Abstract
Purpose
Aromatase plays an important role in ovarian development, the normal progress of the menstrual cycle, and fertility status. Elevated aromatase activity is linked to obesity. There is a bidirectional relationship between obesity and thyroid function. Few studies have investigated the relationship between TSH and ovarian aromatase in obesity. Our aim was to investigate the effect of TSH on aromatase expression of ovarian granulosa cells in obese mice.
Methods
Female mice pups were divided into an obesity group and a control group. Obese parameters and the time of pubertal onset were recorded. At the age of 5 weeks, blood and tissues were obtained. Serum aromatase and hormone concentrations were measured using ELISA. The granulosa cells were isolated and exposed to variable concentrations (0 μM, 1 μM, 10 μM, 100 μM) of TSH. The expression of CYP19A1 mRNA and protein were assessed via RT-qPCR and western blot.
Results
In female mice, body weight, Lee’s obesity index, and serum levels of E2, aromatase, and TSH were significantly higher in the obesity group compared to the control group, whereas the time of pubertal onset and serum T3 and T4 concentrations were significantly lower (all P < 0.001). In granulosa cells, the expression of CYP19A1 mRNA in the obesity group was lower than that in the control group at 1 μM and 100 μM concentrations of TSH (both P < 0.001). The expression of CYP19A1 protein in the obesity group was higher than that in the control group after TSH stimulation (P = 0.014, P < 0.001, and P = 0.004, respectively). With the increase of TSH concentrations, the expression of CYP19A1 mRNA and protein in the two groups significantly increased (all P < 0.001).
Conclusion
Early puberty and elevated serum aromatase and TSH levels were found in obese female mice. In the granulosa cells of obese mice, TSH directly regulates aromatase expression in a dose-dependent manner.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past 50 years, the incidence of childhood obesity has increased in almost all countries around the world [1]. Obesity, which has several adverse direct and indirect effects on the function of multiple tissues and organs, has become a growing global public health problem. Obesity impacts the endocrine system. Multiple studies have demonstrated that girls with obesity have an increased risk of precocious puberty [2]. The relationship between obesity and pubertal onset in boys is an ongoing controversial issue, with some studies showing that obese boys presented with early puberty [3], while other studies found that obese boys experienced delayed puberty [4]. Although there is undoubtedly a link between pediatric obesity and pubertal onset, the pathogenesis behind this is not as yet fully understood. Regarding this issue, up until now, most studies have focused on leptin, a hormone secreted by adipose tissue which can activate the hypothalamic-pituitary–gonadal axis, triggering pubertal onset [5]. However, the leptin theory concerning this link cannot fully explain the gender differences in pubertal onset in obese children. It has been suggested that obesity may affect pubertal onset by regulating sex hormones through peripheral effects.
Within the peripheral mechanism, aromatase is a rate-limiting enzyme, a product of the CYP19A1 gene, that catalyzes the conversion of androgens to estrogens. Aromatase is primarily expressed in gonadal tissue, the latter being the main site for the production of sex hormones, although adipose tissue is considered to be the main extra-gonadal site for production of aromatase in obesity [6]. Aromatase synthesizes estrogens through the combined action of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). Androgens are synthesized by theca cells and transferred to ovarian granulosa cells through the basement membrane. In ovarian granulosa cells, FSH regulates the CYP19A1 gene promoter through the cAMP/PKA/CREB signaling pathway, stimulating aromatase transcription [7]. Aromatase plays an important role in ovarian development, the normal progress of menstrual cycle, and fertility status [8]. In women, it is possible that abnormal estrogen secretion may result in the development of estrogen-dependent disorders such as ovarian, endometrial, or breast cancer, an elevated incidence of PCOS, and infertility problems [7]. Clinical studies have indicated that estrogen levels are higher in obese children than in normal weight children [9]. The high levels of estrogen may result in early puberty in girls and delayed puberty in boys. Further studies are therefore needed to investigate the expression of aromatase in gonads in obesity.
The existence of a complex bidirectional relationship between obesity and thyroid function has been described [10], the latter being more prevalent in the obese population. Serum thyroid-stimulating hormone (TSH) levels tend to be higher and free thyroxine (FT4) levels tend to be lower in obese children compared with normal weight children [11]. Elevated TSH levels are associated with a large number of diseases. TSH can affect the metabolism in obese children regardless of the severity of obesity [12]. Clinical studies have shown that elevated TSH levels may be related to pubertal onset [13]. Chronic and mild stimulation of elevated TSH levels may lead to intermittent estrogen production [14]. In our previous work, we also discovered that girls with primary hypothyroidism showed precocious puberty and elevated levels of TSH and estradiol (E2). Both TSH and E2 levels returned to normal after thyroxine replacement therapy [15, 16]. Boys with primary hypothyroidism showed testicular enlargement and normal levels of serum testosterone (T) [17]. It is therefore reasonable to consider the potential relationship between TSH and aromatase.
