Key summary points
To develop a comprehensive open-source measurement guide of the most prevalent chronic conditions among persons aged 65+ based on registry data of both diagnoses and prescribed drugs [“The Chronic Condition Measurement Guide (CCMG)].
AbstractSection FindingsBased on the Danish population aged 65 years and older we developed the CCMG identifying 83 different chronic conditions based on registry data of both diagnoses and prescribed drugs. By applying the CCMG to a large national cohort of all Danish citizens aged 65 or above, we found that the prevalence of multimorbidity ranged from 10 to 69% using different years of history and in- or excluding information about drug prescribing.
AbstractSection MessageThe CCMG is easily implemented using registry data and we recommend using 10 years of history and drug prescribing information.
Abstract
Purpose
The aim of the study was to develop a comprehensive open-source measurement guide of the most prevalent chronic conditions among persons aged 65+ based on registry data of both diagnoses and prescribed drugs [the chronic condition measurement guide (CCMG)]. Furthermore, to investigate proof of concept of the measurement guide, different years of history and in- and excluding data on prescribed drugs. Finally, to investigate the measurement guide with other measurement guides designed to identify chronic conditions in persons aged 65+.
Methods
The measurement guide was based on the 200 most prevalent chronic ICD10 codes in the Danish population 65+ years in 2015; the 200 most prevalent chronic ICD10 codes and causes of death in a cohort of 209,337 people who died of non-traumatic causes (January 2011–January 2016). Prescribed drugs were included in the measurement guide based on a literature review and specialist opinions.
Results
We identified 83 different chronic conditions based on 1241 unique ICD-10 codes. Multimorbidity prevalence ranged from 10% (1-year history, excluding prescribing information) to 69% (15-year history, including prescribing information). We identified 95% of the persons with multimorbidity using the 29 most prevalent chronic conditions. Inclusion of these 29 conditions affected the prevalence of multimorbidity and 1-year mortality when the CCMG was compared with other measurement guides.
Conclusion
The CCMG is easily implemented using registry data. When implementing the measurement guide 10 years of history and drug prescribing information should be used. Using the CCMG to study multimorbidity, we recommend using at least the 29 most prevalent chronic conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, the population aged 65 years and older is rising and nearly all chronic conditions are strongly related to aging, which have become the biggest challenge for medical and health care systems. Multimorbidity (the existence of two or more chronic conditions within an individual [1, 2]) is a well-established challenge in healthcare today and needs to be addressed to adapt the healthcare resources in the future. Recently, it has been pointed out that there is need for a deeper understanding of the concept beyond counting conditions [3]. Identifying subgroups or patterns of multimorbidity seems promising in the attempt to capture the complexity that lies within multimorbidity. To identify patterns, the chronic diseases underlying the analysis must be defined. At present no uniform or general accepted tool exist which describes and clearly defines multimorbidity, i.e., what it consists of. Hence, pertaining to this notion, the definition of multimorbidity is heterogenous [4, 5] and patterns of multimorbidity vary widely from study to study [6, 7].
For a measure of multimorbidity to be useful for health practitioners, researchers and policy makers the tool must be applicable to electronic health data and have a precise definition of the selected chronic conditions. To measure chronic conditions several approaches have been made [8,9,10,11,12,13]. However, only a few measurement guides have been developed aimed for the older population. Rizzuto et al. and van den Bussche et al. developed measurement guides identifying chronic conditions in older persons based on 38 and 46 chronic conditions, respectively, but only based the definition of chronic conditions on diagnosis codes [14, 15]. Calderón-Larrañaga et al. [16] developed an measurement guide to identify chronic conditions using both diagnosis codes as well as drug prescribing information based on 60 chronic conditions. Nevertheless, the drugs used to identify conditions were not systematically identified. Additionally, the number of years to base the prevalence of multimorbidity on varies across the three before mentioned studies, ranging from 1 to 30 years of history.
Hence, to facilitate a clearer understanding of what multimorbidity precisely consists of there is a need for developing a comprehensive open-source measurement guide to describe the chronic conditions being prevalent today not only regarding registered diagnoses (commonly used) but also regarding drug prescribing information to be used in public health care planning. The aim of the study was, therefore, to develop “the chronic condition measurement guide (CCMG)”, a comprehensive open-source measurement guide of the most prevalent chronic conditions among persons aged 65 years and older based on registry data of both diagnoses and prescribed drugs. Further, to investigate proof of concept of the measurement guide. Finally, to investigate the measurement guide with other measurement guides designed to identify chronic conditions in persons aged 65+.
Materials and methods
Design
To develop “the chronic condition measurement guide (CCMG)”, among persons aged 65 years and older, we performed a nationwide register study in which we included 15 years of history in health care registers for each person. In addition, we investigated proof of concept by applying the CCMG to a large national cohort and differences between the CCMG and other measurement guides designed to identify chronic conditions in persons aged 65 years and older. The study is reported according to the STROBE checklist, using the extension for cross-sectional studies [17].
Setting
Denmark has a public tax-financed health care system, which provides free and equal access to treatment for primary medical care and hospitals, for all people. Patient co-payments are required for prescription drugs [18]. All Danish residents have a uniquely personal and permanent identification number that makes it possible to crosslink data across the different national registries.
