Abstract
Purpose
The effect of exercise on stress has been demonstrated in several studies which have shown that exercise intensity and duration have various effects on the reproductive axis. This study evaluated the effect of different intensities and durations of exercise on the hormonal indices of stress, such as corticosterone (CORT), norepinephrine (NEP), and also reproductive performance indices, including gonadotropin-releasing hormone (GnRH), luteinizing hormone (LH), and testosterone (T).
Methods
In this experimental study, 30 adult Wistar rats were randomly divided into five groups as follows: no-exercise, RME-1 (regular moderate exercise for 1 month), RME-6 (regular moderate exercise for 6 months), RIE-1 (regular intensive exercise for 1 month), and RIE-6 (regular intensive exercise for 6 months). At the end of the experiment, the serum levels of the abovementioned hormones and hypothalamic expression of the Gnrh gene were measured using the enzyme-linked immunosorbent assay and the real-time polymerase chain reaction method, respectively.
Results
The levels of stress hormones, including CORT and NEP, increased only in the RIE-1 group compared with the no-exercise group. In addition, an increase was observed in T hormone levels in the RME-1 group compared with those in the no-exercise group, whereas LH and T hormone levels showed a greater decrease in the RIE-6 group than in the no-exercise group. Gnrh expression levels showed an increase and a decrease in the RME-1 and RIE-6 groups compared with the no-exercise group, respectively.
Conclusion
These results confirmed the effects of different intensities and durations of exercise on sex hormone levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last few decades, the physiological effects of exercise on the endocrine system have been confirmed. It has been shown that the hypothalamic-pituitary–gonadal (HPG) axis, depending on the intensity and duration of exercise, responds to physical activity. Although the effects of exercise on the reproductive axis have been extensively studied in females, these effects have not yet been fully examined in male subjects [1].
The type, duration, and intensity of exercise were found to have different clinical outcomes [2]. Several studies have assessed whether exercise can affect male fertility, some showing that the number of sperms and their ability to move decreased in response to exercise [1]. Moreover, it has been suggested that hypogonadotropic hypogonadism is very common among athletes [3].
Several reports have demonstrated that total and free levels of testosterone (T) are increased by physical activity [4]. It should be noted that the effect of exercise duration and intensity, as well as the type of exercise and the subject’s age on T levels, is paradoxical [4, 5]. For example, the total serum levels of T increased in male cyclists (8 h of exercise per week) compared with recreational athletes (who exercised less than 30 min on most days of the week). In contrast, low levels of T were observed in endurance and resistance athletes, soccer players, and amateur cyclists compared with healthy non-exercisers [1]. Furthermore, in a paraclinical study conducted by Bliss et al. (1972), low plasma testicular T levels were also observed in rats after swimming for 1 h [6]. However, running on a treadmill with a progressive 8 weeks protocol did not change basal serum T levels or testicular concentrations of T [7].
Previous studies have indicated that exercise as a stress stimulus [8] can increase the serum levels of cortisol [9], norepinephrine (NEP), and epinephrine (EP). An increase in the serum levels of cortisol is mediated by the hypothalamic–pituitary–adrenal (HPA) axis. Changes in cortisol levels are strongly related to the intensity of exercise.[10] It has been reported that cortisol levels increased during physical activity; however, other studies suggested that intensive exercise can prevent the secretion of this hormone. This inconsistency in results may be due to the differences in sampling time, as cortisol has a circadian rhythm [9].
While moderate and intensive exercise increased cortisol levels in blood, light exercise did not significantly increase blood levels [9]. In addition, an experiment conducted in rats showed that continuous exercise (i.e., more than 21 days) increased corticosterone (CORT) levels. In contrast, 21 and 42 days of exercise significantly decreased serum and hippocampal CORT levels [11].
Physical activity increases the activity of the adrenergic system, which is followed by an increase in plasma levels of catecholamine (NEP and EP) based on the intensity and duration of exercise [9]. NEP is one of the important transmitters that regulate LH secretion. NEP has both inhibitory and stimulatory effects on gonadotropin-releasing hormone (GnRH) neurons. Therefore, intracerebroventricular injection of NEP in the ovariectomized rats treated with exogenous estradiol stimulates GnRH neurons and increases LH secretion, whereas injection into the preoptic area of NEP inhibits LH secretion [12].
