Abstract
Purpose
The relationship between thyroid hormone sensitivity and albuminuria remains unclear. We aimed to investigate the association between thyroid hormone sensitivity and the risk of albuminuria in a euthyroid population.
Methods
This cross-sectional study included 7634 euthyroid adults collected from the National Health and Nutrition Examination Survey (NHANES) between 2007 and 2012. Central sensitivity to thyroid hormones was evaluated using the thyroid-stimulating hormone index (TSHI), the thyrotrophic thyroxine resistance index (TT4RI), and the thyroid feedback quantile-based index (TFQI). Peripheral sensitivity to thyroid hormones was measured using the free triiodothyronine/free thyroxine (FT3/FT4) ratio. Furthermore, the independent relationship between sensitivity to thyroid hormones and albuminuria was assessed.
Results
The proportion of albuminuria increased with a higher interquartile range of TFQI levels (7.31% vs. 7.89% vs. 7.95% vs. 9.89%, P = 0.024). Furthermore, TFQI was found to be independently associated with the risk of albuminuria after adjusting for confounding factors (OR = 1.28, 95% CI 1.01–1.60, P = 0.037). Subgroup analysis revealed a significant relationship between TFQI and albuminuria, especially among individuals over 60.
Conclusions
In euthyroid subjects, impaired central sensitivity to thyroid hormones is associated with albuminuria. TFQI holds significant potential as an epidemiological tool for quantifying the impact of impaired central sensitivity on the risk of albuminuria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) is a progressive condition that has emerged as a significant cause of mortality and morbidity in the twenty-first century, impacting over 10% of the global population [1]. Albuminuria, which refers to the excessive presence of albumin in the urine, is a crucial marker for glomerular injury and renal dysfunction [2]. It is frequently linked to kidney diseases, including diabetic nephropathy and hypertensive nephropathy [3, 4]. Under normal physiological conditions, the glomerulus filters only a minimal amount of albumin, while the renal tubules reabsorb the majority. However, in the presence of renal dysfunction or impairment, the permeability of the glomerular filtration barrier becomes compromised, resulting in the leakage of albumin into the urine. Moreover, it is strongly associated with an increased risk of progressive decline in renal function, metabolic disease, cardiovascular events, and all-cause mortality [5,6,7,8]. Non-invasive methods, like urine testing, are commonly used to diagnose albuminuria. This type of testing involves measuring the urinary albumin/creatinine ratio (UACR) or the amount of albumin eliminated over a specific period.
Previous studies have indicated an intricate relationship between thyroid hormones and albuminuria [9,10,11,12,13]. Thyroid hormones impact several aspects of kidney function, including kidney structure, hemodynamics, glomerular filtration rate (GFR), and sodium and water homeostasis [14, 15]. Imbalances in the levels of thyroid hormones, specifically hypothyroidism, have been linked to an elevated risk of developing albuminuria [16,17,18]. However, a recent study has suggested a mild form of thyroid hormone resistance in the general population [19]. Thus, a novel metric, TFQI, has been proposed to assess deviations from the average suppression of thyroid hormone in the pituitary gland. However, its clinical relevance remains largely unclear [19].
To our knowledge, no studies have previously examined the connection between sensitivity to thyroid hormones and the likelihood of albuminuria. Therefore, we undertook a study to explore the association between central and peripheral sensitivity to thyroid hormones, primarily measured through TFQI and FT3/FT4, with albuminuria risk among euthyroid individuals from the NHANES database.
Materials and methods
Data source
This population-based study was conducted by sourcing data from the NHANES, conducted by the National Center for Health Statistics of the Centers for Disease Control and Prevention [20]. NHANES conducted a randomized, stratified, multi-stage survey with a nationwide representation; participants underwent physical examinations, questionnaires about their health and nutrition, and laboratory assessments. The research adhered to the guidelines provided by the Ethics Review Board of the National Center for Health Statistics, and written informed consent was obtained from all participants.
