Abstract
This study focuses specifically on the β-C2S polymorph hydrated in deionized water and with the presence of Na2CO3 (2 M) and of water glass. Dicalcium silicate was prepared from calcium carbonate and gel silica. The salts’ activators were dissolved in water and then added to synthesized dicalcium silicate. Hydrated samples after different curing time (from 2 to 90 days) were characterized by X-ray diffraction, infrared spectroscopy, isothermal conduction calorimetry, and scanning electron microscopy, whereas the compressive strength was operated after 28 and 90 days. The results showed that the contact of belite with liquid phase during hydration is strongly influenced by the existence of alkalis. The early hydration was accelerated and this can be clearly viewed during the dissolution of C2S which becomes more fast with a decrease of C2S peaks and the increase of calcium silicate hydrates C–S–H product as well as calcium silicate hydrates containing sodium C–(N)–S–H gel-like. This influence is mainly manifested on the mechanical properties of samples by enhancement in early compressive strength.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Portland cement (PC) is considered as the excellent building material. This is due to its high performance, its good mechanical proprieties and also to good quality/price ratio, because it is possible to find the raw materials almost anywhere around the world (Sánchez-Herrero et al. 2017). The PC consisting mainly of clinker minerals: C3S, C2S, C3A, and C4AF and a little portion of gypsum (Intararit Nilobon et al. 2017). The formation of alite (C3S) phase needs high energy to be burned which is the reason why cement factories are considered one of the industries that release larger amounts of carbon dioxide into the environment overall; the manufacturing process release about 900 kg of CO2 for every tonne of cement produced (Sánchez-Herrero et al. 2017; Intararit Nilobon et al. 2017; Aleksandar Nikolov and Nugteren 2017; Martuscelli et al. 2018; Okoye 2017; Paiste Päärn et al. 2016, RAABSe 2017). The increase of energy cost and necessity of raw materials reinforce the requirement for the development of alternative types of eco-friendly cement. Recently, the elaboration of dicalcium silicate cement has gotten much consideration because of lower carbonate content and consuming energy due to the low burning temperature bringing a decrease of CO2 outflow (Intararit Nilobon et al. 2017).
Dicalcium silicate is one of the major phases which accounts for about 15–30% by weight of PC. This phase can be elaborated by heating the raw materials at 800–1100 °C which needs less power than alite (Intararit Nilobon et al. 2017; Aleksandar Nikolov and Nugteren 2017). Actually, the preparation of dicalcium silicate at low temperature utilizing more friendly raw materials has taken high interest of many kinds of research such as rice husk ash, lignite fly ash, and silica fume (Intararit Nilobon et al. 2017) and also recycled rubber particles (Martuscelli et al. 2018). The main goal of the utilization of these alternative materials is a waste diminution which decreases its environmental impact, on the other hand, a valorization of these raw materials in the elaboration of dicalcium silicate (Intararit Nilobon et al. 2017).
Among the improved research in the alkali activation field, the development of hydration kinetics of belite cement becomes possible with the utilization of activators and also in some new kind of cement according to many researches (Aleksandar Nikolov and Nugteren 2017; Martuscelli et al. 2018; Okoye 2017; Paiste Päärn et al. 2016). Certain of these cement alternatives are those subsequent from the chemical interaction between high alkaline solutions or amorphous aluminosilicates, which can be an industrial by-product or natural waste such as fly ash or blast furnace slag (Okoye 2017; RAABSe 2017; Rie 2016; RMKe 2016). The alkaline solutions used in this process are usually alkaline metal or alkaline-earth hydroxides (ROH, R(OH)2), weak acid salt’s (R2CO3, R2S, RF), strong acid salt’s (Na2SO4, CaSO4·2H2O), and R2O(n)SiO2-type siliceous salts, where R is an alkaline ion such as Na, K, or Li (Paiste Päärn et al. 2016). From the viewpoint of mechanical proprieties of the final elaborated products, the best effective solutions are NaOH, Na2CO3, and hydrated sodium silicates or water glass solutions (Torres-Carrasco et al. 2015).