Regarding the inconsistencies in the effects of exercise on the HPG axis, in this study, the effect of moderate and intensive short- and long-term exercise on the levels of stress hormones and the HPG axis of adult male rats was investigated.
Materials and methods
Inclusion/exclusion criteria
Inclusion/exclusion criteria for this study were as follows:
Inclusion criteria
-
1.
Healthy rats.
-
2.
Adult male rats aged 2–3 months.
-
3.
Weight of over 200 g and lower than 300 g.
-
4.
No movement problems.
-
5.
Before the experiment, all rats were tested on a treadmill, and those able to run on the treadmill were selected.
-
6.
Male fertile rats (by mating with females).
Exclusion criteria
-
1.
Inability to move on the treadmill.
-
2.
Weight of more than 300 g.
-
3.
Weight of less than 200 g.
-
4.
Age of over 3 months.
Thirty male Wistar rats aged 2–3 months (250 ± 50 g) prepared by the animal house of the Shahrekord University of Medical Sciences, Shahrekord, Iran, were used in this experimental study and kept in individual cages. During the experimental period, the rats were maintained at a temperature of 22 °C under a 12-h light–dark cycle (7 AM to 7 PM) and had free access to water and food.
All animal experimental procedures were performed in accordance with the guidelines for the proper conduct of animal experiments issued by the Ethics Committee of the Shahrekord University of Medical Sciences and performed according to the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health, USA (NIH Publication No. 85–23, revised 1985).
Experimental design
The animals were randomly divided into five groups (n = 6) as follows: (1) no-exercise (with no intervention), (2) RME-1 (regular moderate exercise for 1 month), (3) RME-6 (regular moderate exercise for 6 months), (4) RIE-1 (regular intensive exercise for 1 month), and (5) RIE-6 (regular intensive exercise for 6 months). The body weight of the rats at the baseline and end of the experiment is presented in Table 1.
Training protocol
The experimental rats were exposed to running on a treadmill for 60 min/d and at a speed of 22 and 35 m/min during 5 consecutive days. First, the speed was set at 5 m/min to warm up and gradually increased to 22 m/min (in the moderate exercise group) and to 35 m/min (in the intensive exercise group). During the last 5 min, to cool down the rats, the speed decreased to 5 m/min. The rats in the no-exercise group were placed on the non-moving treadmill for 60 min. It should be noted that all exercised rats were treated before 12 AM [13].
Blood sampling and ELISA assay
Finally, all experimental rats were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (5 mg/kg) [14, 15] 24 h after the last day of exercise at 8 to 11 AM. Then, blood sampling was done from orbital sinus and the separated serum was used for the assessment of T (Cusabio-CSB-E05100r; sensitivity range: 0.06 ng/ml), luteinizing (E0179Ra-Bioassay Technology Laboratory; sensitivity range: 0.015mIU/ml), CORT (Cusabio-CSB-E07014r; sensitivity range: 0.1 ng/ml), and NEP (Bioassay Technology Laboratory-E00514Ra; sensitivity range: 2.51 ng/ml) hormones.
Real-time polymerase chain reaction (RT-PCR) technique
The rats were swiftly euthanized 24 h after the behavioral experiments and their hypothalamic arcuate nucleus was immediately removed, placed in liquid nitrogen, and frozen. Using YTzol Pure RNA buffer (Yekta Tajiz, Iran), all RNAs were extracted from the hypothalamic arcuate nucleus samples and their concentration and purity were then detected with the NanoDrop device, and using a reverse transcription kit (BIONEER), RNA underwent the process of reverse transcription. Triple reaction gene-specific primers are used to measure Gnrh mRNA expression with cDNA samples. The sequences of primers and thermal schedule used in this study are presented in Tables 2 and 3. Next, RT-PCR was performed using SYBR Green PCR Master Mix (Takara Bio Inc.). Finally, real-time quantitative PCR analysis was performed using the comparative CT method (2−ΔΔCT method) and the arithmetic formula was used to measure the relative expression of target mRNAs above the reference values [16].
