Abstract
The present research work aims to investigate the seasonal variations in hydrochemistry and water quality of Khacheopalri Lake, a recognized Ramsar site in Sikkim Himalayas, along with determining the dissolved ion sources and the mechanisms influencing lake water chemistry. The result has shown the acidic nature of lake water with distinct seasonal variation (mean pH 5.61 and 6.02 in the dry and wet seasons). Elevated values for EC, TDS, DO, HCO3−, Cl− in the lake water during the wet season are due to the precipitation, dissolution, and diffusion of O2/CO2 in the lake system. The hydrochemical facies of lake water is of Ca2+–HCO3− type, depicts dominance of Ca2+ and HCO3− in ionic composition. Cross plot analyses elucidate that lake water chemistry is majorly governed by bicarbonate weathering, with minor contributions from silicate weathering. Principal component analysis of hydrochemical dataset has confirmed that major cations and anions in lake water have mainly arrived from geogenic sources as a result of weathering and erosion in the lake catchment area. However, run-off water from adjacent croplands, human settlements, and temporal factors also contributed to determining lake water characteristics. Evaluation of water quality index (WQI) has ascertained that the lake water is “good to excellent” in type and pertinent to aquatic life and human uses. The findings from present study can provide essential baseline information which would be crucial for effective management and conservation of this sacred ecological site and can be a good reference for further study on glacial-formed Lesser Himalayan lakes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lakes are one of the most important features on the earth surface, as they supply freshwater resources for consumption, irrigation, recreation; support aquatic/wildlife, and help in other ecological functions. India has a considerable number of natural lakes distributed unequally all over the country, but their presence in the Himalayas has a great importance as these are the central sources of freshwater at high-altitude remote areas. The Himalayan Mountain range in India extends approximately 2400 km from east to west and it is an abode to hundreds of glacial and non-glacial lakes. Here we find the abundance of perennial lakes mostly, those that contain freshwater from monsoon rain and snowmelt source. These lakes and wetlands in the Himalayan Mountain region are geographically and environmentally of immense value, as these are the paradises for tourists, trekkers, and sources of sustenance to the local people and pastoral communities, in addition to wildlife and aquatic life forms (Bhat et al., 2011). But little attention has been given so far to explore the ionic composition, water quality, productivity, and the range of biodiversity of these remote lakes.
The integrity of the lake ecosystem is subjected to its geophysical condition, and it is also related to various natural life processes taking place in the lake environment. Weathering of rocks and erosion in the lake catchment area, sedimentation, and anthropogenic contributions are the prime factors that regulate the ionic composition and quality of lake water. Besides temporal variations and precipitation rate are also known to have an influence on the abundance of major cations (Ca2+, Mg2+, Na+, K+) and anions (HCO3−, SO42−, PO43−, NO3−, Cl−), and contribute determining the terminal water characteristics of the lake (Anshumali & Ramanathan, 2007). With the higher tourist inflow coupled with the increasing population in the lake catchment area, it is imperative to maintain the health of lake ecosystems. Keeping in view with the scenario, it is, therefore, essential to understand the hydrochemical characteristics to protect the water quality and pristine nature of the Himalayan lakes.
