (2020) Study of the filtering properties of fabrics derived from cotton and synthetic fibres. Proceedings of Universities. Applied Chemistry and Biotechnology 10:325–331. https://doi.org/10.21285/2227-2925-2020-10-2-325-331
Blauvelt DG, Abada EN, Oishi P, Roy S (2021) Advances in extracorporeal membrane oxygenator design for artificial placenta technology. Artif Organs 45:205–221. https://doi.org/10.1111/aor.13827
Xu T, Du H, Liu H, Liu W, Zhang X, Si C, Liu P, Zhang K (2021) Advanced nanocellulose-based composites for flexible functional energy storage devices. Adv Mater 33:2101368. https://doi.org/10.1002/adma.202101368
Zhang M, Du H, Liu K, Nie S, Xu T, Zhang X, Si C (2021) Fabrication and applications of cellulose-based nanogenerators. Advanced Composites and Hybrid Materials 4:865–884. https://doi.org/10.1007/s42114-021-00312-2
Liu H, Du H, Zheng T, Liu K, Ji X, Xu T, Zhang X, Si C (2021) Cellulose based composite foams and aerogels for advanced energy storage devices. Chem Eng J 426:130817. https://doi.org/10.1016/j.cej.2021.130817
Liu H, Xu T, Liu K, Zhang M, Liu W, Li H, Du H, Si C (2021) Lignin-based electrodes for energy storage application. Ind Crops Prod 165:113425. https://doi.org/10.1016/j.indcrop.2021.113425
Liu K, Du H, Zheng T, Liu H, Zhang M, Xie H, Zhang X, Ma M, Si C (2021) Recent advances in cellulose and its derivatives for oilfield applications. Carbohyd Polym 259:117740. https://doi.org/10.1016/j.carbpol.2021.117740
Liu K, Du H, Liu W, Liu H, Zhang M, Xu T, Si C (2022) Cellulose nanomaterials for oil exploration applications. Polym Rev. https://doi.org/10.1080/15583724.2021.2007121
Du H, Zhang M, Liu K, Parit M, Jiang Z, Zhang X, Li B, Si C (2022) Conductive PEDOT:PSS/cellulose nanofibril paper electrodes for flexible supercapacitors with superior areal capacitance and cycling stability. Chem Eng J 428:131994. https://doi.org/10.1016/j.cej.2021.131994
Du H, Parit M, Liu K, Zhang M, Jiang Z, Huang T, Zhang X, Si C (2021) Multifunctional cellulose nanopaper with superior water-resistant, conductive, and antibacterial properties functionalized with chitosan and polypyrrole. ACS Appl Mater Interfaces 13(27):32115–32125. https://doi.org/10.1021/acsami.1c06647
Du H, Parit M, Liu K, Zhang M, Jiang Z, Huang T, Zhang X, Si C (2021) Engineering cellulose nanopaper with water resistant, antibacterial, and improved barrier properties by impregnation of chitosan and the followed halogenation. Carbohyd Polym 270:118372. https://doi.org/10.1016/j.carbpol.2021.118372
Liu W, Liu K, Du H, Zheng T, Zhang N, Xu T, Pang B, Zhang X, Si C, Zhang K (2022) Cellulose nanopaper: fabrication, functionalization, and applications. Nano-Micro Letters 14:104. https://doi.org/10.1007/s40820-022-00849-x
Chakrabarti U, Paoli R, Chatterjee S, Megaridis CM (2019) Importance of body stance in fog droplet collection by the Namib desert beetle. Biomimetics 4:59. https://doi.org/10.3390/biomimetics4030059
Zhu H, Huang Y, Lou X, Xia F (2019) Beetle-inspired wettable materials: from fabrications to applications. Materials Today Nano 6:100034. https://doi.org/10.1016/j.mtnano.2019.100034
Mitchell D, Henschel JR, Hetem RS, Wassenaar TD, Strauss WM, Hanrahan SA, Seely MK (2020) Fog and fauna of the Namib Desert: past and future. Ecosphere 11:2996. https://doi.org/10.1002/ecs2.2996
Xu C, Feng R, Song F, Wang X-L, Wang Y-Z (2018) Desert beetle-inspired superhydrophilic/superhydrophobic patterned cellulose film with efficient water collection and antibacterial performance. ACS Sustainable Chemistry & Engineering 6:14679–14684. https://doi.org/10.1021/acssuschemeng.8b03247
Lee JJ, Kim D-Y (2019) Investigation of morphology and surface structure of Stenocara eburnea, Namib desert beetle. Microsc Microanal 25:1096–1097. https://doi.org/10.1017/S1431927619006214