The hydration behavior of the cement-based material is one of the most essential properties for a useful utilization (Okoye 2017). Several studies investigate the hydration product such as calcium silicate hydrates C–S–H gel in which it interacts with the alkaline oxides present in cement. These researches carried out also the effect of alkalis on cement and observed that the presence of ‘soluble’ alkalis hastened the initial C2S hydration reactions, promoting the formation of portlandite and C–S–H gel (Sánchez-Herrero et al. 2017; ÁgdlT et al. 2009; Yanagisawa Kazumichi et al. 2006; Wang et al. 2018). The findings revealed that the presence of alkalis does indeed expedite C2S and C3S hydration kinetics. An analysis of the precipitating gel in such conditions revealed the existence of calcium silicate hydrates containing sodium C–(N)–S–H-like gels in which Na+ replaces the calcium in the structure, compensating the charge on the OH− groups (Sánchez-Herrero et al. 2017; Intararit Nilobon et al. 2017).
In this work, the hydration behavior of synthesized C2S in the presence of the two activators Na2CO3 and water glass is carried out as well as the most significant microstructural changes provoked in the formed products. C2S was prepared using calcium carbonate and gel silica as raw material. The changes in C–S–H gel due to the presence of CaCO32− anions and its possible interaction with the Na+ present in the medium are followed by DRX and SEM/EDS.
Materials and methods
Dicalcium silicate (C2S) was synthesized by solid-state reaction using calcium carbonate and silica gel as starting materials (Bouregba and Diouri 2016; Bouregba et al. 2018). The raw materials are mixed with specific proportions (77% and 23%, respectively) then treated slowly at different temperatures from 500 to 1050 °C.
The hydration reaction was performed on belite cement pastes. Dicalcium silicate samples were mixed with Na2CO3 or water glass as activators. Water/Solid ratio of 0.5 was used for all mixtures. The Na2CO3 activator was prepared by dissolving 20 g of Na2CO3 in 100 ml of distilled water. The water glass is obtained by adding 100 ml distilled water on 100 g of glass powder; after mixing, the mixture is followed by filtration of the obtained solution which can be used as activator. The chemical composition of glass powder before and after water drainage obtained by the Fluorescent X-ray analysis is shown in Table 1.
The prepared activators’ solution was added into the ground sample. The hydrated samples were cured at 2, 7, 28, and 90 days. The changes in the properties of the hydrated samples were monitored by several techniques such as X-ray diffraction (XRD) using a Siemens D5000 diffractometer operating with 40 kV and 20 mA, equipped with a copper anticathode and a secondary monochromator (λ = 1.5406 Å), Fourier Transform Infrared (FT-IR) spectroscopy using a JASCO FT-IR-4600 in the region 400-4000 cm−1, and Scanning Electron Microscopy/energy-dispersive X-ray spectroscopy (SEM/EDS) using JEOL JSM-IT100. The development of compressive strength is evaluated on cylindrical specimens (1.50 × 0.75 cm) at 28 and 90 days. Three values of compressive strength are obtained for each sample and the averages are listed.
Results and discussion
Characterization of synthesized dicalcium silicate
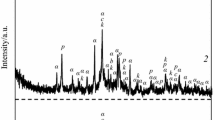
Synthesized dicalcium silicate was characterized by SEM, XRD, and FT-IR, as shown in Fig. 1. The SEM image shows that the grain has a form of pellet with the occupation of crystallized particles on the surface in which corresponds to the crystalline belite. The ß form of belite was present with the highest percentage of larnite Ca2SiO4 according to the ICDD-Card No. 01-070-0388 (JCPDS) with short peaks of wollastonite CaSiO3 (JCPDS card No. 00-027-0088).
The FT-IR spectra of the powder synthesized at 1050 °C introduces the main bands corresponding to the silicate groups which are attributed to the SiO44− tetrahedral. According to Puertas and al. (Puertas and Trivino 1985), the absorption bands of different C2S polymorphs corresponding to (υ1) (symmetric stretching) located around 800–900 cm−1 and (υ3) (antisymmetric stretching) are typically located around 800–1000 cm−1 and those of (υ4) (the triply degenerated out of plane bending) located around 400–500 cm−1 (Puertas and Trivino 1985).
Effect of Na2CO3 and water glass
After synthesizing C2S, we have processed the hydration of samples with Na2CO3 or water glass as activators. The hydration with distilled water was used as reference test.