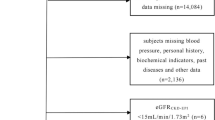
Our analysis finally comprised 7634 eligible participants, obtained by merging data from the following NHANES cycles: 2007–2008, 2009–2010, and 2011–2012 (30,442 participants). All included participants were aged between 19 and 79 years, had no records of pregnancy, exhibited complete indicators of thyroid function, and provided complete data on the UACR and estimated glomerular filtration rate (eGFR). In line with the medical guidelines for clinical practice by the American Association of Clinical Endocrinologists, serum TSH levels between 0.4 and 4.5 mIU/L were considered normal [21]. We excluded subjects with TSH levels < 0.4 mIU/L or > 4.5 mIU/L, as well as individuals with abnormal reference ranges for both FT3 and FT4. Additionally, participants undergoing thyroid hormone replacement therapy (levothyroxine, liothyronine, thyroid desiccated, or other) were excluded, along with those who had a previous history of thyroid disease (Fig. 1).
Outcome definitions
UACR was calculated as the ratio of urinary concentration of albumin (measured in mg/dL) to creatinine (measured in mg/L). UACR values of 30–300 mg/g and ≥ 300 mg/g indicate the presence of microalbuminuria and macroalbuminuria, respectively. The occurrence of albuminuria (UACR ≥ 30 mg/g) served as the outcome variable for our study [22].
Exposure definitions
Three indices were used to evaluate central sensitivity to thyroid hormones, as follows: TSHI, TT4RI, and TFQI. TSHI was calculated as ln TSH (mIU/L) + 0.1345 ⋅ FT4 (pmol/L). TT4RI was measured by multiplying the values for FT4 (pmol/L) by TSH (mIU/L). The formula for calculating TFQI was cumulative distribution function (cdfFT4) − (1 − cdfTSH). The values for TFQI range from − 1 to 1, where negative values indicate greater sensitivity of the hypothalamic-pituitary-thyroid axis to thyroid hormone fluctuations, and positive values denote lesser sensitivity [19]. Furthermore, the ratio of FT3 to FT4 measured peripheral sensitivity to thyroid hormones23.
Covariate definitions
The study collected demographic data, including age, gender, and race. It also included several potential covariates, such as annual income, education level, physical activity, smoking status, hypertension, diabetes, cardiovascular disease, body mass index (BMI, kg/m2), alanine transaminase (ALT, U/L), aspartate transaminase (AST, U/L), gamma-glutamyl transferase (GGT, U/L), triglycerides (TG, mmol/L), total cholesterol (TC, mmol/L), high-density lipoprotein cholesterol (HDL-c, mmol/L), low-density lipoprotein cholesterol (LDL-c, mmol/L), blood urea nitrogen (BUN, mg/dL), serum creatinine (Scr, mg/dL), serum uric acid (SUA, μmol/L), and eGFR. eGFR was calculated using the CKD-EPI creatinine equation, considering factors such as age, gender, race, and Scr [23]. Self-reported diabetes was defined. Hypertension was defined as systolic blood pressure > 140 mmHg or diastolic blood pressure > 90 mmHg or the use of antihypertensive medication. The presence of cardiovascular disease was determined based on self-reported history of heart attack, stroke, congestive heart failure, coronary artery disease, or angina. Detailed measurement procedures for all variables in this study were publicly available in the NHANES database (https://www.cdc.gov/nchs/nhanes/).
Statistical analysis
Population-weighted descriptive statistics were calculated and are presented in this study. Continuous variables are reported as mean with standard error (SE) and categorical variables as percentages. The weighted Student’s t-test and chi-squared test were used to compare continuous and categorical variables in multiple groups. Weighted Pearson correlation analysis was used to assess the association of TFQI with other covariates. A logistic regression model was employed to investigate the associations between thyroid hormone levels and sensitivity-related indicators (FT3, FT4, TSH, FT3/FT4, TSHI, TT4RI, and TFQI) with the risk of albuminuria. Three models were used, as follows: model 1 without covariates; model 2 with adjustments for age, gender, and race; and model 3 with adjustments for age, gender, race, annual income, education level, smoking status, physical activity, diabetes, hypertension, cardiovascular disease, BMI, ALT, AST, GGT, TG, TC, HDL, LDL, BUN, SUA, Scr, and eGFR. Smooth curve fitting analysis was conducted using a generalized additive model for albuminuria, UACR, BUN, Scr, and eGFR, with the TFQI index as the independent variable, after adjusting for age, gender, and race. Receiver operating characteristic (ROC) analysis was also employed to evaluate the impact of thyroid hormone sensitivity–related indices on albuminuria. Subgroup analyses were performed stratified by age (< 60/ ≥ 60 years), gender (female/male), BMI (< 25/25–30/ ≥ 30 kg/m2), diabetes (yes/no), hypertension (yes/no), cardiovascular disease (yes/no), and eGFR (< 60/60–90/ ≥ 90 mL/min/1.73 m2). All statistical analyses followed the guidelines provided by the Centers for Disease Control and Prevention, considering the complex multi-stage cluster survey design and incorporating weights from three cycles. The data analyses used were Empower (http://www.empowerstats.com) and R software (http://www.r-project.org). Statistical significance was determined as a two-sided P value < 0.05.
