Abstract
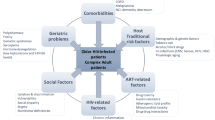
Polypharmacy is a well-described problem in the geriatric population. It is a relatively new problem for people living with HIV (PLWH), as this group now has a life expectancy approaching that of the general population. Defining polypharmacy for PLWH is difficult, since the most common traditional definition of at least five medications would encompass a large percentage of PLWH who are on antiretrovirals (ARVs) and medications for other medical comorbidities. Even when excluding ARVs, the prevalence of polypharmacy in PLWH is higher than the general population, and not just in resource-rich countries. Using a more nuanced approach with “appropriate” or “safer” polypharmacy allows for a better framework for discussing how to mitigate the associated risks. Some of the consequences of polypharmacy include adverse effects of medications such as increased risk of geriatric syndromes, drug–drug interactions, decreased adherence, and over- and undertreatment of medical comorbidities. Interventions to combat polypharmacy include decreasing pill burden—specifically with fixed-dose combination tablets—and medication reconciliation/de-prescription using established criteria. The goal of these interventions is to decrease drug interactions and improve quality of life and outcomes. Some special populations of interest within the community of PLWH include those with chronic pain, substance abuse, or requiring end of life care. A final look into the future of antiretroviral therapy shows the promise of possible two-drug regimens, which can help reduce the above risks of polypharmacy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide, it was estimated that in 2017, approximately 6.7 million people aged 50 and older were living with HIV [1]. In 2015, approximately 47% of PLWH in the United States (US) were aged 50 and older, according to the CDC [2]. This number is expected to rise as the life expectancy of PLWH approaches that of non-infected individuals [3]. Polypharmacy, a well-described problem in the geriatric population, is a concern in the aging population of PLWH, as well. It is crucial for providers to understand the unique risks of polypharmacy within this community and how to mitigate them. This will allow for the effective treatment of HIV and medical comorbidities and the prevention of harm.
Definition
Polypharmacy denotes the prescription of multiple medications [4], but defining it is challenging; a systematic review in 2017 found 138 definitions [5]. The meaning of polypharmacy often depends upon the purpose for which the term is being used. Most commonly, a quantitative definition is used, as this is conceptually simple and supported by a literature that documents an increased risk of complications with increased number of medications [5]. A second conceptualization of polypharmacy centers is around medication choice. In this case, the question is a more complicated one: irrespective of total number of medications, is each drug justified and safely administered? Table 1 lists typical definitions and resources. A more comprehensive list and comparison of appropriateness measures can be found in Whitman et al. [6].
Several things are noteworthy:
A quantitative definition may seem easy to measure and has the advantage of an extensive literature. Nonetheless, when viewing polypharmacy quantitatively, patients and providers may have very different perspectives. The patient may be most concerned about pill burden, while the provider may be most concerned about total number of medications irrespective of dosing frequency or mode of administration. Providers may forget to ask about over-the-counter medications or complementary therapies and underestimate medication burden.
The use of multiple medications does not necessarily indicate poor prescribing, but rather may merely be an indicator of multimorbidity [12]. It does not factor in medication toxicity or potency; older PLWH may meet quantitative definitions of polypharmacy merely by taking a few over-the counter medications along with their antiretroviral medication. Medication appropriateness may be a more viable construct and a more achievable goal, especially in the setting of HIV.
To that end, Duerden et al. [4] distinguish between problematic (as defined in Table 1) and appropriate polypharmacy, which they define as “prescribing for an individual for complex conditions or for multiple conditions in circumstances, where medicines use has been optimised and where the medicines are prescribed according to best evidence.” This framework is relevant to all PLWH. Age, frailty, functional and cognitive status, and multimorbidity should be considered when determining the optimal regimen.
Prevalence
For the purposes of this paper, we will use the quantitative definition of polypharmacy to evaluate prevalence. Even within this context, the prevalence of polypharmacy depends on the definition. Most studies exclude antiretroviral medications from the definition, which enables more realistic comparisons to uninfected populations, but does not reflect the frame of reference of PLWH. Fortunately, the antiretroviral therapy (ART) pill burden among PLWH has decreased drastically over the past decades. A Canadian study showed that among PLWH of all ages, the average number of antiretroviral (ARV) pills (among 365 PLWH) was 10 per day in 1998 compared to 3.4 per day in 2010 (among 1419 PLWH) [13]. Despite a decrease in pill burden in recent years, many patients are on single-tablet regimens, which include three active medications and possibly a pharmacokinetic enhancer, or “booster.”