Data analysis
Statistical analysis was performed using SPSS version 16.0 for Windows (SPSS Inc., Chicago, IL, USA). Comparison among the various groups was performed using nonparametric tests (Kruskal–Wallis followed by pairwise multiple comparison procedures (Dunn’s test) because of the small sample size (i.e., six rats per group). The results are presented using the median (min, max) and the significance level was set at 0.05 in all analyses.
Results
The changes in CORT levels in response to exercise
Statistical analysis using Kruskal–Wallis followed by Dunn’s test showed significant differences between groups (P = 0.002; Fig. 1). Specifically, there was a significant decrease in serum levels of CORT in the RME-1 group (P = 0.047) and a significant increase in the RIE-1 group (P = 0.040) compared with the no-exercise group. Moreover, a significant increase in the RIE-1 group was observed compared with the RME-6 (P = 0.047) group. Furthermore, the levels of CORT in the RME-6 (P = 0.040), RIE-1 (P < 0.001), and RIE-6 (P = 0.015) groups significantly increased compared with the RME-1group. However, no significant difference was observed in the RME-6 (P = 0.948) and RIE-6 (P = 0.657) groups compared with the no-exercise group (Fig. 1). The CORT levels are presented as median (min, max) in Table 4.
Changes in serum levels of corticosterone between the RME-1 (regular moderate exercise for 1 month), RIE-1 (regular intensive exercise for 1 month), RME-6 (regular moderate exercise for 6 months), and RIE-6 (regular intensive exercise for 6 months) groups (n = 6). *P < 0.05, **P < 0.01, and ***P < 0.001 in comparison with the no-exercise group; #P < 0.05, ##P < 0.01, and ###P < 0.001 in comparison with the RME-1 group; $P < 0.05, $$P < 0.01, and $$$P < 0.001 in comparison with the RIE-1 group. Data are expressed as median (min, max) and assessed by the Kruskal–Wallis test followed by Dunn’s multiple comparisons test
Changes in NEP levels in response to exercise
The comparison of exercise duration effects on the levels of NEP showed that there was a significant difference between groups (P = 0.002; Fig. 2). Specifically, there was a significant increase in the RIE-1 group compared with the no-exercise (P < 0.001) and RME-1 (P = 0.002) groups. Furthermore, this parameter was found to be significantly decreased in the RIE-6 (P = 0.008) and RME-6 (P = 0.024) groups compared with the RIE-1 group.
Changes in serum levels of norepinephrine (NEP) between the RME-1 (regular moderate exercise for 1 month), RIE-1 (regular intensive exercise for 1 month), RME-6 (regular moderate exercise for 6 months), and RIE-6 (regular intensive exercise for 6 months) groups (n = 6). *P < 0.05,**P < 0.01, and ***P < 0.001 in comparison with the no-exercise group; #P < 0.05, ##P < 0.01, and ###P < 0.001 in comparison with the RME-1 group; $P < 0.05, $$P < 0.01, and $$$P < 0.001 in comparison with the RIE-1 group. Data are expressed as median (min, max) and assessed by the Kruskal–Wallis test followed by Dunn’s multiple comparisons test
However, no significant difference was observed in the RME-6 (P = 0.115) and RIE-6 (P = 0.238) groups compared with the no-exercise group (Fig. 2). The NEP levels are presented as median (min, max) in Table 4.
Changes in LH levels in response to exercise
A significant difference was observed in LH serum levels in the experimental groups (P = 0.001; Fig. 3). Dunn’s test indicated a significant reduction in the levels of LH in the RIE-6 group compared with the no-exercise group (P = 0.019). Moreover, LH serum levels significantly decreased in the RIE-6 (P < 0.001) and RIE-1(P = 0.001) groups compared with the RME-1 group. The levels of LH in the RME-6 group were significantly decreased compared with those of the RME-1 group (P = 0.012). However, a significant difference was not observed in the RME-6 (P = 0.512) and RIE-1 (P = 0.184) groups compared with the no-exercise group (Fig. 3). The LH serum levels are presented as median (min, max) in Table 4.