Results
Baseline characteristics of the study population
The study included 7634 subjects who met the inclusion criteria, with an average age of 44.91 ± 0.40 years and a male composition of 50.27%. We analyzed and compared the general information and clinical indicators between the groups categorized as non-albuminuria and albuminuria (Table 1). The results demonstrated that the albuminuria group exhibited significantly higher values in regard to age, annual income below $20,000, smoking, diabetes, hypertension, cardiovascular disease, BMI, ALT, AST, GGT, TG, BUN, SUA, Scr, FT4 levels, TSH levels, TSHI levels, TT4RI levels, and urinary albumin in comparison to the non-albuminuria group (P < 0.05). However, the albuminuria group exhibited significant decreases in education level above high school, moderate physical activity, HDL-c, LDL, eGFR, FT3 levels, and FT3/FT4 levels (P < 0.05). Furthermore, significant differences in race distribution were observed between the groups (P < 0.001). Notably, the albuminuria group exhibited higher TFQI levels than the non-albuminuria group (P < 0.001).
Clinical features of the participants according to the quartiles of TFQI
The study participants were categorized into four groups based on their levels of TFQI, as follows: quartile I, quartile II, quartile III, and quartile IV, as shown in Table 2. In comparison to the quartile I-TFQI group, the quartile II-TFQI, quartile III-TFQI, and quartile IV-TFQI groups showed significant increases in age, education level above high school, prevalence of diabetes, hypertension, cardiovascular disease, BMI, ALT, AST, GGT, BUN, SUA, Scr, and levels of FT4, TSH, TSHI, and TT4RI (P < 0.05). Additionally, moderate physical activity, HDL-c, eGFR, FT3/FT4 levels, and urinary creatinine significantly decreased (P < 0.001). There were also significant differences in the distribution of race and smoking status among the various quartile groups (P < 0.001). Furthermore, no differences in gender, annual income, TG, TC, LDL-c, FT3 levels, and urinary albumin were observed between groups. Importantly, as the TFQI levels increased progressively, both the UACR (22.75 ± 3.46 vs. 24.82 ± 4.05 vs. 34.57 ± 6.77 vs. 42.45 ± 8.59, P = 0.026) and the prevalence of albuminuria (7.31% vs. 7.89% vs. 7.95% vs. 9.89%, P = 0.024) also showed a gradual increase.
Correlation of TFQI with clinical parameters
From the weighted Pearson correlation analysis, TFQI exhibited significant associations with multiple variables (Table 3). Specifically, TFQI positively correlated with age, education level above high school, diabetes, hypertension, cardiovascular disease, BMI, BUN, Scr, SUA, UACR, and albuminuria (P < 0.01). In contrast, TFQI negatively correlated with moderate physical activity, smoking status, TC, HDL-c, LDL-c, eGFR, and urinary creatinine (P < 0.05).
Associations between sensitivity to thyroid hormones and albuminuria
Table 4 presents the association between thyroid hormone sensitivity and albuminuria. The unadjusted models revealed positive associations between FT4, TSH, TSHI, TT4RI, and TFQI and an increased risk of albuminuria (P < 0.001). In contrast, FT3 and FT3/FT4 showed a negative association with the prevalence of albuminuria (P < 0.001). Except for FT3, these associations remained statistically significant after adjusting for age, gender, and race (P < 0.05). Following additional adjustment for multiple covariates, only TT4RI and TFQI were related to albuminuria risk. The results indicated a 28% increased risk of albuminuria per unit increase in TFQI (OR = 1.28, 95% CI 1.01–1.60, P = 0.037). Classifying TFQI into quartiles demonstrated that higher TFQI quartiles were associated with a higher prevalence of albuminuria than the lowest quartiles in the fully adjusted model (P for trend = 0.035). Finally, the smooth curve fitting analysis results confirmed a positive correlation between TFQI and albuminuria, UACR, BUN, and Scr while also showing a negative correlation with eGFR (Fig. 2). No significant threshold effect was found.