Several studies have examined prevalence of polypharmacy in PLWH. According to the 2011 Swiss Cohort study, 14.2% of the 450 PLWH over 65 were taking at least 4 different non-ART medications [14]. An examination of polypharmacy and comorbidity in the Italian GEPPO cohort (aged ≥ 65) defined polypharmacy as taking five or more non-ART medications. They found that polypharmacy was present in 37% of the HIV-infected cohort compared to 24% in controls. In addition, this study demonstrated that the risk of polypharmacy increased for those greater than 75 and with longer exposure to HIV, rising to 43% for those who had been diagnosed with HIV for at least 20 years [15].
The Veterans Aging Cohort study of 7200 veterans in the US noted that among those older than 50, PLWH were taking an average of 7 medications, while uninfected counterparts were taking an average of 5. Of PLWH, 55% were taking 5 or more medications (which included ART). This likely underestimates the actual number, as only prescribed medications were counted [16]. While the prevalence of polypharmacy was much higher in this study than prior ones, it still demonstrated a higher rate of polypharmacy in PLWH than in those without HIV, even when excluding ART. When assessing what additional medications PLWH take compared to those without HIV, a Spanish study of 8172 PLWH aged 50 years or older showed that PLWH are prescribed more CNS medications and anti-infectives, but similar amounts of cardiovascular (CV) drugs [17].
Polypharmacy is not restricted to resource-rich countries. In Uganda, where the prevalence of HIV in those aged >=50 is 4%, the rate of polypharmacy in 411 PLWH from one clinic was approximately 15%. Polypharmacy was more common among those who had seen a physician and was not associated with adverse events, reflecting the possibility that polypharmacy in part may be an indicator of access to care [18].
Consequences
Even though most definitions of polypharmacy exclude ARVs, many of the negative outcomes of polypharmacy are a consequence of interactions between ARVs and medications used to treat comorbidities. The following table divides the most common ARVs by drug class and details the adverse effects, geriatric considerations, and major drug interactions (Table 2). A more exhaustive list with specific drug interactions is available online—Liverpool HIV Drug Interactions [19]. The US Department of Health and Human Services (DHHS) website contains a chart of drug interactions between ARV class and commonly prescribed medications [20].
One of the most serious consequences of polypharmacy in this population is drug interactions. In one study of PLWH that included 159 people of all ages in Liverpool, clinically significant drug interactions were recorded in 27%, with 15% of interactions potentially lowering antiretroviral concentrations. Risk of clinically significant drug interactions was significantly related to receipt of protease inhibitors. Only 36% of clinically significant drug interactions were correctly identified by physicians [30].
One can analyze drug interactions further by category (defined by Lexicomp®)—category D means to consider therapy modification, while category X interactions would be completely avoided. Using these criteria, 70% of 89 PLWH aged 60 or older in a San Francisco area study had at least 1 category D drug–drug interaction (DDI), while 11% had a category X interaction. A clinical pharmacist determined 60% of interactions to be clinically significant. Approximately half of the interactions were between ART and non-ART medications, and 35% were between non-ART [31]. One study of 3810 PLWH over 50-year-old living in Liverpool showed that 7% of PLWH had at least one ARV/non-ARV combination that was contraindicated and a third with moderate or high evidence of interaction. The medications that were most often involved included PPIs, statins, and benzodiazepines [32].
As seen in the chart above, pharmacokinetic enhancers, or “boosting” medications, such as ritonavir and cobicistat, which are strong inhibitors of CYP3A4, are among those drugs with the highest risk for interactions [33], see Table 3 for more specific interactions.
Another complication of polypharmacy is medication nonadherence. Although controversial in the literature, a systematic review of studies with rigorous designs found that four out of the five studies examined showed an association between polypharmacy and a greater risk of nonadherence [34]. Polypharmacy has been associated with decreased adherence to ART [35]. However, this is not seen universally. In the Uganda study discussed above, there was no impact on adherence or clinical outcomes [18]. Adverse events are the most frequent reason for first-line antiretroviral therapy discontinuation/switch. Among 1096 PLWH in Italy of all ages, there was a higher rate of discontinuation of ARV secondary to side effects in older PLWH [36].