In vitro, TSH directly inhibits testosterone secretion in Leydig cells by binding to the TSH receptor (TSHR) [18]. In Sertoli cells of male rats, aromatase activity and estrogen secretion increased due to hypothyroidism and decreased after thyroid hormone replacement treatment [19]. These results point to the close relationship between TSH and gonadal aromatase. However, few studies have investigated the relationship between TSH and ovarian aromatase in obesity. Therefore, the aim of the present study was to investigate the changes in aromatase and thyroid function as well as the effect of TSH on aromatase expression of ovarian granulose cells in obese mice.
Materials and methods
Experimental animals
The pregnant C57BL/6 J mice used in this study were purchased from Henan SCBS Biotechnology Co., Ltd. They were allowed free access to water and food. Their environment was controlled in terms of light, temperature, and humidity (12-12 h light–dark cycle; temperature 23 ± 2℃; 55 ± 15% relative humidity). The experimental protocol was approved by the Ethics Committee of Xuzhou Medical University. Animal maintenance and research were conducted in accordance with the Guide for the Care and Use of Laboratory Animals.
On the day of delivery, maternal mice were randomly divided into two groups, the obesity group and the control group. The control group received regular chow (300 kcal/100 g, 17% fat, 29% protein, 54% carbohydrate); the obesity group received high fat chow (460 kcal/100 g, 60%fat, 20% protein, 20% carbohydrate). The pups were weaned in the 3rd postnatal week. The female offspring (n = 8 per group) were selected to be fed the same chow for 2 weeks. The obesity parameters included body weight and body length. Lee’s obesity index was calculated as follows: [body weight (g) × 1000/body length (cm)] 1/3. The vaginal opening is an external signal of pubertal onset. The time of pubertal onset was recorded. In the 5rd postnatal week, the female mice were anesthetized with chloral hydrate. Terminal blood was extracted via heart puncture. The mice were then euthanized by decapitation to obtain tissue samples.
Measurement of aromatase and hormone levels
Serum aromatase, estradiol (E2), testosterone (T), triiodothyronine (T3), thyroxine (T4), and TSH concentrations were measured using enzyme-linked immunosorbent assay (ELISA) quantification kits (Shanghai Fanke Biotechnology Co., Ltd.). Serum samples were diluted and analyzed according to the manufacturer’s instructions. The ELISA assays were run in duplicates for each sample, which were averaged for comparison. The intra-assay and inter-assay coefficients of variation were less than 10%, respectively.
Ovarian morphology and immunohistochemistry
Ovarian specimens were rapidly fixed in 4% paraformaldehyde fixative, embedded in paraffin, and sectioned at 4 μm for hematoxylin and eosin (H&E) and immunohistochemistry (IHC) staining. H&E staining was performed to examine the morphologic structure of the ovaries. After dewaxing in distilled water, the slides were then incubated with the primary polyclonal rabbit antibodies of anti-aromatase (ab18995, Abcam, 1:400) and anti-TSHR (14450–1-AP, ProteinTech Group, Inc., 1:50) for 12 h at 4 °C in a humid environment. Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG secondary antibody (GB23303, Servicebio, 1:200) was applied to the sections and incubated for 1 h incubation at 37 °C. The sections were stained with diaminobenzidine (G1212, Servicebio). IHC staining was conducted to detect aromatase expression and TSHR in ovaries with an optical microscope (Eclipse E100, Nikon, Japan). The positive expression of the targeted protein was brownish yellow. The integral optical density (IOD) and areas of these regions were measured using Image-Pro Plus 6.0. The average optical density (AOD) value was calculated using the following formula: AOD = IOD/area.
Isolation and culture of mouse granulosa cells
The 35-day-old mice were pretreated with 5 IU pregnant mare serum gonadotropin (PMSG) (Ningbo, Zhejiang, China) for 48 h. The female mice were sacrificed and their ovaries were removed. Following Dulbecco’s modified Eagle medium/F12 (DMEM/F12, Hyclone, USA) washing of the ovaries, granulosa cells were collected using the follicle puncture method. The granulosa cells were centrifuged (1000 rpm, 5 min) and washed with DMEM/F12 medium three times. After discarding the supernatants, the pellets were preserved. The granulosa cells were seeded in 6-well plates and cultured in DMEM/F12 supplemented with 10% feta bovine serum (FBS, Hyclone, USA) and 1% penicillin/streptomycin (Hyclone, USA) at 37 °C in a humidified environment containing 5% CO2. During incubation, the culture media were replaced every 24 h. The purity of the isolated granulosa cells was verified via immunofluorescence staining with follicle-stimulating hormone receptor (FSHR), which is a specific marker of granulosa cells. The standard procedure for immunofluorescence staining was similar to the IHC staining described above. The polyclonal anti-FSHR antibody (1:100, Proteintech) was used to determine the expression of FSHR protein in granulosa cells. Finally, the slices were observed using an optical microscope (Eclipse E100, Nikon, Japan).