ROC analysis results of thyroid hormone sensitivity on albuminuria
Figure 3 illustrates the evaluation of thyroid hormone sensitivity–related indices, including FT3/FT4, TSHI, TT4RI, and TFQI, in terms of their predictive power for albuminuria risk using ROC analysis. The corresponding area under the curve (AUC) for each index is as follows: FT3/FT4 (57.79%), TSHI (54.12%), TT4RI (53.71%), and TFQI (60.64%).
Subgroup analysis
We carried out additional investigations of TFQI and the risk of albuminuria in subgroups stratified by various factors, including age (< 60/ ≥ 60 years), gender (female/male), BMI (< 25/25–30/ ≥ 30 kg/m2), diabetes (yes/no), hypertension (yes/no), cardiovascular disease (yes/no), and eGFR (< 60/60–90/ ≥ 90 mL/min/1.73 m2) (Fig. 4). Our findings revealed a significant interaction between age and TFQI in relation to albuminuria (P for interaction = 0.020). Specifically, TFQI exhibited a stronger association with the risk of albuminuria in the elderly population (OR = 1.65, 95% CI 1.20–2.26, P = 0.002) compared to individuals under 60 years old (OR = 1.02, 95% CI 0.78–1.33, P = 0.903). Furthermore, no other significant interactions were observed in the subgroup analyses of TFQI and the risk of albuminuria (P for interaction > 0.05).
Discussion
This is, to our knowledge, the first population-based study investigating the relationship between thyroid hormone sensitivity and albuminuria risk in euthyroid subjects. Our study demonstrated that impaired central sensitivity to thyroid hormones is associated with albuminuria, which is more pronounced in the elderly. Compared with other thyroid hormone sensitivity–related indices, TFQI is an excellent potential epidemiological tool to quantify the role of central thyroid hormone resistance in the risk of albuminuria.
Thyroid hormone sensitivity can be categorized into two distinct types, namely, central sensitivity and peripheral sensitivity [19]. Impaired central sensitivity disrupts the feedback loop within the central nervous system, thereby bringing about modifications in the set point for regulating thyroid hormone levels. In contrast, impaired peripheral sensitivity hampers the signaling and action of thyroid hormones at the cellular level, ultimately diminishing their metabolic effects. Several studies have investigated the relationship between thyroid function and albuminuria/CKD. Yang et al. observed higher TSH levels and lower levels of FT4 in individuals with type 2 diabetes who had diabetic kidney disease (DKD) as compared to those without DKD. Moreover, the FT4 level shows a negative correlation with UACR and eGFR. In turn, TSH exhibits a positive correlation with UACR and eGFR [11]. However, Reinhardt et al. suggest that TSH or FT4 does not significantly correlate with UACR and albuminuria severity in CKD patients [9]. A separate study demonstrated that elevated levels of TT4 and FT4 in individuals with CKD are associated with an elevated risk and severity of albuminuria [10]. Nonetheless, these findings are primarily limited to a specific population and do not account for thyroid hormone resistance, indicating that relying solely on TSH or thyroid hormone levels may not adequately explain the connection between the thyroid system and albuminuria. Moreover, the results demonstrate certain inconsistencies. In the context of thyroid hormone resistance in euthyroid individuals, assessment measures based on thyroid hormone sensitivity have shown stronger correlations with albuminuria compared to those measures alone. This finding could potentially explain the inconsistencies observed in previous studies while also aiding in establishing a link between thyroid function and albuminuria in euthyroid individuals. Among the three indices that assess central thyroid hormone sensitivity, TFQI exhibited a stronger correlation with albuminuria. Moreover, TFQI demonstrated superior stability compared to both TT4RI and TSHI in assessing the abnormal range of thyroid response to TSH, particularly when the deviation is near normal [19]. Notably, this suggests that TFQI may provide a more accurate assessment of thyroid function in individuals with borderline or subclinical thyroid abnormalities, this being consistent with our findings.