Isothermal calorimetry
Figure 2 shows the calorimetric curves for C2S pastes with and without alkalis. The curves show a strong similarity mainly between the water-hydrated paste and those hydrated with water glass (WG).
We note a little difference when the Na2CO3 activator was used. We note the presence of a single peak at the beginning of the measurement, whereas the amount of heat released equal to 6.4 mW/g for the C2S with Na2CO3 and 1.2 and 1.0 mW/g for C2S with water and water glass, respectively. Moreover, C2S is known to react very slowly and we use a range of periods less than 70 h, so it is so hard to see the influence of alkalis during the early age. According to Maria José and al. (Sánchez-Herrero et al. 2017), in the water-hydrated C2S pastes, a calorimetric peak was detected 30 days after the start time; however, in this work, the dicalcium silicate hydration was facilitated and accelerated with the presence of Na2CO3.
Hydration properties
Figures 3, 4, and 5 show the XRD patterns of hydrated products obtained at the room temperature by mixing C2S powder with different liquid mediums (w/b = 0.5).
In all samples, it is noted that the solubility of C2S is more intense when the activator are used. Slow hydration of C2S without activator (Fig. 3) can be seen by the slow decrease of C2S peaks which correspond to the late dissolution. This dissolution induces the formation of small amount of C–S–H phases which may be considered to be gel-like, but it is not necessary amorphous and it can be defined in diffraction pattern (Nonat 2004). We note also the presence of portlandite (Fig. 3) which becomes more intense with the increasing hydration time.
When the hydration was occurred with a solution of Na2CO3 or water glass (Figs. 4 and 5), a fast dissolution of C2S with precipitation of large amounts of C–S–H phases and portlandite was observed. The present phases occurred during the hydration are not identical. For the hydrated samples with water glass, we note the presence of portlandite phase after 2 days of hydration with small amount of rosenhanite [Ca3Si3O8(OH)2]. On the other side, the hydrated sample with Na2CO3 presents large peaks of rosenhanite after 2 days of hydration. For both hydrated samples, we note the presence of tobermorite phase [Ca5(Si6O16)(OH)2] which become more intense with the increasing hydration time.
This difference can be clearly viewed in the SEM micrographs of the hydrated dicalcium silicate using various solutions (deionized water, Na2CO3, and water glass) at the age of 2, 7, 28, and 90 days, as shown in Figs. 6, 7, 8, and 9. The control samples without activators at the age of 2 and 7 days (Fig. 6) revealed spherical and irregular shaped particles; furthermore, we note also the presence of some thin layer at the age of 28 days.
For the hydrated sample with Na2CO3, we note the presence of the spherically shaped particles and some fine hexagonal like crystals of Ca(OH)2 appear at the age of 7 days (Fig. 6), and subsequently some tiny fibrous crystals were precipitated on the surface of the 28-day sample. This clearly enhanced from the age of 28–90 days. We observed also the presence of a small thin gel-like in the surface of the hydrated sample which grows up and becomes more scattered and covers the entire surface (Wang et al. 2018; Janotka 2001). This gel-like is assigned to the C–N–S–H gel-like such as pectolite NaCa2Si3O8(OH). For the hydrated sample with water glass, spherical and irregular shape particles with a gel-like were observed at the age of 28 days which follow the polymerization in the surface at the age of 90 days.
These observations were confirmed by EDS analyses on the 90-day samples. The percentages of the following elements: Ca, Si, O, C, and Na in the sample with deionized water are strongly similar in all zones for every type of activators (Figs. 8 and 9). This finding proves the above results obtained by XRD patterns of hydrated paste with and without activators after 2, 7, 28, and 90 days of hydration. The sample without activator presents portlandite, larnite, and C–S–H phases such as rosenhahnite and reinhardbrannsite. The occurrence of peaks from the samples with activators is similar to that of the control sample. However, the intensity of portlandite peak decreased with the addition of water glass and Na2CO3. The relative intensity of the portlandite peaks is lower in the mixtures with Na2CO3, but it is present in all mixtures. This demonstrates that the development of amorphous C–S–H phase was limited by the presence amount of soluble silicate in the mixture and the excess calcium was precipitated as portlandite phase in the sample hydrated with water glass (Fig. 5) (Paiste Päärn et al. 2016).