Population
Two cohorts were included in this study. Cohort 1 consisted of all Danish citizens alive January 1st 2016 aged 65 or above with no migration status during 2015. Cohort 1 consisted of 1,083,689 persons with a median age of 73 (Interquartile range 69;79) years and 54% were women. To assess the most prevalent conditions in the oldest and most ill persons, we included Cohort 2. Cohort 2 consisted of all Danish citizens, who died from non-traumatic causes in the period from 1st of January 2011 until 1st of January 2016. Persons were included in Cohort 2 if they at time of death were over the age of 65 years and had been living in Denmark for the past 15 years. Cohort 2 consisted of 209,337 people with a median age of 83 (Interquartile range 75;89) years and 53% were women. Cohort 1 and 2 were used for selecting the chronic conditions included in the measurement guide, whereas only Cohort 1 was used in the subsequently analyses.
Data collection
For both cohorts, the following data were collected: (1) Somatic and psychiatric diagnosis codes (both primary and secondary) from The Danish National Patient Register, which contains data on all Danish patients’ hospital contacts including both hospitalizations and out-patient visits (most of these are performed in hospitals in Denmark) [19]. Diagnoses are registered using the ICD-10 system [20]; (2) Prescription drugs from The National Prescription Registry, which contains information on all prescription drugs dispensed at Danish community pharmacies including prescriptions for nursing home residents. Drugs given during hospitalizations and over the counter drugs were not included in the registry [18]. Prescription drugs are in this system coded according to the Anatomical Therapeutic Chemical (ATC) classification system by WHO17 [21]; (3) Information on immigration and emigration from The Register for Migration [22]; (4) Diagnosis codes from The Danish Register of Causes of Death (COD), which contains data on all deaths among citizens dying in Denmark [23]; and (5) Information on sex, date of birth and vital status from The Civil Registration System [24].
Development of chronic condition measurement guide
The development of the chronic condition measurement guide (CCMG) was twofold. Firstly, we developed a list of ICD-10 codes referring to chronic conditions. Secondly, we developed a list of these chronic conditions based on ATC-codes. The two lists were then combined to constitute the CCMG. The following steps were used to develop a list of ICD-10 codes referring to chronic conditions:
Step 1: The chronicity of all the diagnoses in Cohort 1 and 2 was assessed using The Healthcare Cost and Utilization Project’s, Chronic Condition Indicator tool (CCI) [25]. The procedure and its translation to European ICD-10 codes is described elsewhere [26]. In short, the CCI is a dichotomized assessment of United States ICD-10 codes as “chronic” or “non-chronic”.
Step 2: To assess the most prevalent conditions in the general population, we identified the 200 most prevalent chronic ICD-10 codes in Cohort 1 using 10 years of history for each person. To assess the most prevalent conditions in the oldest and most ill persons, we identified the 200 most prevalent ICD-10 codes in Cohort 2 registered using 10 years of history for each person. Finally, to assess the most prevalent causes of death we identified the 200 most prevalent causes of death (ICD-10 codes) in Cohort 2. A list of all the different ICD-10 codes was made and these were finally grouped according to different chronic conditions by the authors: HJ-L and OA.
Step 3: To increase the face validity of the construct of the conditions a medical specialist in internal medicine and the co-authors compared the existing conditions and ICD-10 codes found in Step 2 with the International Statistical Classification of Diseases and Health Related Problems to see if related ICD-10 codes were to be included in the list. For example, if C158 and C159 were identified in Step 2 the ICD-10 codes C150, C151, C152, C153, C154, and C155 were added to “Malignant neoplasm of the esophagus”.
Step 4: To ensure that no relevant ICD-10 codes were missed, we identified all the chronic ICD-10 codes registered for the past 10 years in Cohort 1 that were not included in the measurement guide and compared the ICD-10 codes with the identified chronic conditions. For example, was thrombosis of the vena renalis (I823) added to “Chronic kidney disease” and emphysema interstitial pulmonum (J982) added to “Interstitial pulmonary disease”.
To identify all the ATC-codes that uniquely could be used to identify the chronic conditions identified in Step 2, the following steps were performed:
Step 5: We performed a systematic search using the terms: “(“Anatomical Therapeutic Chemical classification system” OR “ATC”) AND (“ICD10” OR “chronic disease” OR “chronic condition”)” in combination with the filter: “Language = English”. The search was carried out on 15 August 2018 in PubMed, EMBASE (via Ovid), and the Cochrane Library, evidence for healthcare decision-making. In addition, reference searches were carried out to identify other relevant studies. Only studies linking ATC-codes with a chronic condition were eligible. ATC-codes linked to chronic conditions were derived and structured in a list. Medical specialists in Cardiology, in Geriatric medicine, in Internal Medicine, in Respiratory Medicine and three physicians face validated the list of ATC-codes linked to chronic conditions based on experience in the field. They were asked to identify relationships between ATC-code and chronic conditions defined by ICD-10 codes, with only one indication for the specific ATC-code. The medical specialists were blinded from each other’s answers. In case of differences in answers, the authors HJL and LDC investigated the drugs in a database containing information about drugs and treatment instructions for doctors, pharmacists, and other health professionals. In the database, we investigated the indications for the drug. If the indication and the drug did not match the chronic condition, we consulted a medical specialist in geriatric medicine and in internal medicine. A decision was then made based on consensus. In case of doubt the drug was excluded.