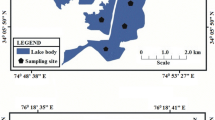
The field study of the present investigation was conducted on Khacheopalri Lake, situated at an altitude of 1700 m (Lat:27 °22′24″N Long: 88 °12′30″E) in the midst of a pristine forest in West Sikkim, India (Fig. 1), has gained much popularity among tourists in the last few years. Owing to its rich biodiversity, features of the landscape, and religio-cultural prominence, the site has been open to multifaceted research opportunities. The places around the lake have become essential tourist zones, but parallel with the entry of the tourists, there is also a fear of the contamination of the lake water. It represents the original region of compact granular snow, a region that was formed by glacier and the depression (estimated 3500 years old) was formed as a result of the scooping action of the glacier. (Jain et al., 2000; Raina & Srivastava, 1980). The lake drainage area (which extends about 12 km) is an integral part of the Ramam Watershed, which covers broad-leaved mixed forests, crop lands and two villages. The lake is divided into the water area (3.79 hectares) and the surrounding peat land is infested with encroached peripheral vegetation. The primary rock type of the study area comprised Kangchenjunga gneisses of the higher Himalaya thrust over the Daling rocks, phyllite and quartzite with streaks of biotite (GSI, 1984; Raina & Srivastava, 1980). The soil type of the study area is sandy loam. Temporal variability influences the hydrochemical characteristics and the distribution of ions/dissolved elementals in water. The temperature ranges from 3 °C in winter to 22 °C in summer. Variations in rainfall are noticeably high, with mean annual precipitation of 2410.4 mm, of which 64% of the annual rainfall is received during the southwest monsoon rainfall (June–September). Based on that, the study area is distinguished by two dominant seasons: the wet or rainy season (May–September, mean precipitation of 1868.0 mn) and the dry season (October–April, mean precipitation of 514.4 mm), which includes winter and short summer (Guhathakurta et al., 2020). Frequent landslides and soil erosion are very common in the lake catchment area during the wet season because of heavy rainfall (Choudhury, 2006).
The lake is considered to be a holy lake for the Buddhist communities and the local name ‘wishing lake’ is also attributed to it. There is also a monastery that is nestled amidst the dense foliage and the greenery surrounding the lake. The well-guarded forest boundary protects the entire Khacheopalri lake. The lake is also an important destination for migratory birds during winter. Due to the presence of rich flora and fauna, the place also provides a basic source of sustenance to a wide variety of aquatic and wild animals, and birds. The concept of the pristine ecosystem is far from an ideal call now, as conservationists are now perturbed by a large number of human footprints in the area. Due to the rampant growth of anthropogenic pressure, researchers have found that the quality of the lake is under threat. It is a promising site for ecotourism, indigenous economic and social development located within the southern part of Khangchendzonga Biosphere Reserve. The lake is a rich platform for religious fairs and cultural festivals which devotees from Nepal and Bhutan also come to attend (Jain et al., 2004; Brown & Mallarach, 2008). The lake also provides strong spiritual support to the local population, especially during the time of any crisis. This lake is of national importance because of its religious and tourist attraction and home to different aquatic, terrestrial animals, and birds. Besides, the water of the lake is used for worship, and rituals; thus, this study has a lot of sociological importance as well.
Several research studies were carried out to investigate the hydrochemical appraisal and water quality of the Himalayan lakes (Anshumali & Ramanathan, 2007; Deka et al., 2015; Singh et al., 2016; Gaury et al., 2018; Kumar et al., 2019), but only a few reports are available on the Sikkim Himalayan lakes (Jain et al., 1999; Roy & Thapa, 1998). Earlier, Jain et al., (1999, 2000) studied nutrient dynamics and changes in land use/land cover of Khacheopalri Lake; and the trophic state of the lake was investigated by Nayek et al. (2018). However, previous findings were not concerned with hydrochemical mechanisms, major ion composition, and evaluation of water quality. Therefore, this study was undertaken (i) to explore the hydrochemical process, major ion chemistry and the influence of temporal variations on lake water, (ii) source identification of dissolved ions/solutes by multivariate statistics, and (iii) assessment of lake water using water quality index (WQI) for human use and wildlife. The literature survey has shown that this study would be the first of its kind to address the hadrochemical processes, delineate the sources of dissolved ions, and influence of temporal variability on water chemistry of glacial-formed lakes in the Sikkim Himalayas.
Materials and methods
Collection of lake water samples
Field investigation and the collection of water samples were conducted during the period of October 2018–January 2020 (until the lockdown was imposed due to COVID). Sampling stations were selected strategically along the peripheral region of the lake as there is no permission for boating to access water from the middle of the lake. Representative water samples were collected from 10 different sites in the dry season (i.e., summer and winter) and from six sampling sites in the wet season (monsoon) as some of the sampling stations become inaccessible in the rainy season due to profuse growth of peripheral bogs and risk of land subsidence. Lake water samples were collected in 1L sterilized polythene containers approximately 1ft depth from the surface water level; and the containers were pre-washed with the lake water before filling up to the capacity. Care was taken during the collection of water to avoid any type of mechanical agitation. Collection of water samples, its preservation and analysis were performed according to standard methods (APHA 2005).