MTT assay
Granulosa cell viability was measured using the MTT assay (Sangon Biotech, Shanghai, China). Briefly, granulosa cells were plated at a density of 2000 cells/well in 96-well plates in DMEM/F12 supplemented with 10% FBS and 1% penicillin/streptomycin for 24 h. After incubation, the granulosa cells were exposed to variable concentrations (0 μM, 1 μM, 10 μM, 100 μM) of TSH (HY-107916, MedChemExpress, China) for an additional 24 h. The relative cell numbers were investigated by incubating the cells with MTT for 4 h. After the reaction, the supernatant was removed and the remaining formazan was dissolved in dimethyl sulfoxide (DMSO) to measure absorbance at 490 nm using a spectrophotometer (Thermo Fisher Scientific, Vantaa, Finland).
Real-time quantitative PCR (RT-qPCR)
The granulosa cells were incubated with different concentrations of TSH for 24 h. Total RNA was extracted from the granulosa cells using an Ultrapure RNA Extraction kit (CW0581M, CWBIO, Beijing, China) following the manufacturer’s instructions for subsequent cDNA synthesis. Real-time quantitative PCR (RT-qPCR) was measured using SYBR Green Premix Pro Taq HS qPCR Kit (Accurate Biology, Shanghai, China). The GAPDH gene was selected as a reference gene in this study. Prime sequences used in the present experiment were as follows: GAPDH forward,5’- GGTGAAGGTCGGTGTGAACG -3’, reverse, 5’-CTCGCTCCTGGAAGATGGTG-3’ (233 bp), CYP19A1 forward, 5’-CACATCATGCTGGACACCTCTAA-3’, reverse, 5’-AGCTGTGGAAACTTTGTGTCTCT-3’ (231 bp). The Ct value for each reaction tube was calculated using a real-time PCR instrument (Bio-Rad, USA) automatically, and the relative gene expression was calculated using the 2-ΔΔCt method.
Western blot analysis
The granulosa cells were incubated with different concentrations of TSH for 24 h. Total protein concentration was extracted from the granulosa cells using a BCA protein assay kit (Beyotime Biotechnology, Beijing, China). After 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, whole-cell lysates were separated and transferred onto polyvinylidene fluoride membranes (Merck Millipore, Temecula, USA). The membranes were blocked in a solution of 5% powdered skim milk in tris-buffered saline (TBS) for 2 h and incubated overnight at 4 °C with primary antibodies against GAPDH (1:5,000, Proteintech), CYP19A1 (1:1,000, ab18995, Abcam), followed by incubation with a secondary antibody (1:10,000, AB-2301, Zsbio, China) for 2 h. The experiments were repeated three times. The membranes were incubated with goat anti-rabbit secondary antibodies (1:10,000, Zsbio, China) for 1 h at 25 °C following washing with Tris-buffered saline with Tween (TBST). Protein bands were visualized using the enhanced chemiluminescence (ECL) detection system and analyzed using Image J software.
Statistical analysis
The data from in vivo and in vitro experiments were expressed as median (interquartile range). The Mann–Whitney test was used to compare the parameters of the obesity group and the control group. Multiple comparisons among different times and concentrations were analyzed using the Kruskal–Wallis test. P < 0.05 was regarded as statistically significant. Data analysis was analyzed using SPSS version 20.0 (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY, USA: IBM Corp).
Results
Obesity parameters and pubertal onset time
The body weights and lengths of the C57BL/6 J mice in the obesity and control groups gradually increased with time (both P < 0.001). Lee’s obesity index in the two groups gradually decreased with time (P < 0.001) (Fig. 1A, B, C). At 2, 3, 4, and 5 weeks, the body weights and Lee’s obesity index were significantly higher in the obesity group when compared to the control group (all P < 0.001). There was no difference in body length between the two groups (all P > 0.05) (Fig. 1B). The time of pubertal onset was significantly earlier in the obesity group than in the control group (P = 0.002). (Fig. 1D).
Parameters and pubertal onset time in C57BL/6 J mice. Curves show the changes of body weights (A), lengths (B), Lee’s index (C), and pubertal onset time (D) in the obesity group and the control group. Data are shown as box and whiskers plots. Box plots show the median, lower, and upper quartiles, and the whiskers as minimum and maximum values. *P < 0.05, **P < 0.001 vs. the control group (Mann–Whitney test, Kruskal–Wallis test)
Aromatase and hormone levels
As shown in Table 1, serum E2, aromatase, and TSH levels were significantly increased in the obesity group compared with the control group (all P < 0.001). Serum T3 and T4 concentrations were significantly decreased in the obesity group compared with the control group (both P < 0.001). There was no significant difference in T concentrations between the two groups (P = 0.083).