Step 6: The list of ICD-10 codes and the list of ATC-codes were combined to constitute the CCMG.
To demonstrate proof of concept of the CCMG, we applied the measurement guide to Cohort 1 using different years of history and in- and excluding drug prescribing information.
Other measurement guides
We compared the prevalence of multimorbidity (defined as 1+, 2+, 3+, 5+ and 10+ chronic conditions) and age, sex and 1-year mortality for patients with multimorbidity using the CCMG with three open-source measurement guides identifying chronic conditions in people aged 65 years and older from health records. The three measurement guides were (the Calderón-Larrañaga-measurement guide; the Rizzuto-measurement guide; and the van den Bussche-measurement guide). Calderón-Larrañaga et al. [16] developed an operational measure of multimorbidity in the population of 60 chronic disease categories including 918 ICD10-codes based on a consensus definition of chronic disease from the ICD10. This operational measure is henceforth named the Calderón-Larrañaga-measurement guide. Rizzuto et al. [14] developed a list of 38 chronic conditions of which six was defined using clinical data and 32 were defined using administrative data (ICD-10 codes) based on the prevalence of chronic conditions in a population aged 75 and older. This list is henceforth named the Rizzuto-measurement guide. A list of 46 chronic conditions was developed by van den Bussche et al. [15] based on the prevalence of the most frequent conditions in general practitioners in a population of people aged 65 and older [15]. This list is henceforth named the van den Bussche-measurement guide.
Ethics approval and informed consent
This study has been approved by The Data Protection Agency (Project no. 704775 at Statistics Denmark). No approval from The Danish Research Ethics Committees for The Capital Region was needed since only national registry data have been used.
Statistical methods
Data are presented as numbers and percentages or as medians depending of the nature of the variables. To study the influence of using different years of history on CCMG the prevalence of all the chronic conditions identified in the measurement guide was calculated using 1, 5, 10, 15 years of history in the registries, respectively, and using both diagnosis and medication information and only using diagnosis information. Moreover, the prevalence of persons with 1+, 2+, 3+, 5+, and 10+ chronic conditions were calculated based on the same datasets. We plotted the percentage of people with multimorbidity—defined as having at least two, three, five or ten chronic conditions—against the number of chronic conditions included in CCG. We did this to study the influence of the number of chronic conditions included in the measure of multimorbidity on the prevalence of persons with multimorbidity. The chronic conditions were ordered according to prevalence, e.g., the number 20 on the x-axis refers to the 20 most prevalent number of chronic conditions. Moreover, we estimated the number of chronic conditions used to identify 95% of the people with multimorbidity. A cumulative incidence plot with 1-year follow-up was used to compare un-adjusted survival among different definitions of multimorbidity. The statistical analyses were conducted using SAS 9.4 software package for Windows. The cumulative incidence plots were created in R version 3.5.0.
Results
Development of the chronic condition measurement guide
After collapsing the 200 most prevalent diagnose codes from each of the three settings we identified 342 unique ICD-10 codes in step 2, 19 were later excluded because they were considered to be of minor relevance for developing the CCMG (G562, G931, G935, G936, H431, I249, I460, I469, I480, I493, K589, M202, M204, R990, Z018, Z966) e.g. intracranial space-occupying lesion or cardiac arrest with successful resuscitation are covered by other ICD10-codes and do not contribute further to morbidity. In total, we identified 83 chronic conditions containing 1241 ICD10-codes after supplementing the diagnose codes in Step 3 (Suppl. Table 1). We were able to identify 64 of the chronic conditions from the 200 most prevalent ICD10-codes in Cohort 1, 63 from Cohort 2, and 58 from the 200 most prevalent reasons for dying in Cohort 2. Of the 83 chronic conditions, 40 were identified in all three datasets.
The search for studies linking ATC-codes to chronic conditions generated a total of 220 hits; PubMed (138 hits), EMBASE (via Ovid) (78 hits), and the Cochrane Library, evidence for healthcare decision-making (four hits). After removing duplicates and conducting a title and abstract screening, 14 studies were eligible for full-text assessment. Five studies were excluded because they did not meet the inclusion criteria of linking chronic disease and ATC-codes. In total, nine studies were included [27,28,29,30,31,32,33,34,35]. The medical specialists were able to link 20 chronic conditions with 136 ATC-codes (Additional file Table S1).
Differences in the prevalence of chronic conditions
The prevalence of multimorbidity, defined as having two or more chronic conditions, ranged from 10% (1 year of history without drug prescribing information) to 69% (using 15 years of history concerning drug prescribing information) (Table 1). The prevalence of multimorbidity did not change substantially when looking at 10 vs. 15 years of history both in- and excluding medication [68% vs. 69% (including drug prescribing information) and 51% vs. 52% (excluding drug prescribing information)]. Additionally, when looking at the single chronic conditions the prevalence did not change more than 1.7% point (CCMG56 atherosclerosis) when comparing 10 and 15 years of history with medication (Additional file Table S2). However, the prevalence of multimorbidity was influenced when including information on drug prescribing the prevalence changed from 51 to 68% using 10 years of history.