Changes in serum levels of luteinizing hormone (LH) between the RME-1 (regular moderate exercise for 1 month), RIE-1 (regular intensive exercise for 1 month), RME-6 (regular moderate exercise for 6 months), and RIE-6 (regular intensive exercise for 6 months) groups (n = 6). *P < 0.05, **P < 0.01, and ***P < 0.001 in comparison with the no-exercise group; #P < 0.05, ##P < 0.01, and ###P < 0.001 in comparison with the RME-1 group. Data are expressed as median (min, max) and assessed by the Kruskal–Wallis test followed by Dunn’s multiple comparisons test
Changes in T levels in response to exercise
The results of the Kruskal–Wallis test showed a statistically significant difference in T serum levels between the experimental groups (P = 0.001; Fig. 4). Pairwise comparisons using Dunn’s test indicated a significant difference between the no-exercise group and RME-1 (P = 0.024) and RIE-6 (P = 0.048) groups. Moreover, the levels of T in the RIE-6 group significantly decreased compared with those of RIE-1 (P = 0.003) and RME-1 (P < 0.001) groups. T serum levels significantly decreased in the RME-6 group in comparison with those of RME-1 group (P = 0.023; Fig. 4). The serum levels of T are presented as median (min, max) in Table 4.
Changes in serum levels of testosterone (T) hormone between the RME-1 (regular moderate exercise for 1 month), RIE-1 (regular intensive exercise for 1 month), RME-6 (regular moderate exercise for 6 months), and RIE-6 (regular intensive exercise for 6 months) groups (n = 6). *P < 0.05, **P < 0.01, and ***P < 0.001 in comparison with the no-exercise group; #P < 0.05, ##P < 0.01, and ### P < 0.001 in comparison with the RME-1 group; $P < 0.05, $$P < 0.01, and $$$P < 0.001 in comparison with the RIE-1 group. Data are expressed as median (min, max) and assessed by the Kruskal–Wallis test followed by Dunn’s multiple comparisons test
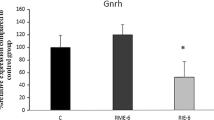
Changes in Gnrh expression in response to exercise
A significant difference was observed between the experimental groups in terms of changes in Gnrh expression levels (P < 0.001; Fig. 5). Pairwise comparisons using Dunn’s test indicated that the level of Gnrh expression significantly increased in the RME-1 group (P = 0.016) and decreased in the RIE-6 (P = 0.029) group compared with the no-exercise group. Furthermore, the level of Gnrh expression in the RIE-6 group significantly decreased compared with the RME-6 (P = 0.003) and RME-1 (P < 0.001; Fig. 5) groups. Also, the expression level of Gnrh significantly decreased in the RIE-1 group compared with the RME-1 group (P = 0.001). The relative values of Gnrh expression are presented as median (min, max) in Table 4.
Changes in the relative expression of Gnrh between the RME-1 (regular moderate exercise for 1 month), RIE-1 (regular intensive exercise for 1 month), RME-6 (regular moderate exercise for 6 months), and RIE-6 (regular intensive exercise for 6 months) groups (n = 6). *P < 0.05,**P < 0.01, and ***P < 0.001 in comparison with the no-exercise group; #P < 0.05, ##P < 0.01, and ###P < 0.001 in comparison with the RME-1 group. Data are expressed as median (min, max) and assessed by the Kruskal–Wallis test followed by Dunn’s multiple comparisons test
Discussion
In the present study, serum levels of CORT and NEP (as biomarkers of stress responses), LH, T, and hypothalamic mRNA levels of Gnrh were measured in response to various types of exercise. Studying hormonal changes in response to exercise is of great importance since it has been revealed that exercise stimulates changes in the neuroendocrine system and basal concentrations of stress hormones (CORT and NEP) in response to physical activity [17]. Previous studies have indicated that, depending on oxygen consumption, exercise has different effects on the HPA axis and modulates CORT secretion and reproductive ability [18].
The results of the present study showed that RME-1 significantly reduced CORT levels compared with the no-exercise group, which is consistent with previous findings. The beneficial effects of moderate and regular exercise on reducing stress have long been known [19]. For example, regular and moderate-intensity physical activity (such as 8 weeks of walking) reduced urine cortisol levels in depressed women and improved the subjects’ depression [20]. In addition, moderate-intensity physical activity reduced serum cortisol levels leading to stimulating neuroplasticity and serotonin production in rats exposed to stress [21, 22].