Polypharmacy increases the risk of geriatric syndromes such as falls, confusion, delirium, and cognitive decline in the general population. A study of 46,946 people of all ages in the US showed that in older individuals with diabetes, taking greater than 4 medications was associated with an increased risk of falls [37]. Another study of 395 PLWH in Colorado ages 45–65 determined that the odds of falling is increased by 1.7 for each comorbidity and 1.4 for each medication [38]. In the geriatric population, there is an association between impaired cognition, difficulty with daily tasks, and polypharmacy for those taking ≥ 10 medications based on 1000 people from the GeMS Study data in Finland [39].
For other systemic side effects unrelated to the geriatric population, a study of 661 people of all ages in Boston found that the number of medications taken was significantly associated with adverse events [40]—the details of possible side effects are shown in Table 2.
Overtreatment is the underlying concern about polypharmacy. However, undertreatment is also an area of concern for PLWH who have high-risk comorbidities. For example, most models of risk for heart disease are based on risk factors that go into the Atherosclerotic Cardiovascular Disease (ASCVD) risk algorithm, which does not include other pro-inflammatory states, such as HIV [41]. The incidence of cancer, liver disease, and cardiovascular disease is higher in treated PLWH than in age-matched HIV-uninfected people [42]. Furthermore, the rate of CV events is higher in untreated PLWH than in treated patients, which is likely related to higher levels of inflammation. IL-6, hsCRP, and D-dimer are three markers of inflammation that are elevated in PLWH and are associated with mortality and CV disease [43]. With this said, treated PLWH still have elevated biomarkers and have higher risk of CV events. For those on protease inhibitors, there are higher rates of hyperlipidemia, insulin resistance, and CV morbidity.
Among people in the Veterans Aging Cohort Study who met NCEP/ATP III criteria for lipid lowering therapy, HIV-infected veterans had a significantly lower prevalence for the receipt of lipid lowering therapy—approximately 60% lower as compared with HIV-uninfected veterans [44]. This shows that even when comparing standard risk factors for CV disease, PLWH were undertreated. Potential interactions between ART and lipid lowering medications may account for some of this discrepancy. When taking into account the fact that PLWH are at higher risk for CV disease in general, the rate of undertreatment is likely higher.
Interventions
Decreasing pill burden can help mitigate polypharmacy in aging PLWH. One way to do this is to encourage fixed-dose combination (FDC) tablets. However, the actual number of active medications may or may not change when these changes are made. Although the previous single-tablet regimens were less likely to be prescribed in those with polypharmacy possibly due to perceived risk of drug–drug interactions (Modena HIV Metabolic Clinic Cohort Study among 2944 people of all ages) [45], two studies have shown that switching from multidrug regimen to FDC can lead to small, but statistically significant increase in adherence. A study of 43 PLWH of all ages in London showed improvement of adherence from 97.7 to 99.4% with the change to a FDC pill [46]. An Italian study of 212 PLWH of all ages showed an improvement of QoL from 68.8 to 72.7% [47]. A review found that five studies (two abstracts and three articles) noted a statistically significant association between regimen complexity and decreased adherence [48]. These findings show that switching to or starting with FDC tablets can improve medication adherence by simplifying drug regimens.
Despite this, many PLWH may be hesitant to switch ART, especially if they have been virologically suppressed for many years. Aside from patient reservations, there are other barriers to switching medications, including time required to obtain prior authorizations (in the US) and potential for higher copays, especially with private insurance. Ideally, newer medications will have even fewer interactions, but since PLWH may be reluctant to risk a new regimen, it is important to consider possible long-term drug interactions at initial counseling. The 2018 recommendations of the International Antiviral Society–USA Panel (IAS–USA) place FDC as preferred for most patients, specifically, combination tablets with a 2-NRTI backbone and an integrase inhibitor. The “boosted” FDC tablets with ritonavir or cobicistat are now considered first line only when regimens without them are not available [49]. In addition, these recommendations prefer TAF (when available) over TDF, given the lower likelihood of renal and bone toxicity. The DHHS guidelines, however, do not recommend one over the other [20]. There are no guidelines from IAS–USA specific for ART in older individuals despite change in pharmacodynamics/kinetics that come with aging [50]. DHHS guidelines recommend tailoring ARV drugs based on aging-associated comorbidities, such as renal or liver dysfunction and bone health, as well as consideration of drug–drug interactions [20].
Medication reconciliation is likely the most important intervention for decreasing polypharmacy or allowing for “safer” polypharmacy. This would help ensure that PLWH are not being over-prescribed unnecessary medications or under-prescribed preventive medications while simultaneously accounting for drug interactions and geriatric concerns. The two most used tools include the STOPP/START and Beers criteria [10, 11].