Analysis of collected water samples
Sampled waters were immediately analyzed for pH, EC, TDS and DO using portable hand analyzer (Systronics 371) and stored in an ice box under 4 °C and transferred to the laboratory for further hydrochemical characterization. In the laboratory collected water samples were filtered through 0.45 μm millipore membrane filter papers (WHA7404002) and analyzed for major ion concentrations. Cations Ca2+, Mg2+, Na+, and K+ were analyzed in atomic absorption spectrophotometer (Varian 220FS), while SO42−, Cl− and NO3− were estimated using ion meter (HSN-90275090). Total hardness (TH), bicarbonate (HCO3−) and PO43− concentration in sampled water were analyzed as per the standard methods (APHA 2005).
Quality control assurance
Proper care was taken during characterization of water samples in the laboratory to ensure the analytical precision. Each water quality parameter was analyzed for three times replication to ensure the accuracy of results. Analytical grade reagents were used for routine laboratory analysis. Suprapure quality standard solutions (E-Mark) were used for calibration of instruments and preparation of standard curves. The glassware used for laboratory analysis were pre-acid washed, rinsed well with Milli-Q water and sterilized in oven under 60 °C. Working standards and intermediate solutions were prepared using Milli-Q water. Blanks were prepared for background correction. After each set of water samples, a set of blank and standard was run to equilibrate the instrument and ensure the quality/accuracy of sample’s results. All experimental results were standardized within 2% standard error (SE) level.
Statistical approach
The variations in the analytical results of measured water parameters were standardized by statistical methods and recorded as Mean ± SD, and the mean values were considered for results interpretation and discussion. The hydrochemical nature of lake water is depicted using conventional graphical methods by plotting the Gibs diagram and Piper diagram in origin software (version 8.5). Multivariate such as Pearson correlations, Principal component analysis (PCA) were performed using SPSS (Version 10) to understand the nature of interlink between the analyzed variables and to predict their possible sources in the lake water. Numerical techniques such as the Water Quality index (WQI) were applied for qualitative assessment of lake health status and classify the lake water with respect to the measured water parameters.
Results and discussion
General characteristics of lake water
The statistical summary of analyzed lake water parameters is presented in Table 1. The recorded temperature of lake water samples showed greater variation during the dry season (8.6 °C–19.4 °C) in comparison to the wet season (11.6 °C–14.8 °C). The solubility of ionic constituents is greatly affected by the pH of the water body. The pH of examined lake water samples found to be in the range of 5.20–6.20 in the dry season and 5.70–6.50 during the wet season. The acidic pH of lake water due to the influence of organic acids arises from decomposition of litters and peripheral bog in the lake environment. Electrical conductivity (EC) is a measure of dissolved ions and inorganic salts in lake water. The EC values in lake water were recorded as 45.80–63.62 µS/cm and 86.40–96.40 µS/cm during the dry season and wet season, respectively. The concentration of total dissolved solids (TDS) ranged between 39.30 and 62.10 mg/L in the dry season, and 84.30 and 94.90 mg/L in the wet season. Higher level of EC and TDS in lake water during the wet season as a result of contributions from the lake catchment through surface run-off. Dissolved oxygen (DO) is an important measure of the healthy water body. In this study, DO level in lake water was on the higher side in the wet season (6.70–8.20 mg/L) in comparison to the dry season (3.40–5.90 mg/L). Elevated DO in lake water may be subjected to water turbulent caused by atmospheric precipitation and influx of run-off water. Total hardness (TH) of the lake water was notably higher in the dry season (mean 29.03 ± 4.50 mg/L) as compared to the wet season (mean 19.81 ± 1.23 mg/L). Nutrient constituents, i.e., PO43− and NO3− showed distinct seasonal variations, with higher PO43− values in dry season (mean 0.08 ± 0.01 mg/L); and NO3− concentration in the wet season (mean 5.55 ± 0.45 mg/L). PO43− and NO3− in the lake water mainly sourced from nearby human settlements and small cultivated fields in the lake watershed.