In comparison, the FT3/FT4 ratio is a valuable indicator of the T4-T3 conversion rate, reflecting the sensitivity of peripheral tissues to thyroid hormones. Previous studies have demonstrated strong associations between FT3/FT4 levels and microangiopathy, metabolic disease, cardiovascular disease, and other disorders [24,25,26,27,28]. In addition, FT3/FT4 is recognized as an autonomous prognostic of all-cause and cardiovascular mortality [28]. After controlling for age, gender, and race, a higher FT3/FT4 ratio was linked to a reduced risk of albuminuria. However, this association lost significance after additional adjustment for multiple confounding factors. Unlike central sensitivity, which can be measured by observing thyroid hormone and TSH concentrations or their derived indicators, assessing thyroid hormone peripheral sensitivity is challenging and susceptible to multiple factors [19, 29]. A previous study in type 2 diabetics also showed no significant association with the risk of albuminuria, although there was an inverse association between FT3/FT4 and UACR [30]. Hence, further investigation is required to explore the connection between thyroid hormone peripheral sensitivity and albuminuria.
Several potential mechanisms may explain the correlation between central thyroid hormone sensitivity and albuminuria. Thyroid hormones can be directly involved in vascular and endothelial dysfunction and the formation of albuminuria [14]. Moreover, interactions between other metabolic hormones and albuminuria may influence thyroid resistance [31]. For instance, thyroid resistance is associated with metabolic processes involving blood glucose and insulin [19, 31,32,33]. Furthermore, thyroid resistance can also potentially induce alterations in the immune microenvironment of kidney disease, thereby augmenting the immune inflammatory response and ultimately causing kidney injury and albuminuria [34].
To summarize, our study sheds light on a potential link between impaired central sensitivity to thyroid hormones and albuminuria in individuals with normal thyroid function. The findings suggest that even in the absence of overt thyroid dysfunction, thyroid hormone resistance may play a role in the development or progression of albuminuria. Moreover, thyroid hormone resistance is associated with an increased risk of kidney disease, cardiovascular events, and mortality. It may also have implications for risk assessment, early detection, and management of kidney disease in individuals with normal thyroid function. Our study has also shown the potential of TFQI as a valuable tool for assessing the impact of impaired central sensitivity on the risk of albuminuria. However, further investigation is needed in the future to determine its clinical applications for targeting euthyroid patients.
It is necessary to acknowledge the limitations of our study. Firstly, the cross-sectional design does not establish a cause-and-effect relationship between sensitivity to thyroid hormones and albuminuria. Cohort studies and intervention trials are needed to determine whether there is a cause-and-effect relationship. Secondly, the study did not include thyroid-related antibodies, which could also potentially be a confounding factor. Thirdly, this study did not specifically exclude subjects with chronic kidney disease (defined as eGFR < 60 mL/min/1.73 m2) or proteinuria. Additional analyses based on eGFR are provided in Attachment 1. Finally, since our study was conducted using a sample from the US population, the generalizability of our findings necessitates further validation.
Conclusion
In a nationally representative study of adults aged 19–79 with normal thyroid function, an association was observed between impaired central thyroid sensitivity and an increased risk of albuminuria.
Data availability
Data was collected from the NHANES database.
References
Kovesdy CP (2022) Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl (2011) 12(1):7–11. https://doi.org/10.1016/j.kisu.2021.11.003
Levey AS, Becker C, Inker LA (2015) Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: a systematic review. JAMA 313(8):837–846. https://doi.org/10.1001/jama.2015.0602