Compressive strength
Figure 10 illustrates the compressive strength values for C2S pastes hydrated with deionized water, Na2CO3, and water glass at 28 and 90 days. The obtained results after 28 days show that the mechanical strength reached low values for hydrated sample with water. However, these values increase for the activated samples from 28 to 90 days, but both samples present a poor mechanical strength compared to control sample at that age.
Discussion
The findings described above show that the two alkaline activators used in this case (Na2CO3 and water glass) do not affect dicalcium silicate (C2S) hydration in the same way. While Na2CO3 expedited hydration, the calorimetric behavior of the C2S pastes hydrated in the presence of water glass was similar to the performance of water-hydrated pastes. Maria José and al., (Sánchez-Herrero et al. 2017) attributed these dissimilar results to the difference of the initial pH values (in our case: 9.25 and 11.50 for water glass and Na2CO3, respectively) and more specifically to the nearly neutral pH of the sodium sulfate medium. Nevertheless, the findings suggest that they may be due to the synergies generated by the reactions during the hydration of calcium silicate in the presence of alkaline salts. More explanation was given by Janotka (2001). These differences might be caused by the precipitation of CaCO3 furthered by the presence of sodium carbonate in the first 24 h of hydration which is not our case according to XRD patterns. This compound significantly modifies the microstructure and porosity of the material, thereby affecting its mechanical strength. The same author observed also that mechanical strength decrease progressively over time due to subsequent carbonation.
From the 28-day materials, the highest strength values for the analyzed calcium silicates were observed for pastes with Na2CO3. The justification of this discovering lies essentially with C–S–H gel characteristics. According to many works, cementitious properties are closely associated with the forces on the C–S–H gel surface. Cement particles bond together due to the formation of a lattice C–S–H gel nanoparticles. During hydration, the C–S–H gel precipitation sets off at the same time with portlandite formation. The latter enhanced the pH in the medium, provoking the ionization of the silanol groups in the gel. The C–S–H particles carry negative charges, compensated by attaching to calcium ions. This increase of the charge density at the surface induces a strong attraction among the C–S–H nanoparticles, thus increasing cement paste cohesion. The presence of ions in the medium may alter C–S–H gel characteristics. Many studies have addressed the effect of the CO32− anion on C–S–H gel. Gel stability is modified by the presence of carbonates requiring Ca2+ to balance the negative charge. Therefore, the interaction of the CO32− anion with the surface of the C–S–H gel induces the formation of more polymerized (tobermorite-like) C–S–H gels and hence better mechanical strength. An analysis of gel precipitating in such conditions revealed the existence of C–(N)–S–H-like gels in which Na+ replaces the calcium in the structure, offset the charge on the OH− groups (Sánchez-Herrero et al. 2017; Intararit Nilobon et al. 2017).
Those results infer that C2S behavior in the presence of Na2CO3/water glass was considerably modified. The contact of the belite cement with liquid medium during the hydration is strongly influenced by the existence of alkalis. This influence is mainly manifested in the early hydration that becomes more quickened which can be clearly viewed in the mechanical properties of cement pastes. In general, an enhancement in early strength and a decrease in final strength are viewed (Torres-Carrasco et al. 2015; Skalny 1978; Venkateswara et al. 2006).
Conclusions
This study focuses specifically on the synthesized β-C2S hydrated in deionized water and with the presence of Na2CO3 (2 M) and water glass. Dicalcium silicate was prepared from commercial calcium carbonate and gel silica. The salts activators were dissolved in water and added to grained cement, then hydrated at different periods from 2 to 90 days. The hydrated sample was characterized by X-ray diffraction (XRD), infrared spectroscopy (FT-IR), isothermal calorimetry and scanning electron microscopy (SEM), whereas the mechanical strength of some samples was operated at 28 and 90 days.
The results show that the contact of belite cement with liquid medium during hydration was strongly influenced by the existence of alkalis. Mostly, early hydration is quickened when the activator is added. This influence is mainly manifested in the mechanical properties of cement pastes by showing. In general, an increase in early strength and a decrease in final strength are viewed.
References
Ágdlt, K. M., Cuberos, A. J. M., Zahir, M., & Aranda, M. A. G. (2009). Preparation and characterization of alkali-activated white belite cements. Materiales de Construcción, 59, 19–29.