Major ion composition and hydrochemical facies of lake water
Dissolved ion concentrations in the lake water primarily arise due to the weathering of parent materials (e.g., carbonate, silicate) and various geophysical and geochemical processes occurring in the lake watershed (Stumm, 1992). The major ionic compositions of lake water are represented in Table 1. The abundance of major cations and anions based on their mean concentrations are: Ca2+ (6.89 ± 2.23 mg/L) > Mg2+ (4.19 ± 1.24 mg/L) > Na+ (3.80 ± 0.75 mg/L) > K+ (2.46 ± 0.66 mg/L) and HCO3− (15.73 ± 5.66 mg/L) > Cl− (5.52 ± 1.15 mg/L) > SO42−(5.34 ± 1.81 mg/L). The concentration of cations like Na+, K+, Ca2+ and Mg2+ and anions viz. SO42− and PO43− are found to be on the higher side during the dry season (Table 1), which can be explained due to the high rate of weathering, mineralization, and evaporation rate during dry season. Contrarily, higher values HCO3−, Cl− and NO3− in wet season as a result of precipitation, diffusion, and influx of run-off water from lake catchment areas.
The analyzed results of water samples reflect the dominance of Ca2+ and Mg2+ in cationic composition, and HCO3– and Cl– in the anionic composition of lake water. A simple plot of TDS against Na+/ (Na+ + Ca2+) can provide valuable insight on the mechanisms governing ion chemistry of lake water (Gibbs, 1970). The plotted diagram (Fig. 2) confirms that the composition and characteristics of studied lake water is primarily subjected to the weathering and erosion in the lake catchment area. Hydro-chemical facies of lake water and relationship between water composition and dissolved ions is identified using Piper (1944) trilinear diagram. The resultant output (Fig. 3) depicts calcium (Ca2+) as the dominant cation and bicarbonate (HCO3−) as the dominant anion; and the lake water is classified as Ca2+–HCO3− type in both seasons.
The mechanism controlling major ion chemistry of lake water
Geochemical weathering, erosion, atmospheric precipitation, and the influx of the melting water are the prime source of dissolved ions in mountain lakes. Carbonate weathering is recognized as the primary contributor of Ca2+ and Mg2+ in the lake water; however, HCO3− arises due to weathering of silicate and carbonate minerals. The molar ratio of (Ca2+ + Mg2+)/ (Na+ + K+) in the silicates of the upper crust is close to 1.0 (Taylor & McLennan, 1985). In this study, equivalent ratios of (Ca2+ + Mg2+)/(Na+ + K+) in lake water samples ranges from 2.55 to 3.95. The higher abundance of (Ca2+ + Mg2+) over (Na+ + K+) in the lake water (Fig. 4a) can be attributed to and weathering of Ca2+/Mg2+ bearing minerals (viz., pyroxene, amphibole, and biotite) in the studied region. The relatively higher proportion of (Ca2+ + Mg2+) with respect to total cations (Tz+) (Fig. 4b) and low ratio of (Na+ + K+) to the total cations (Tz+) (Fig. 4c) implies that the ionic constituents in lake water are majorly subjected to carbonate weathering in the lake catchment area (Das & Kaur, 2001; Anshumali & Ramanathan, 2007). Zhang et al. (1995) reported 0.5 (in molar ratio) as the stoichiometric relationships of Ca2+/HCO3− and Ca2+ + Mg2+/HCO3− in the case of carbonate weathering. (Ca2+ + Mg2+)/HCO3− as equivalent ratios in the monitored lake water ranges from 1.20 to 4.97 including both the season (Fig. 4d). The higher values indicate the excess of Ca2+and Mg2+, and this excess positive charge over bicarbonate (HCO3−) needs to be stabilized by other anions, i.e., SO42– and Cl–. The elevated (Ca2+ + Mg2+)/HCO3− ratios (Table 2) suggest some other non-carbonate sources of Ca2+ and Mg2+, may be due to the weathering of silicates. Lower values of (Na+ + K+)/ (HCO3− + SO42−) in the lake water also denote the contribution from silicate weathering (Table 2). The major proportions of dissolved ions in the lake water are subjected to congruent weathering of carbonates and incongruent weathering of silicates in the lake watershed. This observation is consistent with the earlier findings on the Indian Himalayan lakes (Das & Kaur, 2001; Anshumali & Ramanathan, 2007; Deka et al., 2015; Singh et al., 2016; Gaury et al., 2018; Kumar et al., 2019).