PLWH, especially those who are considered elderly or frail, may be eligible for de-prescription, whereby providers reduce the use of medications, as patients grow older. This is particularly important when PLWH are prescribed medications that either interact or may lead to unwanted adverse events. STOPP/START and Beers criteria can be utilized to help identify medication that should be avoided or at least reduced in elderly patients [10, 11]. For example, the use of psychotropics, such as atypical antipsychotics, is not only likely to increase fall risk in patients, it also poses additional risk in patients with HIV who are receiving pharmacokinetic enhancers ritonavir or cobicistat. Quetiapine levels, for example, can be increased up to fivefold in patients receiving ritonavir [20, 51,52,53,54,55]. This is just one example of how the Beers criteria have important implications on elderly patients with HIV. In addition, a pharmacist-led study used Beers Criteria and STOPP to assess for potentially inappropriate prescribing (PIP) in older PLWH and found that targeting people with 11 or more medications had the highest yield in identifying opportunities for de-prescribing. A little over half of people were found to have a PIP, and after a pharmacist-led visit, approximately 70% of participants had at least one medication discontinued; 10% had at least six medications discontinued [56].
The approval of numerous HIV FDC tablets may reduce the total number of medications that a person is receiving. This should prompt HIV providers to evaluate previously selected regimens that may be changed to newer FDC tablets. For example, patients may still be receiving twice daily darunavir/ritonavir selected years ago when that was the standard of care [57]. Research has demonstrated that once-daily darunavir/ritonavir or darunavir/cobicistat can be used if darunavir resistance associated mutations (RAMS) are absent [57]. This change not only reduces pill burden, but also eliminates a dose of darunavir/ritonavir, which could potentially improve lipids, reduce GI adverse events, and minimize drug interactions [20].
In addition, common medical issues, such as diabetes, hyperlipidemia, and hypertension, need to be evaluated and addressed in all aging patients. Hemoglobin A1c and blood pressure goals may need to be adjusted to reduce the risk of falls in elderly patients. The SPRINT trial showed that more intensive blood pressure control, even in the elderly, was associated with decreased risk of many CV outcomes, but with increased risk of adverse events, including hypotension and syncope [58]. The recommendations from the American Diabetes Association for A1c goal in the elderly depend on functional status and comorbidities, though there are few data on the subject (grade C recommendations) [59]. Finally, LDL goals that require additional medications besides the use of statins may also need to be liberalized to reduce potential for toxicity.
When reviewing medications, it is important to note again that this is a cohort with higher levels of inflammation. Thus, it may be even more critical to aggressively treat comorbidities. While awaiting further studies that may change guidelines specific to those with chronic inflammation, it is important to at least ensure guideline-based CV prevention therapy.
Special populations
PLWH are at high risk for chronic pain [60]. Aging PLWH are especially vulnerable to chronic pain and opiate use [61, 62]. The treatment of chronic pain has shown to improve ART adherence [63,64,65]. Unfortunately, pain medications may increase both the pill burden and the risk for drug interactions/adverse drug events [66, 67]. In addition to polypharmacy, opiate use puts older adults at risk for delirium, falls, and fractures, particularly in the setting of possible underlying neurocognitive deficits and low bone mineral density seen in aging PLWH [10].
A special polypharmacy consideration is the effect of ART on opiate metabolism [68]. Some NNRTIs, such as efavirenz, nevirapine, and rilpivirine may decrease methadone levels by increasing the induction of the metabolism of methadone. This can potentially cause withdrawal in people on methadone for chronic pain or opioid addiction [60, 68]. Other ART, such as ritonavir, may increase the level of opioids by inhibiting the CYP34A metabolism thus causing opiate overdoses [60, 68].
Substance abuse is also not uncommon among PLWH [69]. Injection drug users are more likely to be compliant with ARV if they are medically treated for their addiction [70]. The most commonly used medications to treat opiate addiction are methadone and buprenorphine. As discussed above, some ART can interact with the metabolism of methadone. This also applies to buprenorphine [60].