Duration and intensity of exercise have different effects on stress hormone levels [17]. Light and moderate exercise reduced the levels of cortisol (in humans) and CORT (in rodents) [19]. On the other hand, other researchers have shown that by increasing the intensity of exercise, the levels of stress hormones are increased [23]. Moreover, there was a significant increase in CORT levels in the RIE-1 group compared with the no-exercise group, which is consistent with our results. However, intense exercise did not increase cortisol levels during the daytime hours [24]. This inconsistency may be due to the sampling time in different studies and cortisol circadian rhythm [9].
The results of the present study showed that CORT levels are affected by the pattern of exercise intensity. Thus, after moderate-intensity exercise, this level decreased, and following an increase in the intensity of exercise, a significant increase was observed. Furthermore, there was no difference in CORT levels between the RME-6 and RIE-6 groups compared with the no-exercise group. This finding confirmed that the HPA axis does not change in response to moderate long-term (i.e., a fixed form of exercise for a long time) and intense exercise [17]. The results of the present investigation showed that an increase in exercise duration, regardless of exercise intensity, may alter HPA axis adaptation, as the serum levels of CORT did not change in the rat exercise groups compared with those of the no-exercise rats.
In addition, in our investigation, serum levels of NEP (as a biomarker of the stress response) were measured in response to exercise. NEP is known to be an indicator of sympathetic system function [25] and exercise activates the sympathetic nervous system. NEP can alter the release of GnRH and LH hormones, which affects reproductive activity.
Based on our results, RME-1 could not increase the level of NEP hormone compared with the no-exercise group. Also, other studies have shown that catecholamine levels did not increase in the low-intensity exercise group compared with the no-exercise group [26], and our results confirmed this finding. However, RIE-1 treatment increased the concentration of NEP compared with the no-exercise group. There is a relationship between exercise intensity and plasma levels of NEP [26]. As the intensity of exercise increases, the levels of NEP are increased. These findings are consistent with the results of the present study.
The sympathetic nervous system is one of the factors that regulate the body’s metabolic and physiological responses and is necessary for the energy required by the muscles involved in exercise [26]. During intense exercise, the muscles need more oxygen and substrate; therefore, the sympathetic nervous system becomes more active. In this study, RIE-1 treatment increased serum levels of NEP probably by stimulating the nervous system. The obtained results showed that in both types of intensity, increased duration of exercise prevented significant changes in NEP levels compared with the no-exercise group. In other words, the serum levels of NEP showed no significant increase in the RME-6 and RIE-6 groups compared with the no-exercise group. This finding demonstrated that the nervous system is adapted to long-term exercise because, by an increase in the duration of exercise, the HPA axis and plasma concentration of NEP remain stable [17]. Long-term exercise increases the half-life of this hormone (in the heart and spleen), while causing a reduction in NEP ability for regeneration (turnover) and reducing the activity of peripheral noradrenergic neurons during intensive exercise, important factors for a constant level of peripheral NEP [27]. Serum levels of LH and T hormones are considered indicators of reproductive ability, which were considered in this research. One of the strengths of this study was examining the effects of different types of exercise on the serum levels of these hormones, given that there are inconsistencies regarding their serum levels following different types of exercise.
Our results indicated that RME-1 increased the serum levels of T compared with the no-exercise group. Moreover, RIE-6 treatment decreased the serum levels of T and LH compared with the no-exercise group. Previous studies have shown that intense exercise reduced reproductive ability [28], LH and T levels [1, 29], and the quality of semen [28]. Therefore, our results confirmed that moderate exercise has beneficial effects on reproductive function [1, 28]. However, some studies have shown a marked reduction in sperm count, motility, morphology, and viability after the swimming exercise in male Sprague–Dawley rats. Furthermore, the serum levels of reproductive hormones, i.e., T and LH, significantly decreased and histological examination of the testes and epididymis revealed defective spermatogenesis [30]. A possible cause of a reduction in testicular weight could be decreased food intake [31, 32].