Source identification of ionic constituents in lake water using multivariate statistics
Multivariate analysis is considered one of the convenient methods to figure out the potential sources of dissolved ions in lake water. The correlation analyses execute the degree of association and interdependency of measured variables in a common matrix. In this study, Pearson’s correlation on composite data set of analyzed water parameters executes significant positive association (Table 3) in EC–TDS, TDS–DO, Ca2+–Mg2+, Mg2+–Na+ and K+, HCO3−–Ca2+ and Mg2+, SO42−–Ca2+ and Mg2+, and NO3−–Cl− and PO43− during the dry season. In wet season, positive correlations found to exist for TDS with EC, DO, Ca2+, Mg2+ and K+, Na+ with Ca2+, Mg2+, HCO3− and SO42−, HCO3−–SO42− and NO3−–PO43−. These positive associations between analyzed variables can be explained due to their cognate variability under the influence of the same factor or their dissemination from a common source. The strong association between EC and TDS infers contribution of dissolved solutes through erode materials; and TDS–DO correlations can be attributed to higher oxygen diffusion as a result of water turbulent caused by the influx of run-off water. Meanwhile, HCO3−–Ca2+ and HCO3−–Mg2+ affirm the strong influence of carbonate weathering on lake water system. NO3−–Cl− and NO3−–PO43− depict contribution from nearby farmland and human settlements.
PCA is used to identify the patterns of distribution of analyzed water parameters in a complex hydrochemical dataset by extracting the principal component (PC) with eigen value > 1. The affinity (factor coefficient) of variables in each factor can correspond to their potential origin or controlling mechanism in lake water. PCA analyses (after varimax rotation) of measured water parameters extract three major components (F1, F2, and F3) for both dry and wet seasons exemplify 83.959% and 95.039% of the cumulative variance, respectively (Table 4). In dry season, F1 illustrate higher factor coefficient for temperature, TDS, DO, TH, Ca2+, Mg2+, Na+, K+, HCO3− and SO42−. This observation explains the geo-climatic factors (weathering, mineralization, precipitation) and atmospheric influences (such as diffusion of O2/CO2) on lake water. Variables in F2 such as Cl−, NO3− and PO43− describe contribution from crop lands and human settlements in the lake catchment area. While F3 (pH and EC) can be linked to the release of organic acids due to the decomposition of litter and plant residues in forest-covered lake environments. In the wet season, F1 accounts for temperature, EC, TDS, DO, TH, K+ and Cl− demonstrate temporal factors, atmospheric influences and influx of run-off waters. F2 depicts pH, Ca2+, Mg2+, Na+, HCO3− and SO42− due to geochemical weathering and mineral dissolution in the lake catchment area. However, the higher factor coefficient values for NO3− and PO43− in F3 demonstrate the contribution from adjacent crop fields.
Evaluation of water quality index (WQI)
Being considered as a sacred lake, the water of this lake is used by the local residents for worship, and ritual purposes. Therefore, quality evaluation of lake water is essential and is performed by comparing with WHO (2006) and BIS (2012) standards.
Water quality index (WQI) provides a comprehensive picture of water quality and is widely used to classify the overall quality of surface waters (Edet et al., 2013; Gupta et al., 2016; Yidana & Yidana, 2010). For the calculation of WQI, a weight (W) is assigned to the selected water parameters based on their relative contribution on overall water quality (Table 5). Relative weight (Wr) is determined as: \({W}_{\text{r}}={w}_{\text{i}}/\sum_{i=1}^{n}{w}_{\text{i}}\), where W = assigned weight of water parameter, and \(\sum {w}_{\mathrm{i}}\) = sum of weights of measured variables, which is 32 for this study (Table 5). The Quality rating scale (Qi) for each variable is computed using the formula:
where Ci is the measured value of water parameter; Si is the Standards for drinking water (WHO /BIS).