Lamb T (2018) A fatal agonistic interaction between ant and darkling beetle (Coleoptera: Tenebrionidae: Pimeliinae: Adesmiini) in the Northern Namib Desert. Coleopt Bull 72:314–316. https://doi.org/10.1649/0010-065X-72.2.314
Yu Z, Yun FF, Wang Y, Yao L, Dou S, Liu K, Jiang L, Wang X (2017) Desert beetle-inspired superwettable patterned surfaces for water harvesting. Small 13:1701403. https://doi.org/10.1002/smll.201701403
Liu H, Xu T, Cai C, Liu K, Liu W, Zhang M, Du H, Si C, Zhang K (2022) Multifunctional superelastic, superhydrophilic, and ultralight nanocellulose‐based composite carbon aerogels for compressive supercapacitor and strain sensor. Adv Funct Mater 2113082. https://doi.org/10.1002/adfm.202113082
Liu W, Du H, Liu H, Xie H, Xu T, Zhao X, Liu Y, Zhang X, Si C (2020) Highly efficient and sustainable preparation of carboxylic and thermostable cellulose nanocrystals via FeCl3-catalyzed innocuous citric acid hydrolysis. ACS Sustainable Chemistry & Engineering 8:16691–16700. https://doi.org/10.1021/acssuschemeng.0c06561
Li X, Lu X, Nie S, Wang M, Yu Z, Duan B, Yang J, Xu R, Lu L, Si C (2020) Efficient catalytic production of biomass-derived levulinic acid over phosphotungstic acid in deep eutectic solvent. I Industrial Crops and Products 145:112154. https://doi.org/10.1016/j.indcrop.2020.112154
Li X, Xu R, Yang J, Nie S, Liu D, Liu Y, Si C (2019) Production of 5-hydroxymethylfurfural and levulinic acid from lignocellulosic biomass and catalytic upgradation. Ind Crops Prod 130:184–197. https://doi.org/10.1016/j.indcrop.2018.12.082
Scola V, Ramond J-B, Frossard A, Zablocki O, Adriaenssens EM, Johnson RM, Seely M, Cowan DA (2018) Namib Desert soil microbial community diversity, assembly, and function along a natural xeric gradient. Microb Ecol 75:193–203. https://doi.org/10.1007/s00248-017-1009-8
Liu H, Xu T, Liang Q, Zhao Q, Zhao D, Si C (2022) Compressible cellulose nanofibrils/reduced graphene oxide composite carbon aerogel for solid-state supercapacitor. Advanced Composites and Hybrid Materials. https://doi.org/10.1007/s42114-022-00427-0
Liu W, Du H, Zhang M, Liu K, Liu H, Xie H, Zhang X, Si C (2020) Bacterial cellulose-based composite scaffolds for biomedical applications: a review. ACS Sustainable Chemistry & Engineering 8:7536–7562. https://doi.org/10.1021/acssuschemeng.0c00125
Liu W, Du H, Liu K, Liu H, Xie H, Si C, Pang B, Zhang X (2021) Sustainable preparation of cellulose nanofibrils via choline chloride-citric acid deep eutectic solvent pretreatment combined with high-pressure homogenization. Carbohyd Polym 267:118220. https://doi.org/10.1016/j.carbpol.2021.118220
Wang H, Du H, Liu K, Liu H, Xu T, Zhang S, Chen X, Zhang R, Li H, Xie H, Zhang X, Si C (2021) Sustainable preparation of bifunctional cellulose nanocrystals via mixed H2SO4/formic acid hydrolysis. Carbohyd Polym 266:118107. https://doi.org/10.1016/j.carbpol.2021.118107
Wang H, Xie H, Du H, Wang X, Liu W, Duan Y, Zhang X, Sun L, Si ZXYC (2020) Highly efficient preparation of functional and thermostable cellulose nanocrystals via H2SO4 intensified acetic acid hydrolysis. Carbohyd Polym 239:116233. https://doi.org/10.1016/j.carbpol.2020.116233
Gurera D, Bhushan B (2020) Passive water harvesting by desert plants and animals: lessons from nature. Phil Trans R Soc A 378:20190444. https://doi.org/10.1098/rsta.2019.0444
Basu B, Srinivasan A, Manasa J, Grips V (2012) Improved corrosion protection of aluminium alloy AA 2024 by sol–gel hybrid coatings after surface pretreatment by silanisation. Surf Eng 28:294–299. https://doi.org/10.1179/1743294411Y.0000000074
Liu X, Sun S, Nie R, Ma J, Qu Q, Yang L (2018) Highly uniform porous silica layer open-tubular capillary columns produced via in-situ biphasic sol–gel processing for open-tubular capillary electrochromatography. J Chromatogr A 1538:86–93. https://doi.org/10.1016/j.chroma.2018.01.024