Expression of aromatase and TSHR in ovarian tissues
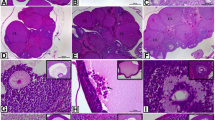
To determine whether aromatase and TSHR were expressed in ovaries, IHC staining was conducted. In the ovarian tissues, the expression of aromatase and TSHR protein were present in the granulosa cells (Fig. 2A). The AOD values of aromatase and TSHR protein were significantly higher in the obesity group than in the control group (P = 0.005, P < 0.001). (Fig. 2B).
Expression of aromatase and TSHR in ovaries. Representative images of IHC staining of aromatase and TSHR expression in the ovaries of C57BL/6 J mice (A). The AOD quantified are presented as box and whiskers plots (B). Box plots show the median, lower, and upper quartiles, and the whiskers as minimum and maximum values. *P < 0.05, **P < 0.001 vs. the control group (Mann–Whitney test)
Effect of TSH on viability of granulosa cells
Compared to the control group, granulosa cell viability was significantly lower at 0 μM, 10 μM, and 100 μM concentrations of TSH in the obesity group (P = 0.002, P = 0.024, and P < 0.001, respectively). With the treatment of increasing concentrations of TSH, granulosa cell viability in the obesity group and the control group increased gradually (all P < 0.001). (Fig. 3).
Effect of TSH on viability of granulosa cells in vitro. After the stimulation of different concentrations of TSH, the viability of granulosa cells was measured using the MTT assay. Data are shown as box and whiskers plots. Box plots show the median, lower, and upper quartiles, and the whiskers as minimum and maximum values. *P < 0.05, **P < 0.001 vs. the control group (Mann–Whitney test, Kruskal–Wallis test)
Effects of TSH on aromatase and hormone levels in granulosa cells
Under the stimulation of different concentrations of TSH, the E2 level of granulosa cells in the obesity group was higher than that in the control group, and the T level was lower than that in the control group (all P < 0.001) (Fig. 4A, B). Aromatase levels in the obesity group were lower than those in the control group at 0 μM concentrations of TSH (P = 0.001). At 1 μM, 10 μM, and 100 μM concentrations of TSH, the aromatase level of granulosa cells in the obesity group was higher than that in the control group (P = 0.024, P < 0.001, and P < 0.001, respectively) (Fig. 4C). With the treatment of increasing concentrations of TSH, the E2 and aromatase of granulosa cells in the obesity group and the control group gradually increased and the T level gradually decreased (all P < 0.001) (Fig. 4).
Effect of TSH on hormone and aromatase levels in granulosa cells. After the stimulation of different concentrations of TSH, the concentrations of E2 (A), T (B), and aromatase (C) in the culture were measured using an ELISA kit. Data are shown as box and whiskers plots. Box plots show the median, lower, and upper quartiles, and the whiskers as minimum and maximum values. *P < 0.05, **P < 0.001 vs. the control group (Mann–Whitney test, Kruskal–Wallis test)
Effects of TSH on CYP19A1 mRNA and protein expression in granulosa cells
The effects of TSH on CYP19A1 mRNA and protein expression are shown in Fig. 5. At 1 μM and 100 μM concentrations of TSH, the expression of CYP19A1 mRNA in granulosa cells in the obesity group was lower than that in the control group (P = 0.001, P < 0.001). There was no significant difference in the expression of CYP19A1 mRNA between the two groups at 0 μM and 10 μM concentrations of TSH (both P > 0.05). After the stimulation of different concentrations of TSH, the expression of CYP19A1 protein in granulosa cells in the obesity group was higher than that in the control group (P = 0.014, P < 0.001, and P = 0.004, respectively). With the increase of TSH concentrations, the expression of CYP19A1 mRNA and protein in granulosa cells in the obesity group and the control group significantly increased (all P < 0.001).
Effect of TSH on CYP19A1 mRNA and protein expression in granulosa cells. Granulosa cells were stimulated with different concentrations of TSH for 24 h. A CYP19A1 mRNA was measured by RT-qPCR. B CYP19A1 protein was assessed by Western blot. Data were normalized to GAPDH mRNA or protein levels. Data are shown as box and whiskers plots. Box plots show the median, lower, and upper quartiles, and the whiskers as minimum and maximum values. *P < 0.05, **P < 0.001 vs. the control group (Mann–Whitney test, Kruskal–Wallis test)
Discussion
In the present study, we identified the occurrence of early puberty and elevated serum aromatase and TSH levels in obese female mice. In the ovarian granulosa cells of obese mice, TSH directly regulates aromatase expression in a dose-dependent manner. Our data confirm the relationship between TSH and aromatase expression in obese mice.