The three single chronic conditions with the largest prevalence differences was hypertension [5% (1 year) vs. 25% (15 years)], disorders of the eyes and ears (7% vs. 21%) and osteoarthritis (2% vs. 15%) (Additional file Table S2). Additionally, we found differences in the three most prevalent chronic conditions among patients with multimorbidity depending on the use of drug prescription information. When including drug prescribing information, disorders of the lipoprotein metabolism (CCMG31) was most prevalent, whereas hypertension (CCMG48) was most prevalent when excluding drug prescribing information (Table 1). Moreover, the prevalence of the single conditions were influenced by the use of drug prescribing information in CCMG: The three single chronic conditions most affected by the use of drug prescribing information (10 years) was disorders of the lipoprotein metabolism [9% (excluding drug prescribing information) vs. 46% (including drug prescribing information)], atherosclerosis (5% vs. 38%) and hyperplasia of prostate (4% vs. 11%) (Additional file Table S2).
Number of conditions needed to measure multimorbidity
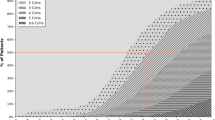
Figure 1 shows the percentage of people with multimorbidity defined as having at least two, three, five or ten chronic conditions in relation to the number of the most prevalent chronic conditions from the CCMG included in the definition of multimorbidity. In total, 736,869 people (68%) had multimorbidity using the definition of at least two chronic conditions among all 83 chronic conditions. When including the 29 most prevalent chronic conditions we were able to identify 95% of the persons with multimorbidity. In addition, when defining multimorbidity as at least three, five or ten chronic conditions we needed 37, 47, and 63 chronic conditions to identify 95% of the persons with multimorbidity (identified using all 83 chronic conditions).
Differences among different measurement guides
Figure 2 shows the prevalence of chronic conditions for the four different measurement guides using 10 years history and including medication. The CCMG had the highest prevalence of the different number of chronic conditions and the Rizzuto-measurement guide had the lowest. The characteristics of populations with different numbers of chronic conditions based on the four different measurement guides using 10 years of history and drug prescribing information are shown in Table 2. When looking at the patient chrematistics in the population with two or more chronic conditions, there was no differences between the four measurement guides regarding age, sex or number of redeemed prescriptions within the last year. There was, however, differences regarding the most prevalent chronic conditions. The three most prevalent chronic conditions in the Calderón-Larrañaga- and the van den Bussche-measurement guide both included conditions from the ICD10 chapters: Diseases of the musculoskeletal system and connective tissue, diseases of the eye and adnexa and, diseases of the ear and mastoid process, whereas the CCMG only included conditions from the ICD10 chapter; diseases of the circulatory system. The Rizzuto-measurement guide was the only measurement guide where neoplasms were included in the three most prevalent chronic conditions. In all four measurement guides, there was a tendency of higher number of drugs as the number of chronic conditions rose. In the van den Bussche-measurement guide, the proportion of females increased with the higher number of chronic conditions, whereas the proportion decreased in the three other measurement guides (Table 2). Figure 3 shows the cumulative incidence of mortality within a year in persons with different numbers of chronic conditions for the four measurement guides. The different numbers of chronic conditions are similar associated with 1-year mortality, except for the Rizzuto-measurement guide, which seems to be strongly associated with 1-year mortality.
Prevalence of persons with chronic conditions from the chronic condition measurement guide; the chronic condition measurement guide based on the 29 most prevalent chronic conditions; the Calderón-Larrañaga-measurement guide; the Rizzuto-measurement guide; and the van den Bussche-measurement guide (n = 1,083,689)
Discussion
Based on the Danish population aged 65 years and older we developed the chronic condition measurement guide (CCMG) identifying 83 different chronic conditions based on registry data of both diagnoses and prescribed drugs. This is a tool to be used when studying chronic conditions including subgroups or patterns to capture this complex phenomenon. By applying the CCMG to a large national cohort of all Danish citizens aged 65 years or above, we found that the prevalence of multimorbidity ranged from 10 to 69% using different years of history and in- or excluding information about drug prescribing. In addition, the number of chronic conditions used to define multimorbidity and, hence, the number of ICD-10 codes and or ATC codes affected the outcomes. Furthermore, we were able to identify 95% of the persons with multimorbidity (2+ chronic conditions) using only the 29 most prevalent chronic conditions. When comparing the measurement guide with other existing measurement guides, we found a higher prevalence of multimorbidity in the CCMG, reflecting inclusion of chronic conditions seen in general practice. In addition, the Rizzuto-measurement guide had a lower prevalence of multimorbidity and seemed to be stronger associated with 1-year mortality.