Rahim T, Mohamad D, Ismail AR, Akil HM (2011) Synthesis of nanosilica fillers for experimental dental nanocomposites and their characterisations. Journal of Physical Science 22:93–105. https://doi.org/10.1021/jphyssci.6b07182
Das C, Bansod ND, Kapgate BP, Reuter U, Heinrich G, Das A (2017) Development of highly reinforced acrylonitrile butadiene rubber composites via controlled loading of sol-gel titania. Polymer 109:25–37. https://doi.org/10.1016/j.polymer.2016.12.018
Huang X, Ke R, Dong Y (2020) Characterization and corrosion protection of nano-titanium dioxide doped BTSE-GPTMS sol–gel coating on cast Al–Si alloy. J Sol-Gel Sci Technol 94:671–680. https://doi.org/10.1007/s10971-019-05211-z
Pinto R, Carmezim M, Ferreira M, Montemor M (2010) A two-step surface treatment, combining anodisation and silanisation, for improved corrosion protection of the Mg alloy WE54. Prog Org Coat 69:143–149. https://doi.org/10.1016/j.porgcoat.2010.04.014
Li J, Xu C, Guo C, Tian H, Zha F, Guo L (2018) Underoil superhydrophilic desert sand layer for efficient gravity-directed water-in-oil emulsions separation with high flux. Journal of Materials Chemistry A 6:223–230. https://doi.org/10.1039/c7ta08076j
Yin X, Wang Z, Shen Y, Mu P, Zhu G, Li J (2020) Facile fabrication of superhydrophobic copper hydroxide coated mesh for effective separation of water-in-oil emulsions. Sep Purif Technol 230:115856. https://doi.org/10.1016/j.seppur.2019.115856
Khorsand S, Raeissi K, Ashrafizadeh F, Arenas M, Conde A (2016) Corrosion behaviour of super-hydrophobic electrodeposited nickel–cobalt alloy film. Appl Surf Sci 364:349–357. https://doi.org/10.1016/j.apsusc.2015.12.122
Liu Y, Chen X, Xin JH (2006) Super-hydrophobic surfaces from a simple coating method: a bionic nanoengineering approach. Nanotechnology 17:3259. https://doi.org/10.1088/0957-4484
Qu Z, Wang F, Liu P, Yu Q, Brouwers H (2020) Super-hydrophobic magnesium oxychloride cement (MOC): from structural control to self-cleaning property evaluation. Mater Struct 53:1–10. https://doi.org/10.1617/s11527-020-01462-3
Guo P, Wang Z, Han X, Heng L (2021) Nepenthes pitcher inspired isotropic/anisotropic polymer solid–liquid composite interface: preparation, function, and application. Mater Chem Front 5:1716–1742. https://doi.org/10.1039/d0qm00805b
Lei J, Guo Z (2020) A fog-collecting surface mimicking the Namib beetle: its water collection efficiency and influencing factors. Nanoscale 12:6921–6936. https://doi.org/10.1039/c9nr10808d
Yong J, Chen F, Yang Q, Fang Y, Huo J, Zhang J, Hou X (2017) Nepenthes inspired design of self-repairing omniphobic slippery liquid infused porous surface (SLIPS) by femtosecond laser direct writing. Adv Mater Interfaces 4:1700552. https://doi.org/10.1002/admi.201700552
Zhu H, Duan R, Wang X, Yang J, Wang J, Huang Y, Xia F (2018) Prewetting dichloromethane induced aqueous solution adhered on Cassie superhydrophobic substrates to fabricate efficient fog-harvesting materials inspired by Namib Desert beetles and mussels. Nanoscale 10:13045–13054. https://doi.org/10.1039/c8nr03277g
Zembyla M, Murray BS, Sarkar A (2020) Water-in-oil emulsions stabilized by surfactants, biopolymers and/or particles: a review. Trends Food Sci Technol 104:49–59. https://doi.org/10.1016/j.tifs.2020.07.028
Lin Y-J, Perrard A, Biswal SL, Hill RM, Trabelsi S (2018) Microfluidic investigation of asphaltenes-stabilized water-in-oil emulsions. Energy Fuels 32:4903–4910. https://doi.org/10.1021/acs.energyfuels.8b00249
Gao Y, Guo M, Yuan K, Shen C, Ren Z, Zhang K, Zhao H, Qiao F, Gu J, Qi Y (2020) Multifunctional silanization interface for high-energy and low-gassing lithium metal pouch cells. Adv Energy Mater 10:1903362. https://doi.org/10.1002/aenm.201903362
