Abstract
Quality of water is as important as its quantity, because it determines the state of health of man to a large extent. To maintain sustainable human development and public health of students’ community, water quality assessment was conducted by characterizing the influence of anthropogenic and natural processes on thirty-one (31) water samples from boreholes, hand-dug wells and surface water from Federal University of Agriculture Abeokuta and its environs. Physico-chemical data, statistical data, ionic-ratios, Gibb’s plot etc. were employed in delineating dissolved ions’ sources in the water from the study-area. Some physicochemical parameters were determined insitu with a potable multimeter, while the others were determined in the laboratory using standard methods. Various ionic plots showed that both carbonate dissolution and silicate weathering were the major contributors of ions in the water samples. Chloro-Alkaline Indices 1 and 2 values of samples collected were positive indicating direct ion exchange predominance. Factor analysis produced three factors accounting for 76% of the total variance. Factor-1 had high loadings (45.9%) on calcium, magnesium, bicarbonate, sulphate, potassium, pH, electrical conductivity and total dissolve solids suggesting water–rock interaction within the aquifer as the primary influence on groundwater quality and a common geogenic source. This was also corroborated by Gibb’s plot. Factors 2 and 3 revealed a moderate-high loading on NO3−, Cl− and pH suggesting that anthropogenic activities influence on water quality was quite significant especially in shallow and uncovered wells and surface water but minimal in borehole samples. Due to intense anthropogenic activities (farming and septic tank placement) and poor well management (open wells, poorly completed wells), groundwater drawn from shallower wells and surface-water appeared to be more polluted compared to those from boreholes. Generally, groundwater quality ranged from fairly to well suitable for drinking purpose except in shallow and poorly constructed hand-dug wells bordering farm areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water is an elixir of life. Although there is abundant water on earth (surface and groundwater), they are not always available in the right place, time and quality. Research into groundwater resource has come to the forefront of geological studies in southwestern Nigeria as a result of continuous rise in urban population, shortage of municipal water supply, contamination, and pollution problems (Ehinola 2002). Groundwater is of great importance as it serves as a dependable source of potable water which can be associated with improvement in public health (Laniyan et al. 2015). This makes groundwater to be considered in most countries as the priority for any sustainable development (Fekkoul et al. 2012). The chemistry of water which can in turn have significant impact on its quality can result from the ions dissolved in it when rocks weather due to rock-water interaction (Ige et al. 2017). The contamination of groundwater by anthropogenic sources has long been recognized (Butow et al. 1989; Alloway and Ayres, 1997; Clark 2006). Anthropogenic activities that affect groundwater quality may be through leakages from underground storage tanks, waste dump sites, effluents, sewage pits, and brine disposal from the petroleum industry, mine waste, animals’ feedlots and farmlands/highway run-offs (Naidu et al. 2011). Furthermore, emission from incomplete fuel combustion which gets into the atmosphere results in acid rain which infiltrates the sub-surface water and alters groundwater chemistry. Groundwater in an unconfined aquifer can be contaminated by the excessive use of pesticides, herbicides and fertilizers (Naidu et al. 2011).
A number of researches on water quality of Abeokuta and environs have been conducted (Adebola et al. 2013; Oloruntola and Adeyemi 2014; Awomeso et al. 2019), there is, however, minimal literature on the hydrochemical character of water consumed by the teeming population of students of the Federal University of Agriculture Abeokuta (FUNAAB). Opafola et al. (2020) evaluated the potability of satchet water sold within the university community using physico-chemical and bacteriological properties. It is, however, important to evaluate the various sources of water consumed by students and staff who are resident within the university community and neighbouring environs. Thus, this research is aimed at identifying and characterizing the processes responsible for the quality of various water sources (boreholes, surface water and hand-dug wells) in Federal University of Agriculture, Abeokuta (FUNAAB) and its environs.