We found that the use of different years of history to estimate chronic conditions strongly affected the extent of multimorbidity. In this study, the prevalence of multimorbidity when excluding drug prescribing information is based on health records from out-patient visits as well as from hospitalizations, both acute and planned. Hence, registration of secondary diagnoses is often not performed in a hospital setting leading to an under-registration of chronic conditions, especially conditions primarily treated at the general practitioner [36]. Using 10 years of history increases the likelihood of more registrations, which leads to a higher prevalence of chronic conditions. Using 15 years of history did not increase the prevalence suggesting that 10 years of history seem sufficient when studying multimorbidity. This is in accordance with Schram et al. who in a general practitioner setting found a higher prevalence of multimorbidity using a 10-year prevalence vs. a 5-year prevalence, though they did not evaluate the prevalence of multimorbidity using 15 years of history [36].
As we expected, the use of drug prescription information changed the prevalence of the chronic conditions as well as the prevalence multimorbidity even further. The three single chronic conditions most affected using drug prescribing information was disorders of the lipoprotein metabolism, atherosclerosis, and hyperplasia of prostate. These three conditions are typically identified and treated by the general practitioner and hence, not registered systematically at the hospital. The use of drug prescription information seems to give a more precise measure of the construct multimorbidity when using healthcare registers. Fortin et al. found that the use of a multi-source method is preferable to a single-source method which is in accordance with our findings [37]. We, therefore, recommend using both a 10-year prevalence as well as drug prescribing information when studying multimorbidity using data from health records. If this is not possible we give an estimate on how much the prevalence of multimorbidity in Denmark is affected by drug prescribing information and the use of different years of history to be accounted for in future studies.
We included chronic conditions based on prevalence both from people aged 65 years and older, but also from a cohort of people who had died to gain a comprehensive picture of older peoples’ chronic conditions—not only of people who are at different stages of life but also of people who have lived a full life and have a full history of chronic conditions. This approach turned out to be effective as only 64 of the chronic conditions were identified from Cohort 1. The Calderón-Larrañaga-measurement guide is based on the ICD-10 codes from the entire ICD-10 catalog and not by looking at data, henceforth they have included chromosomal abnormalities that is a rare condition in a population of older people and have a prevalence of 0.0 in their own study [16]. By including chronic conditions in the CCMG based on their prevalence, we believe that the list is more clinically relevant than the lists based on expert opinion as the former represents chronic conditions seen by the doctors in the clinic. Furthermore, the CCMG adds to the Calderón-Larrañaga-measurement guide by including drug prescribing information especially on disorders of the lipoprotein metabolism, atherosclerosis, and cardiac arrhythmias based on a large national dataset reflecting chronic conditions primarily seen in general practice. These highly prevalent chronic conditions are primarily treated in general practice and is, therefore, often underestimated when using registry data based on hospital admissions. This is especially of importance when using the list to study the association to the underlying pathophysiology, i.e., patterns of multimorbidity. Identification of patterns of multimorbidity is of great importance as the finding not only generate new hypotheses on the shared pathophysiology or biological processes of the chronic conditions, but also essential information on how to adapt guidelines and treatment for people with multimorbidity [6, 38].
Our study indicates that choosing the most prevalent chronic conditions to base your analysis on could be more sufficient than the total number of chronic conditions. To identify 95% of the people with multimorbidity, 29 most prevalent chronic conditions from the CCMG are needed. This estimate captures the most prevalent chronic conditions and those conditions that have the highest impact on the prevalence and the patterns of multimorbidity. Finding the most influential chronic conditions to base studies of multimorbidity on will help comparison across studies. Fortin et al. has suggested using a list of at least 12 chronic conditions based on a systematic review [37] and another recent systematic review found that using 25–74 chronic conditions yielded the maximal prevalence of multimorbidity [39], which is well within the 29 conditions found to contain 95% of the persons with multimorbidity in this study. These 29 chronic conditions can also explain the difference in the prevalence of multimorbidity among the four included measurement guides. The Calderón-Larrañaga-measurement guide and the van den Brussche-measurement guide contain all and 27 of the 29 most prevalent chronic conditions from the CCMG, respectively, whereas the Rizzuto-measurement guide only contains 20 chronic conditions of the 29 most prevalent chronic conditions from the CCMG and only two of the top five conditions.
When comparing the four measurement guides we found that the Rizzuto-measurement guide had a lower prevalence of chronic conditions, a stronger association with 1-year mortality and a higher no. of redeemed prescriptions within the last year. This is probably caused by the diversity in the included chronic conditions in the four measurement guides. A recent systematic review investigating the role of diseases, risk factors and symptoms in the definition of multimorbidity found that the inclusion of chronic conditions based on risk factors contributes to higher prevalence estimates of multimorbidity [4]. The Rizzuto-measurement guide have included fewer chronic conditions based on risk factors such as disorders of the lipoprotein metabolism, which is the most prevalent chronic condition when applying the CCMG to Cohort 1, obesity and hearing impairments (as this was operationalized by clinical examination). Hence, the Rizzuto-measurement guide have included more disease-based chronic conditions which are stronger associated with mortality as risk factor-based chronic conditions are. Nevertheless, the CCMG includes a large variety of both diseases, risk factors and symptoms and it has been suggested that using symptoms in the definition of multimorbidity brings out the patients’ perspective thus making the measure of multimorbidity more usable for patients and clinicians [4].