Wu G, Chen L, Liu L (2017) Effects of silanization and silica enrichment of carbon fibers on interfacial properties of methylphenylsilicone resin composites. Compos A Appl Sci Manuf 98:159–165. https://doi.org/10.1016/j.compositesa.2017.03.024
Frka-Petesic B, Vignolini S (2019) So much more than paper. Nat Photonics 13:365–367. https://doi.org/10.1038/s41566-019-0448-9
Wan C, Jiao Y, Li J (2017) Flexible, highly conductive, and free-standing reduced graphene oxide/polypyrrole/cellulose hybrid papers for supercapacitor electrodes. J Mater Chem A 5:3819–3831. https://doi.org/10.1039/c6ta04844g
Hu H, Hua T (2017) An easily manipulated protocol for patterning of MXenes on paper for planar micro-supercapacitors. J Mater Chem A 5:19639–19648. https://doi.org/10.1039/C7TA04735E
Tuteja A, Choi W, Mabry JM, Mckinley GH, Cohen RE (2008) Robust omniphobic surfaces. Proc Natl Acad Sci 105:18200–18205. https://doi.org/10.1073/pnas.0804872105
Glavan AC, Martinez RV, Subramaniam AB, Yoon HJ, Nunes RM, Lange H, Thuo MM, Whitesides GM (2014) Omniphobic “RF paper” produced by silanization of paper with fluoroalkyltrichlorosilanes. Adv Func Mater 24:60–70. https://doi.org/10.1002/adfm.201300780
Zhang P, Zhang L, Chen H, Dong Z, Zhang D (2017) Surfaces inspired by the Nepenthes peristome for unidirectional liquid transport. Adv Mater 29:1702995. https://doi.org/10.1002/adma.201702995
Li Q, Song Y, Xu R, Zhang L, Gao J, Xia Z, Tian Z, Wei N, RüMmeli MH, Zou X (2018) Biotemplating growth of Nepenthes-like N-doped graphene as a bifunctional polysulfide scavenger for Li–S batteries. ACS Nano 12:10240–10250. https://doi.org/10.1021/acsnano.8b05246
Li Z, Liu J, Jiang K, Thundat T (2016) Carbonized nanocellulose sustainably boosts the performance of activated carbon in ionic liquid supercapacitors. Nano Energy 25:161–169. https://doi.org/10.1016/j.nanoen.2016.04.036
Park JK, Kim S (2019) Three-dimensionally structured flexible fog harvesting surfaces inspired by Namib desert beetles. Micromachines 10:201. https://doi.org/10.3390/mi10030201
Nanev CN, Saridakis E, Govada L, Kassen SC, Solomon HV, Chayen NE (2019) Hydrophobic interface-assisted protein crystallization: theory and experiment. ACS Appl Mater Interfaces 11:12931–12940. https://doi.org/10.1021/acsami.8b20995
Tyson W, Miller W (1977) Surface free energies of solid metals: estimation from liquid surface tension measurements. Surf Sci 62:267–276. https://doi.org/10.1016/0039-6028(77)90442-3
Nishino T, Meguro M, Nakamae K, Matsushita M, Ueda Y (1999) The lowest surface free energy based on− CF3 alignment. Langmuir 15:4321–4323. https://doi.org/10.1021/acs.langmuir.6b01922
Park B-G, Park I-J, Han J-S, Lee S-M, Lee C-G, Ha C-S (2013) Characterization of optical properties in water-in-oil emulsion. J Dispersion Sci Technol 34:560–565. https://doi.org/10.1080/01932691.2012.680833
Long M, Peng S, Deng W, Miao X, Wen N, Zhou Q, Deng W (2018) Highly efficient separation of surfactant stabilized water-in-oil emulsion based on surface energy gradient and flame retardancy. J Colloid Interface Sci 520:1–10. https://doi.org/10.1016/j.jcis.2018.02.061
Funding
This work was supported by the National Key R&D Program of China, Grant No. 2019YFC1905900; National Natural Science Foundation of China, Grant No. 31870566; Shandong Key Research and Development Program, Grant No. 2019JZZY010501; and Taishan Scholars Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MP4 4386 KB)
Supplementary file3 (MP4 3028 KB)
Supplementary file4 (MP4 27726 KB)
Supplementary file5 (MP4 22518 KB)
Rights and permissions
About this article
Cite this article
Ding, Z., Tian, Z., Ji, X. et al. Bio-inspired catalytic one-step prepared R-siloxane cellulose composite membranes with highly efficient oil separation. Adv Compos Hybrid Mater 5, 2138–2153 (2022). https://doi.org/10.1007/s42114-022-00517-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42114-022-00517-z