Wang XL, Lu JM, Pan CY, Tian H, Li CL (2005) A comparison of urinary albumin excretion rate and microalbuminuria in various glucose tolerance subjects. Diabet Med 22(3):332–335. https://doi.org/10.1111/j.1464-5491.2004.01408.x
Griffin KA (2017) Hypertensive kidney injury and the progression of chronic kidney disease. Hypertension 70(4):687–694. https://doi.org/10.1161/hypertensionaha.117.08314
Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT (2010) Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 375(9731):2073–2081. https://doi.org/10.1016/s0140-6736(10)60674-5
Sun DQ, Jin Y, Wang TY, Zheng KI, Rios RS, Zhang HY, Targher G, Byrne CD, Yuan WJ, Zheng MH (2021) MAFLD and risk of CKD. Metabolism 115154433. https://doi.org/10.1016/j.metabol.2020.154433
Wijarnpreecha K, Thongprayoon C, Boonpheng B, Panjawatanan P, Sharma K, Ungprasert P, Pungpapong S, Cheungpasitporn W (2018) Nonalcoholic fatty liver disease and albuminuria: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol 30(9):986–994. https://doi.org/10.1097/meg.0000000000001169
Lambers Heerspink HJ, Gansevoort RT (2015) Albuminuria is an appropriate therapeutic target in patients with CKD: the pro view. Clin J Am Soc Nephrol 10(6):1079–1088. https://doi.org/10.2215/cjn.11511114
Reinhardt W, Mülling N, Behrendt S, Benson S, Dolff S, Führer D, Tan S (2021) Association between albuminuria and thyroid function in patients with chronic kidney disease. Endocrine 73(2):367–373. https://doi.org/10.1007/s12020-021-02640-1
Du X, Pan B, Li W, Zou Y, Hua X, Huang W, Wan X, Cao C (2017) Albuminuria is an independent risk factor of T4 elevation in chronic kidney disease. Sci Rep 741302. https://doi.org/10.1038/srep41302
Yang Z, Duan P, Li W, Nie R, Lou X, Wang L, Wu K, Liu J, Tu P, Lai X (2022) The correlation between thyroid hormone levels and the kidney disease progression risk in patients with type 2 diabetes. Diabetes Metab Syndr Obes 1559–67. https://doi.org/10.2147/dmso.S347862
Kwong N, Medici M, Marqusee E, Wassner AJ (2021) Severity of proteinuria is directly associated with risk of hypothyroidism in adults. J Clin Endocrinol Metab 106(2):e757–e762. https://doi.org/10.1210/clinem/dgaa872
Gondil VS, Chandrasekaran A, Rastogi A, Yadav AK, Sood A, Ramachandran R, Kumar V, Rathi M, Kohli HS, Jha V, Gupta KL (2021) Proteinuria in severe hypothyroidism: a prospective study. J Clin Endocrinol Metab 106(2):e749–e756. https://doi.org/10.1210/clinem/dgaa871
Mariani LH, Berns JS (2012) The renal manifestations of thyroid disease. J Am Soc Nephrol 23(1):22–26. https://doi.org/10.1681/asn.2010070766
Iglesias P, Díez JJ (2009) Thyroid dysfunction and kidney disease. Eur J Endocrinol 160(4):503–515. https://doi.org/10.1530/eje-08-0837
Yasuda T, Kaneto H, Kuroda A, Yamamoto T, Takahara M, Naka T, Miyashita K, Fujisawa K, Sakamoto F, Katakami N, Matsuoka TA, Shimomura I (2011) Subclinical hypothyroidism is independently associated with albuminuria in people with type 2 diabetes. Diabetes Res Clin Pract 94(3):e75–e77. https://doi.org/10.1016/j.diabres.2011.08.019
Toda A, Hara S, Tsuji H, Arase Y (2020) Subclinical hypothyroidism is associated with albuminuria in Japanese nondiabetic subjects. Endocrine 68(3):592–598. https://doi.org/10.1007/s12020-020-02220-9
Levy RP, Roess TJ (1956) Proteinuria and hypothyroidism. N Engl J Med 255(11):519–520. https://doi.org/10.1056/nejm195609132551108
Laclaustra M, Moreno-Franco B, Lou-Bonafonte JM, Mateo-Gallego R, Casasnovas JA, Guallar-Castillon P, Cenarro A, Civeira F (2019) Impaired sensitivity to thyroid hormones is associated with diabetes and metabolic syndrome. Diabetes Care 42(2):303–310. https://doi.org/10.2337/dc18-1410
Ahluwalia N, Dwyer J, Terry A, Moshfegh A, Johnson C (2016) Update on NHANES dietary data: focus on collection, release, analytical considerations, and uses to inform public policy. Adv Nutr 7(1):121–134. https://doi.org/10.3945/an.115.009258
Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, Pessah-Pollack R, Singer PA, Woeber KA (2012) Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract 18(6):988–1028. https://doi.org/10.4158/ep12280.Gl