In the CCMG we have chosen to divide cancer into 22 single chronic conditions (Additional file Table S1). This can lead to a higher prevalence of multimorbidity than if all cancer conditions were collapsed into one condition. It can, however, be argued that if we study the complexity of multimorbidity, i.e., patterns of multimorbidity you need to account for the fact that there are different risk factors for different types of cancer. By collapsing the different cancer types, one loose valuable knowledge when studying patterns of multimorbidity where several cancer types might fall into different patterns.
Strengths and limitations
The main strengths of this study include the large unselected population, no risk of recall bias because of the use of register data, and access to high-quality data going back 15 years. The use of high-quality registry data is not available in all countries at the present time, but there is a global trend towards using large-scale data to obtain knowledge on, e.g., public health. The main limitations include lack of diagnoses given by general practitioners, lack of the use of laboratory data, and the possible use of chronic conditions diagnosed 10–15 years ago and not replicated later. The Danish national registries do not include diagnoses given by general practitioners, which could lead to misclassification of chronic conditions. Nevertheless, by including drug prescription information we have included data that mostly come from general practitioners in the primary care setting. Furthermore, by collapsing the ICD10-codes into conditions, the risk of misclassification decreases. We could have used laboratory data to define the chronic conditions as a supplement to the ICD10- and the ATC-codes to increase the validation of the chronic conditions. We believe, however, that using data from two different cohorts and different sources, the chronic conditions included in the CCMG capture the chronic conditions seen in the clinic. Nonetheless, there is always a risk of underestimating multimorbidity from registry data as not all people consult the doctor. To ease calculation of measurement guide, we used the same period for all the chronic conditions, though one could argue that some chronic diagnoses given 10 years ago and not replicated later is not of importance any longer and can lead to risk of misclassification. Furthermore, the diagnoses identified from the out-patient visits are based on trajectories with very long durations and some trajectories can be started in the time frame used in this study, but not be finished at the 1st of January 2016 and hereby, risking the inclusion of chronic conditions diagnosed after the inclusion date. We did not stratify out findings based on sex. However, as the prevalence of men and women are almost similar, we do not believe the selection of the chronic conditions in the CCMG is affected. Finally, this study was carried out in Denmark, a high-income country. The selected chronic conditions in the CCMG cannot be generalized to populations of low- and middle-income countries and the results should be interpreted accordingly.
Applicability of the chronic condition measurement guide
In the past decades, multimorbidity has become a major challenge for the healthcare systems worldwide. A development that seems to continue. Being able to develop prevention and treatment strategies to improve the trajectories for patients with multimorbidity is of great importance. However, a prerequisite for examining the effect of the treatment of patients with multimorbidity is to understand their complexity and to be able to compare results across countries. One of the most comprehensive measures is the John Hopkins Adjusted Clinical Groups Case-mix System [13]. It is a risk-adjustment system that predicts individuals’ future health and healthcare utilization. Unfortunately, this index is not open-source and hence, not free of charge. The CCMG is a standardized tool that can be easily implemented in many western countries using ICD-10 and ATC codes and applied to datasets with or without drug prescribing information. In addition, the open-source nature makes it highly feasible. The need to better understand patterns of chronic conditions among older people will grow in the upcoming years. This is caused by the aging population and the rising number of people with multiple chronic conditions will lead to patient trajectories that are increasingly complex and challenging to manage. Large-scale data combined with machine learning techniques, which can handle complexity, might enhance our ability to better understand multimorbidity. This understanding can inform us on how best to target our limited health care resources, which would benefit both patients and the healthcare system.
In conclusion, we developed “The Chronic Condition Measurement guide (CCMG)”, a comprehensive open-source measurement guide of the most prevalent chronic conditions among persons aged 65 years and older based on registry data of both diagnoses and prescribed drugs. The clinically relevant measurement guide is distinct in its use of drug prescribing information where knowledge is gained on chronic conditions primarily treated in the primary sector and its use of prevalent chronic conditions. The CCMG is easily implemented using registry data and performs well when compared to other measurement guides. Based on these results, we recommend using at least the 29 most prevalent chronic conditions, a 10-year prevalence, and drug prescribing information, when using the CCMG to study chronic conditions using registry data.
Data availability
The Danish national registries are protected by the Danish Act on Processing of Personal Data and can only be assessed following formal approval.