The RME-1 treatment increased the relative expression of Gnrh, whereas RIE-6 decreased it. It has been suggested that acute high-intensity exercise (> 60% of maximal aerobic capacity) can increase the circulation of cortisol through its inhibitory effect on GnRH and LH hormones, which is followed by a decrease in T secretion from Leydig cells [33]. Although there is no certain consensus, acute and chronic exercise can suppress endocrine functions at the hypothalamic and testicular levels, specifically, the suppression of GnRH and T [34, 35].
Some studies have shown that chronic swimming exercise suppressed the kisspeptin-GnRH signaling pathway, leading to a reduction in the production of male reproductive hormones [30].
Conclusion
Overall, the results of this study indicated that increasing exercise duration returned the NEP and CORT serum levels closer to the no-exercise group levels. Consequently, the present finding demonstrates the ability of the sympathetic nervous system and HPA axis to adapt to long-term exercise. However, exercise intensity and duration have different effects on the reproductive axis. Further, sexual behavioral studies are needed to confirm the various impacts of different exercise protocols on the male reproductive axis.
Data availability
The datasets generated and/or analyzed during the current study are available.
References
Cano Sokoloff N, Misra M, Ackerman KE (2016) Exercise, training, and the hypothalamic-pituitary-gonadal axis in men and women. Front Horm Res. 47:27–43
Clark JE (2016) The impact of duration on effectiveness of exercise, the implication for periodization of training and goal setting for individuals who are overfat, a meta-analysis. Biol Sport 33(4):309–333
Pauli SA, Berga SL (2010) Athletic amenorrhea: energy deficit or psychogenic challenge? Ann N Y Acad Sci 1205:33–38
Kumagai H et al (2018) Vigorous physical activity is associated with regular aerobic exercise-induced increased serum testosterone levels in overweight/obese men. Horm Metab Res 50(1):73–79
Steeves JA et al (2016) Cross-sectional association between physical activity and serum testosterone levels in US men: results from NHANES 1999–2004. Andrology 4(3):465–472
Bliss EL, Frischat A, Samuels L (1972) Brain and testicular function. Life Sciences. 11(5, Part 1):231–238
Härkönen M et al (1990) Pituitary and gonadal function during physical exercise in the male rat. J Steroid Biochem 35(1):127–132
Chen JX et al (2007) Influence of acute and chronic treadmill exercise on rat plasma lactate and brain NPY, L-ENK, DYN A1–13. Cell Mol Neurobiol 27(1):1–10
Hill EE et al (2008) Exercise and circulating cortisol levels: the intensity threshold effect. J Endocrinol Invest 31(7):587–591
Anderson T, Wideman L (2017) Exercise and the cortisol awakening response: a systematic review. Sports medicine - open 3(1):37–37
Radahmadi M et al (2015) Effect of forced exercise and exercise withdrawal on memory, serum and hippocampal corticosterone levels in rats. Exp Brain Res 233(10):2789–2799
Szawka RE et al (2013) Release of norepinephrine in the preoptic area activates anteroventral periventricular nucleus neurons and stimulates the surge of luteinizing hormone. Endocrinology 154(1):363–374
Molaei A et al (2020) Synergistic effects of quercetin and regular exercise on the recovery of spatial memory and reduction of parameters of oxidative stress in animal model of Alzheimer’s disease. EXCLI J 8(19):596–612
Shahidi S, et al (2018) Intracerebroventricular microinjection of the 5-HT1F receptor agonist LY 344864 inhibits methamphetamine conditioned place preference reinstatement in rats. Pharmacol Biochem Behav 173(1873–5177 (Electronic)):27–35
Shahidi S, et al (2019) Different doses of methamphetamine alter long-term potentiation, level of BDNF and neuronal apoptosis in the hippocampus of reinstated rats. J Physiol Sci. 69(2)(1880–6562 (Electronic)):409–419
Khajehnasiri N, Khazali H, Sheikhzadeh F (2018) Various responses of male pituitary–gonadal axis to different intensities of long-term exercise: Role of expression of KNDY-related genes. J Biosci 43(4):569–574
Hackney AC (2006) Stress and the neuroendocrine system: the role of exercise as a stressor and modifier of stress. Expert Rev Endocrinol Metab 1(6):783–792