Aleksandar Nikolov, I. R., & Nugteren, H. (2017). Geopolymer materials based on natural zeolite. Case Studies in Construction Materials, 6, 198–205.
Bouregba, A., & Diouri, A. (2016). Potential formation of hydroxyapatite in total blood and dicalcium silicate elaborated from shell and glass powders. Materials Letters, 183, 405–407.
Bouregba, A., Hassan, E. Z., Abdeljebbar, D., & Omar, S. (2018). β-Dicalcium Silicate Cement Modified with β-Tricalcium Phosphate. In Vitro Bioactivity and Mechanical Strength. Journal of Biomimetics, Biomaterials and Biomedical Engineering, 35, 9–19.
Intararit Nilobon, S. A., Sinyoung, S., & Kunchariyakun, K. (2017). Effect of Na2SiO3 and Na2CO3 on hydration properties of dicalcium silicate prepared from black rice husk ash. The Journal of Applied Science, 16, 68–74.
Janotka, I. (2001). Hydration of the cement paste with Na2CO3 addition. Ceramics-Silikaty, 45, 16–23.
Martuscelli, C. C., dos Santos, J. C., Oliveira, P. R., Panzera, T. H., Aguilar, M. T. P., & Garcia, C. T. (2018). Polymer-cementitious composites containing recycled rubber particles. Construction and Building Materials, 170, 446–454.
Nonat, A. (2004). The structure and stoichiometry of C–S–H. Cement and Concrete Research, 34(34), 1521–1528.
Okoye, F. N. (2017). Geopolymer binder: A veritable alternative to Portland cement. Materials Today: Proceedings, 4, 5599–5604.
Paiste Päärn, M. L., Heinmaa, Ivo, Vahur, Signe, & Kirsimäe, Kalle. (2016). Alkali activated construction materials: Assessing the alternative use for oil shale processing solid wastes. Construction and Building Materials, 122, 458–464.
Puertas, F., & Triviňo, F. (1985). Examinations by Infra-Red spectroscopy for the polymorphs of dicalcium silicate. Cement and Concrete Reserch, 15, 127–133.
RAABSe, A. L. (2017). Geopolymeric cements obtained by alkaline activation of aluminosilicates from industrial waste. Materials Science Forum, 899, 431–435.
Rie, A. L. (2016). The use of fermipan in the production of lightweight geopolymer as an environmentally friendly and fire-resistant concrete. Materials Science Forum, 841, 72–78.
RMKe, A. L. (2016). Synthesis of geopolymer paste as coating material based on kaolinite and rice husk ash. Materials Science Forum, 841, 79–82.
Sánchez-Herrero, M. J., Fernández-Jiménez, A., & Palomon, A. (2017). C3S and C2S hydration in the presence of Na2CO3 and Na2SO4. Journal of the American Ceramic Society, 100, 1–11.
Skalny, I. A. J. (1978). Alkalies in cement: A review. Cement and Concrete Research, 8, 37–52.
Torres-Carrasco, M., Rodríguez-Puertas, C., del Mar Alonso, M., & Puertas, F. (2015). Alkali activated slag cements using waste glass as alternative activators. Rheological behaviour. BOLETÍN DE LA SOCIEDAD ESPAÑOLA DE CERÁMICA Y VIDRIO, 54, 45–57.
Venkateswara, V., Reddy, H. S. R., & Jayaveera, K. N. (2006). Influence of strong alkaline substances (sodium carbonate and sodium bicarbonate) in mixing water on strength and setting properties of concrete. Indian Journal of Engineering & Materials Sciences, 13, 123–128.
Wang, L. H. Q. Y., Zhou, S. H., Chen, E., & Tang, S. W. (2018). Hydration, mechanical property and C–S–H structure of early-strength low-heat cement-based materials. Materials Letters, 217, 151–154.
Yanagisawa Kazumichi, X. H., Onda, Ayumu, & Kajiyoshi, Koji. (2006). Hydration of β-dicalcium silicate at high temperatures under hydrothermal conditions. Cement and Concrete Research, 36, 810–816.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bouregba, A., Ez-zaki, H., Diouri, A. et al. Dicalcium silicate hydration behavior in the presence of Na2CO3 and water glass. Asian J Civ Eng 20, 857–867 (2019). https://doi.org/10.1007/s42107-019-00150-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42107-019-00150-0