Finally, WQI is calculated as: \(\mathrm{WQI}=\sum {SI}_{\mathrm{i},}\) where \({SI}_{\mathrm{i}}= {W}_{\mathrm{r}}* {Q}_{\mathrm{i}}.\)
Based on calculated WQI, water samples are classified as: Excellent (WQI < 25), Good (WQI: 25–50), Moderate (WQI: 51–75), Poor (WQI: 76–100), and Very Poor (WQI > 100). The WQI of studied lake water samples recorded to be 18.38–22.43 during the dry season and 23.21–25.56 in the wet season (i.e., monsoon). The results have shown monitored lake water samples as excellent type in the dry season and recognized as excellent to good category in the rainy season (Fig. 5), and suitable for aquatic habitats and human uses.
Implication for conservation and management of sacred Khacheopalri Lake, a Ramsar wetland site in Sikkim Himalaya
Since the Khacheopalri Lake area is marked by rich biodiversity, there is a constant need for revised environmental policies for its protection. As per the basic framework of the Ramsar Convention, there was a need for intergovernmental accord for the wise and sustainable use of wetlands all over the world (O’Neill et al., 2020). The overall ecological system, including the water, flora, fauna, and the cultural atmosphere, and integrity should be protected. A large number of people are dependent on the lake and its surrounding areas for economic growth, and due to the pressure of tourism, there is already a growing menace to sensible use of this wetland. The local stakeholders are equally responsible to demarcate this area for the protection of the wetland ecosystem. The development of micro industries and local cottage crafts has sprung up. Going by the convention of the Ramsar wetland, there is an urgent need to balance environmental and economic concerns simultaneously (Choudhury, 2006).
The lake area is one of the essential high-altitude wetlands which is crucial to sustaining the diverse Himalayan peatland. The glacial history of the place is rich, and the lake is separate from any fen or bog. Khacheopalri Lake is also a habitat for vertebrate and invertebrate fauna and nesting places for migratory birds (Panigrahy, 2010). The uniqueness of this lake is because of the interconnection between the soil, water, biota, and visible water bodies (Brown & Mallarach, 2008). The lake is a major tourist attraction and source of sustenance for the indigenous population and already under pressure due to anthropogenic interference. The dominant nature of the peatland is temperate and there is an urgent need for community-based conservation that is locally feasible. Although the Sikkim government looks after the overall health of the lake, the local Khacheopalri Welfare Committee retains most of the operational rights. Multiple environmental organizations that have collaborated with cultural institutions to preserve the natural resources of this wetland. The forest soil around the wetland is rich in nutrients but its remoteness of location has often hindered proper ecological surveys. The wetland positional correlation combined with the climatic and topographical features is integral to environmental management. The lake is crucial for maintaining inter-linkages between social, cultural, and ecological perspectives and it plays an important role in flood control. So, effective planning and measures to conserve this lake are necessary.
Conclusion
The investigation has shown noticeable temporal variations in the hydrochemical characteristics of Khacheopalri Lake water, with EC, TDS, DO and HCO3− values being recorded higher during the rainy season. The ionic composition of lake water is dominated by Ca2+ and HCO3−, comprising 31.84–43.89% of total cations and 29.42–73.82% of total anions. Gibbs’s plot has depicted rock weathering as the key factor which controls the composition and quality of the lake water to a major extent. Molar equivalent ratios and scattered plot analysis have specified carbonate weathering as the dominant source of major ions, with minor contribution from silicate weathering. However, Cl−, NO3− and PO43− in the lake water are contributed by anthropogenic sources and crop fields in the lake catchment area. Strong positive correlations between ionic components explicate their origin from a common source. PCA analysis of the hydrochemical dataset has revealed that major cations and anions in lake water are mainly derived from geogenic sources as a result of weathering and erosion in the lake catchment area. Besides, run-off water from adjacent croplands, human settlements, and atmospheric/temporal factors also contributed to determining the chemical characteristics of lake water. WQI analysis of lake water has specified that lake water is of excellent type in the dry season and falls under the "excellent to good" category during the wet season in accordance with aquatic life and human uses.