References
Nicholson K, Makovski TT, Griffith LE, Raina P, Stranges S, van den Akker M (2019) Multimorbidity and comorbidity revisited: refining the concepts for international health research. J Clin Epidemiol 105:142–146. https://doi.org/10.1016/j.jclinepi.2018.09.008
van den Akker M, Buntinx F, Knottnerus JA (1996) Comorbidity or multimorbidity. Eur J Gen Pract 2:65–70. https://doi.org/10.3109/13814789609162146
Marengoni A, Vetrano DL, Onder G (2019) Target population for clinical trials on multimorbidity: is disease count enough? J Am Med Dir Assoc 20:113–114. https://doi.org/10.1016/j.jamda.2018.10.012
Willadsen TG, Bebe A, Køster-Rasmussen R, Jarbøl DE, Guassora AD, Waldorff FB et al (2016) The role of diseases, risk factors and symptoms in the definition of multimorbidity—a systematic review. Scand J Prim Health Care 34:112–121. https://doi.org/10.3109/02813432.2016.1153242
Diederichs C, Berger K, Bartels DB (2011) The measurement of multiple chronic diseases—a systematic review on existing multimorbidity indices. J Gerontol A Biol Sci Med Sci 66:301–311. https://doi.org/10.1093/gerona/glq208
Prados-Torres A, Calderón-Larrañaga A, Hancco-Saavedra J, Poblador-Plou B, van den Akker M (2014) Multimorbidity patterns: a systematic review. J Clin Epidemiol 67:254–266. https://doi.org/10.1016/j.jclinepi.2013.09.021
Violan C, Foguet-Boreu Q, Flores-Mateo G, Salisbury C, Blom J, Freitag M et al (2014) Prevalence, determinants and patterns of multimorbidity in primary care: a systematic review of observational studies. PLoS One 9:e102149. https://doi.org/10.1371/journal.pone.0102149
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Elixhauser A, Steiner C, Harris DR, Coffey RM (1998) Comorbidity measures for use with administrative data. Med Care 36:8–27
Linn BS, Linn MW, Gurel L (1968) Cumulative illness rating scale. J Am Geriatr Soc 16:622–626
Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH et al (1992) Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res 41:237–248. https://doi.org/10.1016/0165-1781(92)90005-N
Parkerson GR, Broadhead WE, Tse CK (1993) The Duke severity of illness checklist (DUSOI) for measurement of severity and comorbidity. J Clin Epidemiol 46:379–393
Salisbury C, Johnson L, Purdy S, Valderas JM, Montgomery AA (2011) Epidemiology and impact of multimorbidity in primary care: a retrospective cohort study. Br J Gen Pract J R Coll Gen Pract 61:e12–e21. https://doi.org/10.3399/bjgp11X548929
Rizzuto D, Melis RJF, Angleman S, Qiu C, Marengoni A (2017) Effect of chronic diseases and multimorbidity on survival and functioning in elderly adults. J Am Geriatr Soc 65:1056–1060. https://doi.org/10.1111/jgs.14868
van den Bussche H, Koller D, Kolonko T, Hansen H, Wegscheider K, Glaeske G et al (2011) Which chronic diseases and disease combinations are specific to multimorbidity in the elderly? Results of a claims data based cross-sectional study in Germany. BMC Public Health 11:101. https://doi.org/10.1186/1471-2458-11-101
Calderón-Larrañaga A, Vetrano DL, Onder G, Gimeno-Feliu LA, Coscollar-Santaliestra C, Carfí A et al (2017) Assessing and measuring chronic multimorbidity in the older population: a proposal for its operationalization. J Gerontol A Biol Sci Med Sci 72:1417–1423. https://doi.org/10.1093/gerona/glw233
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP et al (2007) Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 335:806–808. https://doi.org/10.1136/bmj.39335.541782.AD
Pottegård A, Schmidt SAJ, Wallach-Kildemoes H, Sørensen HT, Hallas J, Schmidt M (2017) Data resource profile: the danish national prescription registry. Int J Epidemiol 46:798-798f. https://doi.org/10.1093/ije/dyw213
Lynge E, Sandegaard JL, Rebolj M (2011) The Danish national patient register. Scand J Public Health 39:30–33. https://doi.org/10.1177/1403494811401482
World Health Organization (2014) International statistical classification of diseases and related health problems 10th revision. Available at: http://apps.who.int/classifications/icd10/browse/2015/en. Accessed 20 Jan 2019
WHO Collaborating Centre for Drug Statistics Methodology (2017) Guidelines for ATC classification and DDD assignment 2018. World Health Organization, Oslo, Norway. https://www.whocc.no/atc_ddd_index/. Accessed 20 Jan 2019
Norredam M, Kastrup M, Helweg-Larsen K (2011) Register-based studies on migration, ethnicity, and health. Scand J Public Health 39:201–205. https://doi.org/10.1177/1403494810396561
Helweg-Larsen K (2011) The danish register of causes of death. Scand J Public Health 39:26–29. https://doi.org/10.1177/1403494811399958
Pedersen CB (2011) The Danish civil registration system. Scand J Public Health 39:22–25. https://doi.org/10.1177/1403494810387965
Perrin EC, Newacheck P, Pless IB, Drotar D, Gortmaker SL, Leventhal J et al (1993) Issues involved in the definition and classification of chronic health conditions. Pediatrics 91:787–793