The prevalence of childhood obesity and precocious puberty has increased significantly over the past few decades. A number of studies have shown that obesity is associated with early puberty in girls [20]. Animal experiments also showed earlier vaginal opening in obese mice than in normal-weight mice [21]. Our results were similar to those of the above research studies.
At present, the potential mechanisms of obesity-induced early pubertal onset include the following. (1) Leptin is a protein-like endocrine factor secreted by peripheral adipocytes. Leptin stimulates the release of kisspeptin from the hypothalamus by binding its receptor, indirectly affecting the release of gonadotropin-releasing hormone (GnRH) and promoting pubertal onset [22]. However, injections of leptin alone into young mice and children with leptin deficiency did not induce the onset of puberty [23]. Leptin has a permissive role in pubertal onset, but it is not the trigger for pubertal onset [24]. (2) Insulin resistance in obesity can activate the hypothalamic–pituitary–adrenal axis, increase androgen synthesis, reduce sex hormone-binding globulin (SHBG), and elevate the production of sex hormone, this potentially leading to premature adrenarche and precocious puberty [25]. (3) Moreover, large amounts of inflammatory cytokines secreted by adipocyte tissue in obesity may also promote early puberty [26] (4) Meanwhile, other factors such as genetic background, prenatal and postnatal environments, and endocrine disruptors are also associated with obesity and early puberty [27]. However, the above mechanisms cannot fully explain the inconsistencies between girls and boys at pubertal onset. (5) The peripheral effects of obesity are worthy of note. Given that adipose tissue exerts aromatase activity which increases the conversion of androgens to estrogens [2], the excess estrogens may promote early puberty in girls and delay puberty in boys. More research is certainly needed to determine how the peripheral hormones affect pubertal onset.
One study has shown that E2 levels increase due to enhanced aromatase expression from peripheral adipose tissue in obese postmenopausal females [28]. Furthermore, Chen et al. found that when women’s body mass index (BMI) increased, there was an increase in aromatase activity (E2-to-T ratio) [29]. In men with abdominal obesity, serum aromatase levels were increased, accompanied by increased E2 and decreased T levels [30]. On the other hand, few studies have reported the aromatase profiles in obese children. However, in our preliminary experiments, we noted increased levels of aromatase in obese children, while in the present study, we found elevated aromatase and E2 in obese female mice, this being consistent with the above reports. Taken together, these results demonstrate a link between obesity and aromatase.
Aromatase deficiency, a very rare autosomal recessive disorder, has been described in hyperandrogensim, sexual differentiation, reproductive disease, delayed bone maturation, progressive linear growth, osteoporosis, insulin resistance, and in individuals with an abnormal lipid profile. Aromatase excess syndrome is a rare autosomal dominant disorder. Recombination arrangements in the upstream region and the use of alternative more active promoters might result in a gain of function mutation in the CYP19A1 gene [31]. The CYP19A1 gene is not only affected by uncommon mutations. It has been shown that the CYP19A1 gene has more common genetic polymorphisms. The variations may influence expression of CYP19A1 gene, activity of the enzyme, and susceptibility to cancer development [32]. Elevated aromatase activity is linked to aging, obesity, hyperthyroidism, idiopathic gynecomastia, and tumors such as testicular tumors, breast cancer, and endometrial cancer [31]. Aromatase is expressed in many tissues, such as the gonads, brain, adipose, and placental tissues [7]. Most studies focused on the increased expression of aromatase in adipose tissue in obesity [33]. Many studies reported that aromatase was expressed in ovarian tissues, mainly in patients with polycystic ovary syndrome, ovarian hyperstimulation, ovarian cancer, and other diseases [7, 29, 34]. However, there are few reports of aromatase expression in the ovaries of obese women. In the present study, our results demonstrated enhanced aromatase expression in the ovarian tissues of obese mice.