This lake has spiritual and cultural significance to indigenous people, as they come here for rituals and worship, and it also has great economic importance for local communities as well as for the State. Being a Ramsar site under the Kanchenjunga Biosphere Reserve, the studied lake has immense ecological value and, hence, needs to be monitored on a regular basis for the qualitative assessment of its health. The current research work can provide important insight to the major ion chemistry and quality of the lake water and the governing processes occurring in the lake environment. Hence, this would be useful to take appropriate measures for effective conservation and sustainable management of this important wetland, considering its ecological importance and tourism potential.
Availability of data and material
The authors ensure about the transparency of data obtained through experimental results, and will be able to provide the Data on demand.
Code availability
Not applicable.
References
Anshumali, & Ramanathan, A. L. (2007). Seasonal variation in the major ion chemistry of Pandoh lake, Mandi 297 district, Himachal Pradesh, India. Applied Geochemistry, 22, 1736–1747.
APHA, AWWA, WEF. (2005). Standard methods for examination of the water and wastewater (21st ed.). American Public Health Association.
Bhat, F. A., Yousuf, A. R., Aftab, A., Arshid, J., Mahdi, M. D., & Balkhi, M. H. (2011). Ecology and biodiversity in Pangong Tso (Lake) and its inlet stream in Ladakh, India. International Journal of Biodiversity and Conservation, 3(10), 501–511.
Brown, J., & Mallarach, J. (2008). Protected landscapes and cultural amb [i.e. and] spiritual values. Kaparek.
Bureau of Indian Standards (2012). Indian Standard for Drinking Water—Specification (Second Revision). New Delhi: Bureau of Indian Standards.
Choudhury, M. (2006). Sikkim. A Mittal Publications.
Das, B. K., & Kaur, P. (2001). Major ion chemistry of Renuka lake and weathering processes, sirmaur district, Himachal Pradesh, India. Environmental Geology, 40, 908–917.
Deka, J. P., Baruah, B., Singh, S., Chaudhury, R., Prakash, A., Bhattacharyya, P., Selvan, M. T., & Kumar, M. (2015). Tracing phosphorous distributions in the surficial sediments of two eastern Himalayan high altitude lakes through sequential extraction, multivariate and HYSPLIT back trajectory analyses. Environmental Earth Sciences, 73(11), 7617–7629.
Edet, A., Ukpong, A., & Nganje, T. (2013). Hydrochemical studies of Cross river basin (Southern Nigeria) river system using cross plots, stastics, and water quality index. Environmental Earth Sciences, 70, 3043–3056.
Gaury, P. K., Meena, N. K., & Mahajan, A. K. (2018). Hydrochemistry and water quality of Rewalsar Lake of Lesser Himalaya, Himachal Pradesh, India. Environmental Monitoring and Assessment, 190(2), 84–105.
Gibbs, R. J. (1970). Mechanisms controlling worlds water chemistry. Science, 170, 1088–1090.
GSI. (1984). Geology and Mineral resources of West District of Sikkim state. Geological Society of India.
Guhathakurta, P., Bandgar, A., Menon, P., Prasad, A.K., Sangwan, N., & Advani, S.C. (2020). Observed Rainfall Variability and Changes Over Sikkim State. Met Monograph no. ESSO/IMD/HS/Rainfall Variability/23(2020)/47. India Meteorological Department: Government of India.
Gupta, S., Nayek, S., & Chakraborty, D. (2016). Hydrochemical evaluation of Rangit river, Sikkim, India: Using Water Quality Index and multivariate statistics. Environmental Earth Sciences, 75, 567–580.
Jain, A., Rai, S. C., Pal, J., & Sharma, E. (1999). Hydrology and nutrient dynamics of a sacred lake in Sikkim Himalaya. Hydrobiologia, 416, 13–22.