Klausen HH, Petersen J, Bandholm T, Juul-Larsen HG, Tavenier J, Eugen-Olsen J et al (2017) Association between routine laboratory tests and long-term mortality among acutely admitted older medical patients: a cohort study. BMC Geriatr 17:62. https://doi.org/10.1186/s12877-017-0434-3
Kuo RN, Dong Y-H, Liu J-P, Chang C-H, Shau W-Y, Lai M-S (2011) Predicting healthcare utilization using a pharmacy-based metric with the WHO’s Anatomic Therapeutic Chemical algorithm. Med Care 49:1031–1039. https://doi.org/10.1097/MLR.0b013e31822ebe11
Vivas-Consuelo D, Usó-Talamantes R, Trillo-Mata JL, Caballer-Tarazona M, Barrachina-Martínez I, Buigues-Pastor L (2014) Predictability of pharmaceutical spending in primary health services using clinical risk groups. Health Policy Amst Neth 116:188–195. https://doi.org/10.1016/j.healthpol.2014.01.012
Vivas D, Guadalajara N, Barrachina I, Trillo J-L, Usó R, de-la Poza E (2011) Explaining primary healthcare pharmacy expenditure using classification of medications for chronic conditions. Health Policy Amst Neth 103:9–15. https://doi.org/10.1016/j.healthpol.2011.08.014
Halfon P, Eggli Y, Decollogny A, Seker E (2013) Disease identification based on ambulatory drugs dispensation and in-hospital ICD-10 diagnoses: a comparison. BMC Health Serv Res. https://doi.org/10.1186/1472-6963-13-453
Pratt NL, Kerr M, Barratt JD, Kemp-Casey A, Kalisch Ellett LM, Ramsay E et al (2018) The validity of the Rx-risk comorbidity index using medicines mapped to the anatomical therapeutic chemical (ATC) classification system. BMJ Open 8:e021122. https://doi.org/10.1136/bmjopen-2017-021122
Lamers LM, van Vliet RCJA (2004) The Pharmacy-based cost group model: validating and adjusting the classification of medications for chronic conditions to the Dutch situation. Health Policy Amst Neth 68:113–121. https://doi.org/10.1016/j.healthpol.2003.09.001
Huber CA, Szucs TD, Rapold R, Reich O (2013) Identifying patients with chronic conditions using pharmacy data in Switzerland: an updated mapping approach to the classification of medications. BMC Public Health 13:1030. https://doi.org/10.1186/1471-2458-13-1030
Chini F, Pezzotti P, Orzella L, Borgia P, Guasticchi G (2011) Can we use the pharmacy data to estimate the prevalence of chronic conditions? A comparison of multiple data sources. BMC Public Health 11:688. https://doi.org/10.1186/1471-2458-11-688
Johansen, NB, Lykke, MB, Bekker-Jeppesen, M, Buhelt, LP, Allesoe, K, Andreasen, AH, et al. [Sundhedsprofil for Region Hovedstaden og kommuner 2017—Kronisk sygdom. In English: Health profile for the Capitol Region of Denmark and municipalities 2017—Chronic disease] In Danish. Centre for Clinical Research and Prevention, University Hospital of Bispebjerg and Frederiksberg, The Capitol Region of Denmark. 2018. https://www.regionh.dk/fcfs/sundhedsfremme-og-forebyggelse/Documents/Sundhedsprofil_2017_Kronisk%20sygdom.pdf. Accessed 12 Dec 2018
Schram MT, Frijters D, van de Lisdonk EH, Ploemacher J, de Craen AJM, de Waal MWM et al (2008) Setting and registry characteristics affect the prevalence and nature of multimorbidity in the elderly. J Clin Epidemiol 61:1104–1112. https://doi.org/10.1016/j.jclinepi.2007.11.021
Fortin M, Stewart M, Poitras M-E, Almirall J, Maddocks H (2012) A systematic review of prevalence studies on multimorbidity: toward a more uniform methodology. Ann Fam Med 10:142–151. https://doi.org/10.1370/afm.1337
Marengoni A, Rizzuto D, Wang H-X, Winblad B, Fratiglioni L (2009) Patterns of chronic multimorbidity in the elderly population. J Am Geriatr Soc 57:225–230. https://doi.org/10.1111/j.1532-5415.2008.02109.x
Holzer BM, Siebenhuener K, Bopp M, Minder CE (2017) Evidence-based design recommendations for prevalence studies on multimorbidity: improving comparability of estimates. Popul Health Metr 15:9. https://doi.org/10.1186/s12963-017-0126-4
Acknowledgements
The Authors like to thank Henrik Hedegaard Klausen MD, PhD, Beata Malmqvist MD, PhD, Ejvind Frausing MD, Ane Kathrine Skielbo MD, PhD, Thomas Huneck Haupt MD, PhD for validating the drugs used in the Chronic Condition Measurement Guide.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest in this work.
Ethical approval
This study has been approved by The Data Protection Agency (Project no. 704775 at Statistics Denmark). No approval from The Danish Research Ethics Committees for The Capital Region was needed since only national registry data have been used.
Informed consent
As this is a registry study based on national registers no informed consent exists.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Juul-Larsen, H.G., Christensen, L.D., Andersen, O. et al. Development of the “chronic condition measurement guide”: a new tool to measure chronic conditions in older people based on ICD-10 and ATC-codes. Eur Geriatr Med 10, 431–444 (2019). https://doi.org/10.1007/s41999-019-00188-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41999-019-00188-y