Description of study location
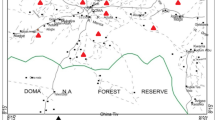
The study area borders the Ogun-Osun River Basin Development Authority. It is situated along Osiele-Abeokuta road, off Abeokuta-Ibadan road, Alabata (Fig. 1). It lies on latitude 7.20° N–7.24° N and longitude 3.41° E–3.46° E. The study area is associated with a rugged terrain as it boasts of well pronounced undulating topography with an altitude ranging 30–180 m above mean sea level (Bello et al. 2009). The area is drained by rivers Oshinko, Ole/Alakata, Arakanga, Pala, Pap/Olu and Tigba/Ajigbayin. Alternate wet and dry seasons characterize the climate of the area. During the raining season (March–October), rainfall is about 750––1000 mm, while November–March is dry season with amount of rainfall between 250 and 500 mm (Akanni 1992). The drainage pattern is dendritic and the area is dominantly drained by Rivers Ogun, Oyan and many other tributaries. The study area is underlain by the Precambrian rocks of the southwestern basement complex consisting of granites suite, migmatite and gneisses (Oyawoye 1972; Kayode 1976). Field observations show that quartz, feldspar, biotite, muscovite are common minerals. The regional geology of the area has also been well documented by Oloruntola and Adeyemi (2014), Akinse and Gbadebo (2016).
Material and Methods
Sampling location and samples analysis
Total of 31 water samples were randomly collected from boreholes, well water and surface water sources located within and outside FUNAAB premises (Table 1, Fig. 2). GPS was used to take coordinates of sampled points and the samples were labelled appropriately. The samples were collected with minimum of 300 m distance between each location to ensure wide representation of the whole target area and to prevent duplication of samples with similar anthropogenic interference. The water samples were collected for cations and anions tests in 60 cl and 100 ml bottles, respectively (APHA 2005). These bottles were thoroughly rinsed about 4–5 times with the water to be sampled, while samples for the cations were acidified with concentrated nitric acid (HNO3). Physico-chemical properties of the water such as: pH, TDS, EC and salinity were measured in-situ using field portable device (Jenway 3505), while cations and anions’ presence (Ca2+, Mg2+, Na+, K+,Fe,NO3−, PO4−,SO42−, HCO3−, CO3−,Cl−) were analyzed at FATLAB Nigeria company, Ibadan, Nigeria. The accuracy of the obtained results from laboratory analysis for major ions was checked by calculating the charge balance error (CBE), which was generally within ± 5 (Eq. 1). All the samples were kept in a cool environment to maintain a temperature of about 4˚C before analysis (Malomo et al. 1990). The concentrations of anions were measured using ion chromatography techniques, Ca and Mg were measured using standard Ethylenediaminetetraacetic acid (EDTA) titration, and K and Na were measured using flame photometry (model: JENWAY PFP7)
Results and discussion
The results of the physico-chemical parameters are presented in Table 2. Physico-chemical data highlighted distinct differences between hand-dug wells, boreholes, sachet water and surface water. The order of abundance of the cations’ concentration in hand-dug wells, sachet-water and boreholes was the same: K+ > Ca2+ > Na+ > Mg2+ > Fe2+,while surface water is: Mg2+ > K+ > Na+ > Ca2+ > Fe2+. For the anions, the order of abundance for surface-water, hand-dug wells and borehole samples was the same: Cl− > NO3− > HCO3− > PO43− > SO42− > CO3−, while that of sachet-water is: Cl− > NO3− > HCO3− > SO42− > PO43− > CO3−.
A high coefficient of variation (CV > 50%, Table 2) and spatial distribution indicated the heterogeneous abundance of most physico-chemical parameters in hand-dug wells. This can result from direct contamination during use, seepage from septic tanks or pit toilets. Atmospheric contamination could also be possible as many wells were not covered. However, most borehole, sachet and surface water samples had CV < 50% (except TDS, SO4 and HCO3) and indicated homogeneity.
Suitability of the groundwater sources for drinking purposes
The quality of the collected water samples and its potential impacts on human health have been assessed by comparing their physico-chemical properties with standards provided by the World Health Organization (2011) and the Standard Organization of Nigeria (2007) as shown in Table 3.
pH values ranged from 3.30 to 11.75(highly acidic–alkaline) with an average of 6.99. These extremes of pH are indications of pollution. pH values of the majority of the well and surface-water samples fall outside WHO recommendation for potable water. Four samples: two surface water samples and two hand-dug well samples with no farming activity nearby fall within the WHO recommendation of 6.5–8.5. However, pH of borehole samples range between 6.9–6.91 and, therefore, fall within the WHO standard for potable water. Eleven (11) water samples sourced from sachet-water and hand-dug wells were categorized as alkaline.