Jain, A., Rai, S. C., & Sharma, E. (2000). Hydro-ecological analysis of a sacred lake watershed system in relation to landuse/cover change from Sikkim Himalaya. CATENA, 40, 263–278.
Jain, A., Singh, H., Rai, S., & Sharma, E. (2004). Folklores of Sacred Khecheopalri Lake in the Sikkim Himalaya of India. Asian Folklore Studies, 63, 291–302.
Kumar, P., Meena, N. K., & Mahajan, A. K. (2019). Major ion chemistry, catchment weathering and water quality of Renuka Lake, north-west Himalaya, India. Environmental Earth Sciences, 78, 319–334.
O’Neill, A., Chhetri, P., Chhetri, B., & Rana, S. (2020). Establishing ecological baselines around a temperate Himalayan peatland. Wetlands Ecology and Management, 28(2), 375–388.
Panigrahy, S. (2010). National Wetland Atlas Sikkim. Ministry Of Environment And Forest. Retrieved 24 June, 2021, from http://moef.gov.in/wp-content/uploads/2019/09/NWIA_Sikkim50k_Atlas.pdf
Piper, A. M. (1944). A graphic procedure in the geochemical interpretation of water analysis. Transactions of the American Geophysical Union, 25(6), 914–928.
Raina, V. K., & Srivastava, B. S. (1980). A reappraisal of the geology of the Sikkim Lesser Himalaya. In K. S. Valdiya & S. B. Bhatia (Eds.), Stratigraphy and Correlations of Lesser Himalayan Formations (pp. 231–241). Hindustan Publishing Corporation.
Roy, B. N., & Thapa, M. P. (1998). Lakes of Sikkim: A limnological study. In S. C. Rai, R. C. Sundriyal, & E. Sharma (Eds.), Sikkim: Prespectives for Planning and Development (pp. 189–204). Sikkim Science Society and Bishen Singh Mahendra Pal Singh.
Singh, V. B., Ramanathan, A. L., & Mandal, A. (2016). Hydrogeochemistry of high-altitude lake: A case study of the Chandra Tal, Western Himalaya, India. Arabian Journal of Geosciences, 9, 308–316.
Stumm, W. (1992). Chemistry of the solid–water interface. Wiley.
Taylor, S. R., & McLennan, S. M. (1985). The continental crust: Its composition and evolution. Blackwell.
WHO. (2006). Drinking Water Guidelines World Health Organization standard. World Health Organization.
Yidana, S. M., & Yidana, A. (2010). Assessing water quality using water quality index and multivariate analysis. Environmental Earth Sciences, 59, 1461–1473.
Zhang, J., Huang, W. W., Letolle, R., & Jusserand, C. (1995). Major element chemistry of the Huanghe (Yellow River), China: Weathering processes and chemical fluxes. Journal of Hydrology, 168, 173–203.
Acknowledgements
The authors wish to thank Dr. P.C. Rai for his valuable suggestion during this research work, and acknowledge DST, GOI, for partial financial support (Project No. DST/TMD/EWO/WTI/2K19/EWFH/2019/217).
Funding
This research study partially was supported by DST, Government of India, (Project No. DST/TMD/EWO/WTI/2K19/EWFH/2019/217).
Author information
Authors and Affiliations
Contributions
NA: Conceptualization, Sample collection, Methodology, Formal analysis, Visualization, Writing—original draft preparation. KKP: Conceptualization, Formal analysis, Software, Investigation, Writing—original draft preparation. SC: Investigation, Visualization, Writing—original draft preparation. SN: Supervision, Visualization, Investigation, Validation, Writing, Reviewing and Editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
The authors are here by giving the consent the publication of manuscript as well as experimental data (represented in tabular, graphical and in image form). Authors also ensure that the manuscript or the experimental results/data have not been submitted elsewhere for the publication.
Rights and permissions
About this article
Cite this article
Auddy, N., Pobi, K.K., Chakraborty, S. et al. Ion chemistry and quality assessment of a post-glacial sacred lake in Sikkim Himalaya: source identification and conservation measures. Int J Energ Water Res 6, 303–314 (2022). https://doi.org/10.1007/s42108-021-00155-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42108-021-00155-z