St-Pierre D, Richard D (2020) The Effect of Exercise on the Hypothalamic-Pituitary-Adrenal Axis. In: Hackney AC, Constantini NW (eds) Endocrinology of Physical Activity and Sport. Springer International Publishing, Cham, pp 41–54
Sandlund ES, Norlander T (2000) The effects of Tai Chi Chuan relaxation and exercise on stress responses and well-being: an overview of research. Int J Stress Manag 7(2):139–149
Nabkasorn C et al (2006) Effects of physical exercise on depression, neuroendocrine stress hormones and physiological fitness in adolescent females with depressive symptoms. Eur J Public Health 16(2):179–184
Jiang P et al (2014) The impacts of swimming exercise on hippocampal expression of neurotrophic factors in rats exposed to chronic unpredictable mild stress. Evidence-Based Complementary and Alternative Medicine 2014:729827
Liu W et al (2013) Swimming exercise ameliorates depression-like behavior in chronically stressed rats: relevant to proinflammatory cytokines and IDO activation. Behav Brain Res 242:110–116
Bobbert T et al (2005) Adaptation of the hypothalamic-pituitary hormones during intensive endurance training. Clin Endocrinol (Oxf) 63(5):530–536
Tsatsoulis A, Fountoulakis S (2006) The protective role of exercise on stress system dysregulation and comorbidities. Ann N Y Acad Sci 1083:196–213
Christensen NJ, Galbo H (1983) Sympathetic nervous activity during exercise. Annu Rev Physiol 45:139–153
Greiwe JS et al (1999) Norepinephrine response to exercise at the same relative intensity before and after endurance exercise training. J Appl Physiol (1985) 86(2):531–5
Ostman I, Nybäck H (1976) Adaptive changes in central and peripheral noradrenergic neurons in rats following chronic exercise. Neuroscience 1(1):41–47
Vaamonde D et al (2009) Response of semen parameters to three training modalities. Fertil Steril 92(6):1941–1946
Wang O (2012) Glucocorticoids Regulate Kisspeptin Neurons during Stress and Contribute to Infertility and Obesity in LeptinDeficient Mice. Doctoral dissertation. Harvard University
Arisha AH, Moustafa A (2019) Potential inhibitory effect of swimming exercise on the Kisspeptin-GnRH signaling pathway in male rats. Theriogenology 133:87–96
Bakos HW et al (2011) The effect of paternal diet-induced obesity on sperm function and fertilization in a mouse model. Int J Androl 34(5 Pt 1):402–410
Ghanayem BI et al (2010) Diet-induced obesity in male mice is associated with reduced fertility and potentiation of acrylamide-induced reproductive toxicity. Biol Reprod 82(1):96–104
Alves J, Toro V, Barrientos G, Bartolome I, Munoz D, Maynar, (2020) Hormonal Changes in High-Level Aerobic Male Athletes during a Sports Season. Int. J Environ Res Public Health 17(16):5833
Kujala UM, Alen M, Huhtaniemi IT (1990) Gonadotrophin-releasing hormone and human chorionic gonadotrophin tests reveal that both hypothalamic and testicular endocrine functions are suppressed during acute prolonged physical exercise. Clin Endocrinol (Oxf) 33(2):219–225
Vaamonde D, Garcia-Manso JM, Hackney AC (2017) Impact of physical activity and exercise on male reproductive potential: a new assessment questionnaire. Revista andaluza de medicina del deporte 10(2):79–93
Funding
The authors thank the Shahrekord University of Medical Sciences for the preparation of laboratory equipment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All animal experimental procedures were performed in accordance with the guidelines for the proper conduct of animal experiments issued by the Ethics Committee of the Shahrekord University of Medical Sciences and performed according to The Guide for Care and Use of Laboratory Animals published by the National Institutes of Health, USA (NIH Publication No. 85–23, revised 1985).
Conflicts of interests
The authors declare no competing interests.
Competing financial interests
The authors declare no competing financial interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khajehnasiri, N., Dehkordi, M.B., Amini-Khoei, H. et al. Effect of exercise intensity and duration on the levels of stress hormones and hypothalamic-pituitary–gonadal axis in adult male rats: an experimental study. Hormones 20, 483–490 (2021). https://doi.org/10.1007/s42000-021-00303-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42000-021-00303-4