It is well known that FSH is the most important inducer of aromatase in ovarian granulosa cells. FSH binds to the G protein-coupled receptors (GPCRs) on the surface of gonadal cells, activates the cAMP/PKA/CREB signaling pathway, and promotes CYP19A1 gene expression [8]. FSH is regulated by many factors, with T3 and T4 being inhibitory factors [35]. In Sertoli cells of male rats, aromatase activity and estrogen secretion were increased in hypothyroid patients and decreased after thyroid hormone replacement treatment [19]. TSH and FSH are both glycoprotein hormones, consisting of an identical α-subunit and a unique β-subunit. TSH has a cross effect with FSH in promoting the proliferation of granulosa cells [36]. Both TSHR and FSHR belong to the subfamily of GPCRs. TSHR is expressed in several tissues, and also in gonadal tissue [37]. TSH may promote the expression of the CYP19A1 gene by binding to TSHR and FSHR on the surface of gonadal cells, inducing the transcription of aromatase. Serum TSH levels tend to be higher and FT4 levels tend to be lower in obese children compared with normal weight children [11]. Thus, there is a link between abnormal thyroid function and aromatase expression in obesity. In our study, we found that TSH levels were increased in obese female mice and their granulosa cells. In obese female mice, TSHR protein was expressed in ovarian tissues and granulosa cells. TSH stimulated granulosa cell viability in a dose-dependent manner. The viability of granulosa cells was better in the control group than in the obesity group. TSH plays a role in promoting proliferation of gonadal cells [38]. However, emerging evidence indicates that obesity impairs granulosa cell function [39]. It is possible that elevated estrogen in obesity inhibits granulosa cell viability [40]. In our study, we found that TSH stimulated the expression of CYP19A1 mRNA and protein in a dose-dependent manner in both groups. Compared to the control group, CYP19A1 mRNA levels were higher at 1 μm TSH, indifferent at 10 μm TSH, and lower at 100 μm TSH in ovarian granulosa cells of obese mice. It has been reported that TSH mediates a biphasic effect on gene expression in a number of cell systems. Low doses of TSH upregulate and higher TSH doses decrease the levels of mRNAs [41]. In the obesity group, the expression of CYP19A1 mRNA was lower than that in the control group, but the expression of CYP19A1 protein was higher than that in the control group. These findings might be explained by differentially expressed miRNAs regulating CYP19A1 mRNAs in the obese mice and normal-weight mice [7]. The specific molecular mechanism remains unknown. Further in-depth research is needed.
This study has some limitations. First, it is only a preliminary exploration of the effect of TSH on aromatase expression of ovarian granulosa cells. Extensive research is needed to investigate the deeper molecular mechanisms of these effects. Moreover, we decided not to explore aromatase activity in the gonadal tissues of male mice due to the existence of findings in previous research. We intend to carry out further research to enrich our knowledge concerning the molecular mechanism if sufficient funds are available.
In conclusion, our study demonstrates the relationship between TSH and ovarian aromatase and the effect of TSH on aromatase expression of granulosa cells in obese mice. While our results provide new ideas for the promotion of clinical and basic research, further studies are required to investigate the specific molecular mechanisms.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Jebeile H, Kelly AS, O’Malley G, Baur LA (2022) Obesity in children and adolescents: epidemiology, causes, assessment, and management. Lancet Diabetes Endocrinol 10:351–365. https://doi.org/10.1016/S2213-8587(22)00047-X
Reinehr T, Roth CL (2019) Is there a causal relationship between obesity and puberty? Lancet Child Adolesc Health 3(1):44–54. https://doi.org/10.1016/S2352-4642(18)30306-7
Busch AS, Højgaard B, Hagen CP, Teilmann G (2020) Obesity Is Associated with Earlier Pubertal Onset in Boys. J Clin Endocrinol Metab 105:dgz222. https://doi.org/10.1210/clinem/dgz222
Brix N, Ernst A, Lauridsen LLB, Parner ET, Arah OA, Olsen J, Henriksen TB, Ramlau-Hansena CH (2020) Childhood overweight and obesity and timing of puberty in boys and girls: cohort and sibling-matched analyses. Int J Epidemiol 49:834–844. https://doi.org/10.1093/ije/dyaa056