Water hardness was evaluated using Sawyer and McCarthy (1967) classification (Table 4). 77.42% of the samples fall below 75 mg/L; therefore, they are categorized as soft. Soft water with low alkalinity can aid potential toxic heavy metals mobility in the aquifer (Cravotta and Kirby 2004). 19.35% of the samples were classified as moderately hard, while a hand-dug well sample was categorized as hard; this was confirmed by its ionic concentration outlier values for both Ca2+ (62.14 mg/L) and Mg2+ (24.65 mg/L) which are above the maximum permissible limits specified by SON (2007). This sample though good for drinking, since it is rich in Ca is not economical for domestic use, since soaps do not form lather easily in it (save detergents are used). They also result in furring of kettles. While consumption of hard waters can also play a role in heart diseases and prenatal mortality (Agarwal and Jagetai 1997), soft water intake can also increase the risk of high blood pressure (WHO 2011).
NO3− is generally high ranging from 0 to 70.32 mg/L and a mean concentration of 42.92 mg/L. Majority of the areas with concentration of NO3− greater than 30 mg/L are also associated with high Cl− concentration (> 100 mg/L). Interestingly, the highest Cl− concentration recorded was obtained, where the NO3− value was also very high (61.33 mg/L) and this occurred at the Constitution of the Committee on Centre for Community Based Farming Scheme’s (COBFAS) pineapple farm within FUNAAB. This invariably suggests similar contamination sources. About 68% of the groundwater samples have nitrate concentration greater than 30 mg/L, the cut-off for possible nitrate from natural sources (Hernandez-Garcia and Custodio 2004) meaning the NO3−was from anthropogenic sources (animal waste and fertilizer most likely). NO3− concentration of more than 50 mg/L which is greater than WHO standard was observed in about 48% of the samples collected. These samples were collected from hand-dug wells close to farms and soak-away. These points to possible anthropogenic influence caused by infiltration of fertilizers used in the farmland and seepage from septic tanks (Singh et al. 2016). Samples (hand-dug wells) from animal farms have higher nitrate values than samples taken in plant farms (where fertilizers were not applied). This is probably as a result of high nitrate content of animal wastes. Surface water samples also have high NO3− values and this results from run-offs of nearby farms and anthropogenic activities like washing near the streams. The values spread across the study area and hence, it can be deduced that nitrate pollution is a major pollution source in the study area. Nitrate has known adverse impact on human health, primarily in infants. Nitrate affects hemoglobin in the blood and reduces the babies’ ability to transport oxygen; infants so affected are said to have ‘blue baby syndrome’. The most common origin of nitrate in groundwater within the study area are agricultural activities and improper disposal of animal wastes and seepages from septic tanks.
The concentration of iron (Fe) in the water samples range from 0 to 4.09 mg/L. 32.3% samples exceeded WHO recommended value for Fe; Higher levels of Fe beyond the permissible limit can result into liver, kidney, neurological disorders (Mandour 2012; Farina et al. 2013) etc.
The Cl− limit set by SON (2007) was exceeded by about 38.7% of the samples, but none exceeded the WHO standard. The elevated concentration of Cl− and NO3− is largely due to downward percolation of water contaminated by livestock manure as well as washing activities of the inhabitants in the residential areas. Higher levels of Cl− beyond the permissible limit can impact taste to the water.
Sachet-water, boreholes and surface-water samples have EC values range (average) of 53–127 (95 μS/cm); 264–576 (368.67 μS/cm) and 317–568 (408 μS/cm), respectively, which imply low-moderate contamination. However, well samples EC values ranged 173–1181 μS/cm (496.5 μS/cm) indicative of high contamination.
The TDS followed the trend of EC because of their relationship. What affect one affects the other. The range of TDS values (average) for sachet-water, boreholes and surface-water samples are: 35–90 mg/L (62.50 mg/L); 114–307 mg/L (179 mg/L) and 212–377 mg/L (271.67 mg/L), respectively. These values indicate they are potable water sources (Table 5). On the contrary, Some Hand-dug well samples make up the remaining 15.63% with values falling between 500 and 1000 mg/L with TDS values between 115 and 786 mg/L (328.64 mg/L), making them just permissible. Based on TDS concentration, 84.37% of the waterfall between 0 and 500 mg/L making them suitable for drinking (Table 5). Therefore, the water sources are fair to well suitable for drinking purposes.