Cho YT, Chen CW, Chen MP, Hu JL, Su H, Shiea J, Wu WJ, Wu DC (2013) Diagnosis of albuminuria by tryptic digestion and matrix-assisted laser desorption ionization/time-of-flight mass spectrometry. Clin Chim Acta 42076–81. https://doi.org/10.1016/j.cca.2012.12.016
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006
Zhang H, Che W, Shi K, Huang Y, Xu C, Fei M, Fan X, Zhang J, Hu X, Hu F, Qin S, Zhang X, Huang Q, Yu F (2022) FT4/FT3 ratio: a novel biomarker predicts coronary microvascular dysfunction (CMD) in euthyroid INOCA patients. Front Endocrinol (Lausanne) 131021326. https://doi.org/10.3389/fendo.2022.1021326
Wang C, Han S, Li Y, Tong F, Li Z, Sun Z (2022) Value of FT3/FT4 ratio in prognosis of patients with heart failure: a propensity-matched study. Front Cardiovasc Med 9859608. https://doi.org/10.3389/fcvm.2022.859608
Raets L, Minschart C, Van den Bruel A, Van den Bogaert E, Van Crombrugge P, Moyson C, Verhaeghe J, Vandeginste S, Verlaenen H, Vercammen C, Maes T, Dufraimont E, Roggen N, De Block C, Jacquemyn Y, Mekahli F, De Clippel K, Loccufier A, Laenen A, Devlieger R, Mathieu C, Decallonne B, Benhalima K (2022) Higher thyroid fT3-to-fT4 ratio is associated with gestational diabetes mellitus and adverse pregnancy outcomes. J Clin Med 11(17). https://doi.org/10.3390/jcm11175016
Türker F, Oral A, Şahin T, Türker B, Koçak E, Ataoğlu HE, Ahbab S (2021) Does the FT3-to-FT4 ratio easily predict the progression of NAFLD and NASH cirrhosis? J Int Med Res 49(11):3000605211056841. https://doi.org/10.1177/03000605211056841
Lang X, Li Y, Zhang D, Zhang Y, Wu N, Zhang Y (2022) FT3/FT4 ratio is correlated with all-cause mortality, cardiovascular mortality, and cardiovascular disease risk: NHANES 2007–2012. Front Endocrinol (Lausanne) 13964822. https://doi.org/10.3389/fendo.2022.964822
Liu B, Wang Z, Fu J, Guan H, Lyu Z, Wang W (2021) Sensitivity to thyroid hormones and risk of prediabetes: a cross-sectional study. Front Endocrinol (Lausanne) 12657114. https://doi.org/10.3389/fendo.2021.657114
Shi W, Song D, Chen D, Duan W, Zhang J (2023) The association of thyroid parameters with markers of chronic kidney disease in euthyroid patients with type 2 diabetes. Endocr J. https://doi.org/10.1507/endocrj.EJ22-0643
Yu L, Li Z, Yang R, Pan G, Cheng Q, He Y, Liu Y, Liu F, Ma M, Yang T, Wang Y, Su J, Zheng Y, Gao S, Xu Q, Li L, Yu C (2022) Impaired sensitivity to thyroid hormones is associated with elevated blood glucose in coronary heart disease. Front Endocrinol (Lausanne) 13895843. https://doi.org/10.3389/fendo.2022.895843
Ding X, Wang Y, Liu J, Wang G (2022) Impaired sensitivity to thyroid hormones is associated with elevated homocysteine levels in the euthyroid population. J Clin Endocrinol Metab 107(9):e3731–e3737. https://doi.org/10.1210/clinem/dgac371
Mehran L, Delbari N, Amouzegar A, Hasheminia M, Tohidi M, Azizi F (2022) Reduced Sensitivity to thyroid hormone is associated with diabetes and hypertension. J Clin Endocrinol Metab 107(1):167–176. https://doi.org/10.1210/clinem/dgab646
Han Z, Chen L, Peng H, Zheng H, Lin Y, Peng F, Fan Y, Xie X, Yang S, Wang Z, Yuan L, Wei X, Chen H (2023) The role of thyroid hormone in the renal immune microenvironment. Int Immunopharmacol 119110172. https://doi.org/10.1016/j.intimp.2023.110172
Acknowledgements
We wish to acknowledge all participants in this study and the support provided by Jiangsu University.
Funding
This study was supported by the Guang Ren Foundation Research Project of the Affiliated Hospital of Jiangsu University (KRY-YN2022017) and the Suzhou Science and Technology Planning Project (STL2021006).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The Research Ethics Review Board of the NCHS reviewed and approved this study involving human participants.
Informed consent
The patients/participants provided written informed consent for participation in this study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Z., Wu, M., Pan, T. et al. Impaired sensitivity to thyroid hormones is associated with albuminuria in the euthyroid population: results from NHANES. Hormones 23, 245–255 (2024). https://doi.org/10.1007/s42000-024-00533-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42000-024-00533-2