Takle ZJ, Legesse TG (2017) The effect of leptin on the hypothalamic-pituitary gonadal axis and puberty. Int J Heal Sci Res 7:332–344
Yuxin L, Chen L, Xiaoxia L, Yue L, Junjie L, Youzhu L, Huiliang Z, Qicai L (2021) Research progress on the relationship between obesity-inflammation-aromatase axis and male infertility. Oxid Med Cell Longev 2021:6612796. https://doi.org/10.1155/2021/6612796
Liu T, Huang Y, Lin H (2021) Estrogen disorders: Interpreting the abnormal regulation of aromatase in granulosa cells (Review). Int J Mol Med 47:73. https://doi.org/10.3892/ijmm.2021.4906
Stocco C (2008) Aromatase expression in the ovary: hormonal and molecular regulation. Steroids 73:473–487. https://doi.org/10.1016/j.steroids.2008.01.017
Crocker MK, Stern EA, Sedaka NM, Shomaker LB, Brady SM, Ali AH, Shawker TH, Hubbard VS, Yanovski JA (2014) Sexual dimorphisms in the associations of BMI and body fat with indices of pubertal development in girls and boys. J Clin Endocrinol Metab 99:E1519-1529. https://doi.org/10.1210/jc.2014-1384
Sanyal D, Raychaudhuri M (2016) Hypothyroidism and obesity: An intriguing link. Indian J Endocrinol Metab 20:554–557. https://doi.org/10.4103/2230-8210.183454
An YM, Moon SJ, Kim SK, Suh YJ, Lee JE (2018) Thyroid function in obese Korean children and adolescents: Korea National Health and Nutrition Examination Survey 2013–2015. Ann Pediatr Endocrinol Metab 23:141–147. https://doi.org/10.6065/apem.2018.23.3.141
Patel R, Dave C, Mehta S, Mendpara H, Shukla R, Bajpai A (2021) Metabolic Impact of Subclinical Hypothyroidism in Obese Children and Adolescents. Indian J Pediatr 88:437–440. https://doi.org/10.1007/s12098-020-03463-0
Jung G, Oh SB, Lee WY, Kim HR, Nam HK, Kim JH, Rhie YJ, Lee KH (2019) Thyroid function in girls with central precocious puberty. Ann Pediatr Endocrinol Metab 24:124–128. https://doi.org/10.6065/apem.2019.24.2.124
Yigit O, Sert TK, Ekinci D, Kirankaya A, Kilinc S (2023) The effect of subclinical hypothyroidism on ovarian volume in prepubertal girls. North Clin Istanb 10:48–52. https://doi.org/10.14744/nci.2021.78300
Hu Y, Wang Q, Li G, Sun X, Liu C (2013) Ultrasonic morphology of uterus and ovaries in girls with pituitary hyperplasia secondary to primary hypothyroidism. Horm Metab Res 45:669–674. https://doi.org/10.1055/s-0033-1345141
Hu YY, Li GM, Hu WW, Wang Y (2014) Characteristics of girls with pituitary hyperplasia and sexual precocity secondary to primary hypothyroidism. Acta Paediatr 103:e43-48. https://doi.org/10.1111/apa.12444
Lee SJ, Moon JE, Lee GM, Cho MH, Ko CW (2020) An Alport syndrome boy with Van Wyk-Grumbach syndrome induced by prolonged untreated congenital hypothyroidism. Ann Pediatr Endocrinol Metab 25:132–136. https://doi.org/10.6065/apem.1938074.037
Dhole B, Gupta S, Shekhar S, Kumar A (2020) A novel antigonadotropic role of thyroid stimulating hormone on leydig cell-derived mouse leydig tumor cells-1 line. Ann Natl Acad Med Sci 56:30–37. https://doi.org/10.1055/s-0040-1709091
Yuan Z, Shen X, Yan H, Jiang J, Liu B, Zhang L, Wu Y, Liu Y, Liu Q (2021) Effects of the thyroid endocrine system on gonadal sex ratios and sex-related gene expression in the pufferfish takifugu rubripes. Front Endocrinol (Lausanne) 12:674954. https://doi.org/10.3389/fendo.2021.674954
Liu Y, Yu T, Li X, Pan D, Lai X, Chen Y, Wang X, Yu X, Fu S, Huang S, Lin C, Liu S (2021) Prevalence of precocious puberty among Chinese children: a school population-based study. Endocrine 72:573–581. https://doi.org/10.1007/s12020-021-02630-3
Bohlen TM, Silveira MA, Zampieri TT, Frazão R, Donato J Jr (2016) Fatness rather than leptin sensitivity determines the timing of puberty in female mice. Mol Cell Endocrinol 423:11–21. https://doi.org/10.1016/j.mce.2015.12.022
Sanchez-Garrido MA, Tena-Sempere M (2013) Metabolic control of puberty: roles of leptin and kisspeptins. Horm Behav 64:187–194. https://doi.org/10.1016/j.yhbeh.2013.01.014
Elias CF (2012) Leptin action in pubertal development: recent advances and unanswered questions. Trends Endocrinol Metab 23:9–15. https://doi.org/10.1016/j.tem.2011.09.002
Ahmed ML, Ong KK, Dunger DB (2009) Childhood obesity and the timing of puberty. Trends Endocrinol Metab 20:237–242. https://doi.org/10.1016/j.tem.2009.02.004
Goldsammler M, Merhi Z, Buyuk E (2018) Role of hormonal and inflammatory alterations in obesity-related reproductive dysfunction at the level of the hypothalamic-pituitary-ovarian axis. Reprod Biol Endocrinol 16:45. https://doi.org/10.1186/s12958-018-0366-6