Statistical correlation
The Correlation coefficient is commonly used to measure the relationship between two variables (Table 6). It is simply a measure to exhibit how well one variable predicts the behaviour of the other. TDS shows a strong positive correlation (0.99) with EC, and with cations such as Ca (0.81), Mg (0.73), K (0.72). It can be inferred that these ions were derived from the same source. The weak correlation between HCO3− and Cl− (− 0.05) reflects Cl source from atmospheric inputs such as: rainfall (Manjusree et al. 2017). The impact of anthropogenic activities on water quality can be under-studied using the relationship of NO3−, K+ SO42− and PO43−.
NO3− did not significantly correlate with K+ (− 0.10), SO42− (0.24) and PO43− (0.07) implying agricultural activities is not the principal influence on the water chemistry. The average NO3− value is 42.92 mg/L indicating that the groundwater samples are not principally influenced by pollution from agricultural sources (Zaidi et al. 2017). K+ concentration in the groundwater samples varied from 0.80 to 35.23 mg/L; high K concentrations could have been derived from anthropogenic sources such as potash feldspar in the agricultural lands. The strong correlations between K+ and EC (0.70), TDS (0.72), Ca (0.67) and Mg (0.72) suggested that potassium mostly originated from other sources asides k-feldspar or k-bearing minerals.
A weak relationship (0.29) between total cations (TZ+) and total anions (TZ−) (Fig. 3a) implied that contribution of some non-measured ions to charge balance was significant. The strong relationship between EC and TDS (0.987; Fig. 3b) suggests that the water-samples were less likely to contain substantial amounts of uncharged soluble compounds (e.g., SiO2, Mn, Al, Fe) which may contribute to its TDS concentration (Prasanna et al. 2011).
Due to intense anthropogenic activities (farming and septic tank placement) and poor well management (open wells, poorly completed wells) groundwater drawn from shallower wells and surface-water appeared to be more polluted compared to those from satchet water and boreholes. This is responsible for higher concentrations of ZT+ and ZT− in well samples compared to samples from satchet water and boreholes.
Ionic plots and saturation-index
Bivariate plots identified silicate weathering and carbonate dissolution as the principal hydro-geochemical processes controlling water chemistry in the study area. Few hand-dug wells and surface-water samples plot close to the silicate end member, while most well samples and boreholes plot towards the carbonate end member (Fig. 3c). The molar ratio of Mg2+/Ca2+was employed to further assess the degree of water–rock interaction of the samples. Most samples have Mg/Ca ratio less than 2 which indicate calcite dissolution (Tahoora et al. 2014).
The Ca2+/Mg2+ molar ratio can be used to show the dissolution of calcite and dolomite in water (Tahoora et al. 2014). 84% of the samples have Ca2+/Mg2+ ≥ 1 & ≤ 2 indicating calcite and dolomite dissolutions (Mayo and Loucks 1995) with both processes contributing equally, while 16% of the water samples have Ca/Mg > 2, indicating silicate minerals dissolution (Katz et al. 1997).
Carbonic acid and sulfide mineral oxidation weathering can be differentiated using the HCO3−/(HCO3− + SO42−) ratios (Pandey et al. 2001). HCO3−/(HCO3− + SO42−) = 1 indicates carbonic acid as the main proton source for chemical weathering, but a ratio of 0.5 suggests both carbonic acid and the proton from pyrite oxidation were responsible for the water ion acquisition.
77% of the samples have HCO3−/(HCO3− + SO42−) varying from 0.75 to 1.00, suggesting that carbonic acid weathering of carbonate, dolomite and gypsum controlled the abundance of Ca2+, Mg2+, HCO3− and SO42− in the water samples, while 23% were influenced by sulfide weathering. The linear plot (Fig. 4f) of Na+ + K+–Cl−Vs(Ca2+ + Mg2+)–(SO42− + HCO3−) revealed a weak relationship (0.28) with a slope of (0.33) which is a sharp deviation from Fisher and Mullican (1997) theoretical correlation of (0.90) and slope of − 1. It can then be assumed that chemical weathering is the most important hydrogeochemical process controlling the distribution of (Ca2+, Mg2+, SO42− and HCO3−) in the waters. These ions’ abundance in the water-samples is a function of carbonate mineral serving as the matrix in the host aquifer material. As a result, all the samples are saturated with carbonate minerals. Calcite saturation indices correlated with TDS and Ca2+ (Fig. 4c and d) suggesting that calcite continued to dissolve in the aquifer after its saturation (super-saturated) and possibly precipitated (Trunsdell and Jones 1974).
Moderate correlation exists between dolomite saturation indices and Ca2+ (0.59), and TDS (0.51). Therefore, these samples have the capacity to dissolve dolomite. Halite and Gypsum saturation indices correlate weakly with TDS (0.42 and 0.037; Fig. 4e and f). This indicates that the water has the minimal capacity to dissolve halite and gypsum.
Ion-exchange-process
Groundwater undergoes variety of changes in chemical composition as it travels in the subsurface through varying lithology. Schoeller (1977) suggests this possibility by the use of the Chloro-Alkaline Indices: CAI-1: Cl(Na + K))/Cl and CAI-2: Cl–(Na + K)/SO4 + HCO3 + CO3 + NO3 which can be used to indicate ion exchange between the groundwater and its host environment during residence or travel. The value of these indices can be positive or negative. A negative ratio indicates that Ca2+ and Mg2+ have been adsorbed onto the aquifer materials and Na + /& K+ are released in the groundwater (i.e., reverse ion exchange). However, positive indices reveal that alkaline earth ions (Ca2+ and Mg2+) have been released in the groundwater and alkalis retained by the aquifer materials (i.e., direct ion exchange; Schoeller 1967). CAI 1&2 values of samples collected were positive indicating direct ion exchange predominance (Fig. 3e). The samples have relatively high Cl concentration; but a weak correlation occurs between Na+ and Cl− (0.15) and halite is under-saturated in the samples. Therefore, halite dissolution is not the main source of Cl−in the groundwater. The ratio of Cl−/TZ− was, therefore, used to trace the source of Cl in the samples. A low ratio of (< 1) ratio indicated atmospheric origin of Cl−, e.g., dust, rainfall or sea-spray (Freeze and Cherry 1979).
An index of anthropogenic activities is the use of Na+ and NO3−correlation (David and Gentry 2000). The weak correlation (− 0.06) implies that untreated sewage effluent has less contribution to Na+ loading into the groundwater system. Patterson (1997) stated that laundry-detergent can contribute about 40% of Na+ in wastewater. Na concentrations in hand-dug wells samples were higher compared to boreholes tapping deeper aquifer due to in-appropriate covering/opening and poor well completion. This buttresses the point that anthropogenic activities contribute significantly to Na + loading.
Factor analysis
R-mode factor analysis, employing varimax rotation resulted in three factors (with eigenvalues > 1) which explains 76% of the total variance. Factor-1 has a total variance of 45.9% making it the most important factor influencing water quality. It has high loadings on Ca2+, Mg2+, HCO3−, pH, SO42−, K+, EC and TDS. Ca correlates strongly with Mg (0.88); therefore, it can be suggested that Factor 1 reflects water–rock interaction within the aquifer (Mayo and Loucks 1995).
While Factor 2 revealed a moderate-high loading on NO3−, Cl− and pH, Factor 3 showed a moderate loading on NO3−. Both indicated that anthropogenic activities influence on water qualitywas quite significant especially in shallow and uncovered wells and surface water but minimal in borehole and satchet water. Since R-mode factor analysis only explained 76% of the total variance, the unaccounted 24% probably indicated the presence of other untested parameters (such as dissolved oxygen and biochemical oxygen demand in surface water samples; heavy metals, phosphates, total suspended solids, turbidity, total coliform count in surface and groundwater samples) which could contribute to the quality of the water in the study area. Further studies are thus recommended.
According to Rail (2000) Potassium concentrations in groundwater ≤ 10 mg/L are linked to orthoclase weathering to clay; however, concentrations > 10 mg/L may indicate external sources of K+ abundance. A weak correlation between K+ and Cl− (0.17) and Na+ (0.33) implied that anthropogenic sources contributed more to K+ loading in the waters.