Thaler JP, Schwartz MW (2010) Minireview: inflammation and obesity pathogenesis: the hypothalamus heats up. Endocrinology 151:4109–4115. https://doi.org/10.1210/en.2010-0336
Huang A, Reinehr T, Roth CL (2020) Connections between obesity and puberty: invited by Manuel Tena-Sempere, Cordoba. Curr Opin Endocr Metab Res 14:160–168. https://doi.org/10.1016/j.coemr.2020.08.004
Cleary MP, Grossmann ME (2009) Minireview: obesity and breast cancer: the estrogen connection. Endocrinology 150:2537–2542. https://doi.org/10.1210/en.2009-0070
Chen J, Shen S, Tan Y, Xia D, Xia Y, Cao Y, Wang W, Wu X, Wang H, Yi L, Gao Q, Wang Y (2015) The correlation of aromatase activity and obesity in women with or without polycystic ovary syndrome. J Ovarian Res 8:11. https://doi.org/10.1186/s13048-015-0139-1
Xu X, Wang L, Luo D, Zhang M, Chen S, Wang Y, Zheng D, Feng L, Gao L, Yu C, Guan Q (2018) Effect of testosterone synthesis and conversion on serum testosterone levels in obese men. Horm Metab Res 50:661–670. https://doi.org/10.1055/a-0658-7712
Czajka-Oraniec I, Simpson ER (2010) Aromatase research and its clinical significance. Endokrynol Pol 61:126–134
Warsy AS, Almukaynizi FB, AlDaihan S, Alam S, Daghastani M (2017) Genetic Polymorphisms in Aromatase (CYP19) Gene and Cancer. InTech 27. https://doi.org/10.5772/intechopen.69208
Mair KM, Gaw R, MacLean MR (2020) Obesity, estrogens and adipose tissue dysfunction - implications for pulmonary arterial hypertension. Pulm Circ 10:2045894020952019. https://doi.org/10.1177/2045894020952023
Cheng JC, Fang L, Li Y, Wang S, Li Y, Yan Y, Jia Q, Wu Z, Wang Z, Han X, Sun YP (2020) Melatonin stimulates aromatase expression and estradiol production in human granulosa-lutein cells: relevance for high serum estradiol levels in patients with ovarian hyperstimulation syndrome. Exp Mol Med 52:1341–1350. https://doi.org/10.1038/s12276-020-00491-w
Cecconi S, Rucci N, Scaldaferri ML, Masciulli MP, Rossi G, Moretti C, D’Armiento M, Ulisse S (1999) Thyroid hormone effects on mouse oocyte maturation and granulosa cell aromatase activity. Endocrinology 140:1783–1788. https://doi.org/10.1210/endo.140.4.6635
Kabodmehri R, Sharami SH, Sorouri ZZ, Gashti NG, Milani F, Chaypaz Z, Ghalandari M (2021) The relationship between thyroid function and ovarian reserve: a prospective cross-sectional study. Thyroid Res 14:22. https://doi.org/10.1186/s13044-021-00112-2
Deal CK, Volkoff H (2020) The Role of the Thyroid Axis in Fish. Front Endocrinol (Lausanne) 11:596585. https://doi.org/10.3389/fendo.2020.596585
Vieira IH, Rodrigues D, Paiva I (2022) The Mysterious Universe of the TSH Receptor. Front Endocrinol (Lausanne) 13:944715. https://doi.org/10.3389/fendo.2022.944715
Wu RX, Dong YY, Yang PW, Wang L, Deng YH, Zhang HW, Huang XY (2019) CD36- and obesity-associated granulosa cells dysfunction. Reprod Fertil Dev 31:993–1001. https://doi.org/10.1071/RD18292
Pierre A, Mayeur A, Marie C, Cluzet V, Chauvin J, Frydman N, Grynberg M, Cohen-Tannoudji J, Guigon CJ, Chauvin S (2021) Estradiol Regulates mRNA Levels of Estrogen Receptor Beta 4 and Beta 5 Isoforms and Modulates Human Granulosa Cell Apoptosis. Int J Mol Sci 22:5046. https://doi.org/10.3390/ijms22095046
Jang D, Morgan SJ, Klubo-Gwiezdzinska J, Banga JP, Neumann S, Gershengorn MC (2020) Thyrotropin, but not thyroid-stimulating antibodies, induces biphasic regulation of gene expression in human thyrocytes. Thyroid 30:270–276. https://doi.org/10.1089/thy.2019.0418
Funding
The study was supported by the Shandong Provincial Medical and Health Science and Technology Development Program (202306010730).
Author information
Authors and Affiliations
Contributions
YH designed the original experiments and performed the experiments. LZ and XZ performed the experiments and wrote the manuscript. LM analyzed the experimental data and edited of the final manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Animal maintenance and research were conducted in accordance with the Guide for the Care and Use of Laboratory Animals. The experimental protocol was approved by the Ethics Committee of Xuzhou Medical University.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, L., Zhou, X., Ma, L. et al. Effect of TSH on aromatase expression of ovarian granulosa cells in obese mice. Hormones (2024). https://doi.org/10.1007/s42000-024-00571-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42000-024-00571-w