Hydro-chemical facies
The evolution of hydro-chemical parameters of groundwater can be understood by plotting the concentration of major cations and anions in the Piper (1944) diagram (Fig. 5a). It revealed the analogies, dissimilarities and different types of waters in the area. The plot showed that most of the samples plot as Ca–Cl type (non-carbonate hardness water with secondary salinity), while 5% plot as K–Cl water type (non-carbonate alkali water). In the cation triangular plot field water samples plot at the centre of the plot which suggested mixed cation (no dominant cation-type). The anion triangular field showed that all the samples were within chloride-axis. Schoeller’s diagram revealed that K+ and Cl− are the dominant ions in most parts of the study area (Fig. 5b, c).
Gibbs plots provide information on the mechanism controlling the chemistry of the study area. It showed rock–water interaction as the dominant process affecting water chemistry. Only 2% of the samples (a sachet-water) plot in the precipitation field (Fig. 5d). This observation suggested that dissolution of carbonate and silicate minerals largely control the water chemistry in the study area. The rock–water interaction process includes the chemical weathering of rocks, dissolution–precipitation of secondary carbonates, and ion exchange between water and clay minerals (Kumar et al. 2009).
Conclusion
A high coefficient of variation (CV > 50%) and spatial distribution indicated the heterogeneous levels of most physico-chemical parameters in hand-dug wells. However, most borehole, sachet and surface water samples had CV < 50% and indicated homogeneity. Classification based on hardness properties revealed that over 70% of the samples were soft water type which is an indication of a potential medium for mobility of toxic elements. Evidence of pollution was intense in well water samples than other sources. The water samples had relatively high Cl− concentration which could impact on taste and corrosion effect. Ca–Cl water type was the most dominant in the study area. Cl > NO3 > HCO3 and Ca > K > Na were the order of ionic dominance for anions and cations, respectively. The study further identified silicate weathering and carbonate dissolution as the principal hydro-geochemical processes controlling water chemistry in the study area. Water–rock interaction and anthropogenic activities were the major factors affecting water quality, with the later having more impact on surface water and shallow and uncovered wells. Generally, groundwater is fairly to well suitable for drinking purpose except the shallow and poorly constructed hand-dug wells bordering farm areas.
References
Adebola, A. A., Adedayo, O. B., & Abiola, A. (2013). Pollution studies on groundwater contamination: Water quality of Abeokuta, Ogun State, South West Nigeria. Journal of Environment and Earth Science., 3(5), 161–166.
Agarwal, V., Jagetai, M. (1997). Hydrochemical assessment of groundwater quality in Udaipur city, Rajasthan, India. In: Proceedings of national conference on dimensions of environmental stress in India. Department of geology, MS University, Baroda, India, pp. 151–154.
Akinse, A. G., & Gbadebo, A. M. (2016). Geologic Mapping of Abeokuta Metropolis, Southwestern Nigeria. International Journal of Scientific and Engineering Research., 7(8), 979–983.
Alloway, B.J., Ayres, D.C. (1997). Chemical principles of environmental pollution, In Nd Wastes and their disposal, (2 Ed), Blackie A-cad. London: Professional, pp. 353–357.
APHA. (2005). Standard methods for the examination of water and wastewater (21st ed.). American Water Works Association, Water Environment Federation, Washington: American Public Health Association.
Akanni, C. O. (1992). Relief, drainage, soil and climate of Ogun state in maps. In S. O. Onakomaiya, O. O. Oyesiku, & F. J. Jegede (Eds.) Rex Charles Publication, Ibadan, p. 207.
Awomeso, J. A., Ogundairo, E. S., Taiwo, A. M., Idowu, O. A., Gbadebo, A. M., & Oyetunde, O. A. (2019). Assessment of water quality of Ogun River in Southwestern Nigeria. Ife Journal of Science., 21(2), 375–388.
Bello, N. J., Erudola, A., Gideon, U., & Awomeso, J. (2009). An assessment of water supply potential for crop production: Comparative study of River basins under the jurisdiction of two River Basin Development Authority Areas located in Different Ecological Zones of Nigeria. Journal of Meteorology and Climate Sciences., 7, 49–57.
Butow, E., Holzbecher, E., Kob, E. (1989). Approach to model the transport of leachates from a landfill site including geochemical processes, contaminant transport in groundwater. In Kobus and Kinzelbach, Balkema, Rotterdam, pp. 183–190.
Cravotta, C.A., Kirby, C.S. (2004). Effects of abandoned coal-mine drainage on streamflow and water quality in the Shamokin Creek Basin, Northumberland and Columbia Counties, Pennsylvania, 1999–2001. In US Geol. Survey Water Resources Inv. Rep. WRIR 03-4311, p 53.
Clark, J. (2006). Rivers and their catchments: impact of landfill on water quality, Earth Sciences (p. 2). Scottish Natural Heritage.
David, M. D., & Gentry, L. E. (2000). Anthropogenic inputs of nitrogen andphosphorus and riverine export for Illinois, USA. Journal of Environmental Quality, 29, 494–508.
Ehinola, O. A. (2002). Hydrochemical characteristics of groundwater in parts of the basement complex of southwestern Nigeria. Journal of Mining and Geology, 38(2), 125–133.
Farina, M., Avila, D. S., Da Rocha, J. B. T., & Aschner, M. (2013). Metals, oxidative stress and neurodegeneration: a focus on iron, manganese and mercury. Neurochemistry International, 62, 575–594.
Fekkoul, A., Zarhloule, Y., Boughriba, M., Barkaoui, A., Jilali, A., & Salem, B. (2012). Impact of anthropogenic activities on the groundwater resources of the unconfined aquifer of Triffa plain (Eastern Morocco). Arabian Journal of Geosciences. https://doi.org/10.1007/s12517-012-0740-1.
Fisher, S. R., & Mullican, W. F. (1997). Hydrogeochemical evaluation of sodium-sulphate and sodium-chloride groundwater beneath the northern Chihuahua desert, Trans-Pecos, Texas, USA. Hydrogeology Journal., 5, 4–16. https://doi.org/10.1007/s100400050102.
Freeze, R. A., & Cherry, J. A. (1979). Groundwater prentice. Englewood Cliffs: Engle wood Cliffs.
Hernandez-Garcia, M. E., & Custodio, E. (2004). Natural baseline quality of madrid tertiary detrital aquifer groundwater (Spain): A basis for aquifer management. Environmental Geology., 46, 173–188.
Ige, O.O., Okunola, N., Obasaju, D.O., & Omorinoye, O.A. (2017). Hydrochemical evaluation of water sources in Iwo southwestern Nigeria. Ethiopian Journal of Environmental Studies and Management 10, 958–967
Katz, B. G., Coplen, T. B., Bullen, T. D., & Hal Davis, J. (1997). Use of chemical and isotopic tracers to characterize the interactions between ground water and surface water in mantled karst. Ground Water, 35(6), 1014–1028.
Kayode, A.A. (1976). The genesis of the small and large-feldspar porphyritic Older Granites in the Igbo Ora Complex, southwestern Nigeria. In C. A. Kogbe (ed) Geology of Nigeria, p.75–84.
Kumar, K. S., Ram, M. V., Dajkumar, S. J., & Jeevanandam, M. (2009). Assessment of groundwater quality and hydrogeochemistry of Manimuktha River basin, Tamil Nadu, India. Environmental Monitoring and Assessment, 159(1–4), 341–351.
Laniyan, T. A., Ajibade, O. M., Bayewu, O. O., & Oshomoji, A. O. (2015). Impact of urban activities on groundwater qualities in Abeokuta Southwestern, Nigeria. Ife Journal of Science, 17(2), 239–246.
Malomo, S., Okufarasin, Y. A., Olorunnio, M. A., & Omode, A. (1990). Groundwater chemistry of weathered zone aquifers of an area underlain by basement complex rocks. Journal of African Earth Science., 11, 357–371.
Mandour, R. A. (2012). Human health impacts of drinking water (surface and ground) pollution Dakahyia Governorate, Egypt. Applied Water Science, 2, 157–163.
Manjusree, T., Sabu, J., Marco, P., & Jobin, T. (2017). Integrated approach for identifying the factors controlling groundwater quality of a tropical coastal zone in Kerala, India. Journal of Environment and Earth Science, 76(486), 1–18.
Mayo, A. L., & Loucks, M. D. (1995). Solute and isotopic geochemistry and ground water flow in the central Wasatch Range, Utah. Journal of Hydrology, 172(1–4), 31–59.
Naidu, R., Datta, B., & Chadalavada, S. (2011). Optimization approach for pollution sources identification approach for pollution source identification ingroundwater an overview. International Journal of Environment and Waste Management, 8, 40–61.
Oloruntola, M. O., & Adeyemi, G. O. (2014). Geophysical and hydrochemical evaluation of groundwater potential and character of Abeokuta Area, Southwestern Nigeria. Journal of Geography and Geology, 6(3), 162–177.
Opafola, O. T., Oladepo, K. T., Ajibade, F. O., & David, A. O. (2020). Potability assessment of packaged sachet water sold within a tertiary institution in southwestern Nigeria. Journal of King Saud University—Science., 32, 1999–2004.
Oyawoye, M. N. (1972). The basement complex of Nigeria. In African Geology. In edited by T.F.J Dessauvage and A. J Whiteman, pp. 60—102, University of Ibadan.
Pandey, S. K., Singh, A. K., & Hasnain, S. I. (2001). Hydrochemical characteristics of meltwater draining from Pindari glacier, Kumon Himalaya. Journal of the Geological Society of India, 57, 519–527.
Patterson, R.A. (1997). Domestic Wastewater and the Sodium Factor, site characterization and design of on-site septic systems. In ASTM STP 1324, Bedinger MS, Johnson AI, Fleming JS (eds). American Society for Testing and Materials, 1997, pp 23–35
Piper, A. M. (1944). A graphical procedure in the chemical interpretation of groundwater analysis. Transactions American Geophysical Union, 25, 914–928.
Prasanna, M. V., Chidambaram, S., Shahul, H. A., & Srinivasamoorthy, K. (2011). Hydrogeochemical analysis and evaluation of groundwater quality in the Gadilam river basin, Tamil Nadu, India. Journal of Earth System Science, 120, 85–98. https://doi.org/10.1007/s12040-011-0004-6.
Rai, L. C. D. (2000). Groundwater contamination: sources and hydrology. Boca Raton: CRC Press.
Sawyer, C. N., & McCarty, P. I. (1967). Chemistry of Sanitary Engineers, 2nd edn. New York: McGraw Hill.
Schoeller, H. (1967). Qualitative Evaluation of Groundwater Resources In Methods and Techniques of Groundwater Investigation and Development. Water Resource Series, 33, 44–52.
Schoeller H (1977). Geochemistry of groundwater. In Brown RH, Konoplyantsev AA, Ineson J, Kovalevsky VS (eds) Groundwater Studies—An International Guide for Research and Practice. UNESCO, Paris, vol 15, pp. 1–18.
Singh, H., Raju, N. J., Gossel, W., & Wycisk, P. (2016). Assessment of pollution potential of leachate from the municipal solid waste disposal site and its impact on groundwater quality, Varanasi environs, India. Arabian Journal of Geosciences, 9(2), 131.
SON. (2007). Nigerian standard for drinking water quality. Nigerian Industrial Standard (NIS 554–2007). Standards Organisation of Nigeria (SON), Abuja, Nigeria.
Tahoora, S. N., Mohammad, F. R., Ahmad, Z. A., Wan Nor, A. S., Hafizan, J., & Kazem, F. (2014). Identification of the Hydrogeochemical Processes in Groundwater Using Classic Integrated Geochemical Methods and Geostatistical Techniques, in Amol-Babol Plain, Iran. The Scientific World Journal, 2014, 15. https://doi.org/10.1155/2014/419058.
World Health Organization. (2011). Guidelines for drinking water quality. 4th. Ed. Vol. 1, Geneva, Switzerland pp. 117–153, 404–433
Zaidi, F. K., Mogren, S., Mukhopadhyay, M., & Ibrahim, E. (2017). Factors influencing the major ion chemistry in the Tihama coastal plain of southern Saudi Arabia: Evidences fromhydrochemicalfacies analyses and ionic relationships. Journal of Environmental & Earth Sciences, 76, 472.
Acknowledgements
The Authors wish to express their profound gratitude to FATLAB company, Ibadan, Nigeria for conducting quality and reliable laboratory analyses as well as the contributions of the editor and anonymous reviewers that have significantly improved the quality of this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interests to declare that are relevant to the content of this article.
Rights and permissions
About this article
Cite this article
Ige, O.O., Owolabi, T.A., Fatoyinbo, I.O. et al. Characterization of factors influencing water quality in Federal University of Agriculture Abeokuta and its Environ, Southwestern Nigeria. Int J Energ Water Res 5, 205–218 (2021). https://doi.org/10.1007/s42108-020-00093-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42108-020-00093-2