Another vulnerable population of PLWH at risk of polypharmacy is those near the end of life [71]. Prior to ART, HIV infection may have justified hospice or palliative care soon after diagnosis, but PLWH are now living close to the life expectancy of people without HIV [72, 73], and HIV is an infrequent primary diagnosis for hospice [63, 74]. This does not necessarily mean that people with HIV forego hospice, but that they are admitted to hospice for other life-limiting illnesses [72]. The current hospice admission criteria for end-stage AIDS include having a CD4 < 25 cells/mL or viral load > 100,000, an opportunistic infection, HIV-related malignancies or illnesses, Karnofsky performance status of < 50%, in addition to supporting findings such as HIV not responsive to ART, or forgoing ART [71]. This could mean that there are some who may be eligible for hospice secondary to end-stage AIDS due to resistant strains. In these situations, the question of whether a person benefits from continuing ART comes into play. Even if a PLWH is on hospice secondary to a non-HIV disease such as stroke or heart failure, the decision whether to continue ART is challenging, as currently, there are no guidelines [71]. ART decreases the risks of opportunistic infections [75,76,77], which may lower their symptom burden and potentially decrease the caregiver’s exposure to resistant strains or high viral loads. Informal caregivers have the added burden of fear of infection related to caregiving activities of PLWH [78, 79]. Continuing ART, in addition to enhancing symptom management, may also alleviate some of the fear that caregivers have regarding transmission. Despite the argument that ART should be given to help alleviate symptoms and avoid suffering from certain pain and opportunistic infections, there are situations where discontinuing ART may be necessary. Utilization of standard de-prescribing techniques as described above may be helpful. Providers should evaluate the role of ART in symptom management, the risk of interactions, pill burden, and dysphagia [71].
A look into the future of ART
Initial HIV treatment regimens, which began with the first drug zidovudine in 1986, were characterized by high pill burden, side effects, and complicated dosing regimens that often resulted in decreased adherence [80]. Over the past 3 decades, HIV treatment has evolved greatly, now with options for one pill once a day. Any single-tablet regimen is a combination therapy, typically including three-to-four different medications co-formulated into one tablet. As discussed previously, ease of dosing and adherence to ARV therapy have increased with single-tablet regimens and once-daily dosing schedules. Nonetheless, clinicians must consider multiple factors when selecting a single tablet that contains three-to-four medications within each tablet, particularly in an older population.
Ongoing research offers potential for two-drug combination regimens, notably without a protease inhibitor that may require a pharmacologic “booster”. Recently, the SWORD 1 and 2 trials evaluated the combination of dolutegravir/rilpivirine, as maintenance therapy compared to “current” ARV three-drug regimens such as two NRTIs and an INSTI, NNRTI, or PI. At time of enrollment, all participants had HIV-1 RNA < 50 copies/mL for at least 6 months. The primary endpoint was proportion of participants with HIV-1 RNA < 50 copies/mL at 48 weeks [81] and a follow-up study of 100 weeks [82]. These data show that the combination of dolutegravir/rilpivirine was non-inferior to current ARV regimens, as defined above, for ongoing viral suppression of HIV-1. Ongoing studies evaluating lipid profiles, bone mineral density, and fractures may offer further insight into the metabolic effects and potential benefits of this two-drug regimen.
There are currently no recommended two-drug regimens for initial therapy of HIV infection. However, this may change, as ongoing research is evaluating the combination of dolutegravir/lamivudine compared to a dolutegravir plus two NRTI regimen in the GEMINI 1&2 phase III clinical trials [83, 84], whose estimated completion date is March 2020. If effective, this could offer newly infected people with HIV an opportunity to initiate therapy with a novel two-drug combination free of either NNRTI or PI medications that often carry a relatively higher burden of side effects, drug interactions, and metabolic complications.
Change history
20 May 2019
The original version of this article unfortunately contained a mistake.
References
UNAIDS UNAIDS—AIDSinfo. http://aidsinfo.unaids.org/. Accessed 8 Oct 2018
CDC HIV among people aged 50 and older. https://www.cdc.gov/hiv/group/age/olderamericans/index.html. Accessed 29 Sept 2018
Antiretroviral Therapy Cohort Collaboration (2017) Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV 4:e349–e356. https://doi.org/10.1016/S2352-3018(17)30066-8
Duerden M, Avery T, Payne R (2013) Polypharmacy and medicines optimisation. Making it safe and sound. The King’s Fund, London. https://www.kingsfund.org.uk/sites/default/files/field/field_publication_file/polypharmacy-and-medicinesoptimisation-kingsfund-nov13.pdf. Accessed 12 Sept 2018
Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE (2017) What is polypharmacy? A systematic review of definitions. BMC Geriatr 17:230. https://doi.org/10.1186/s12877-017-0621-2
Whitman AM, DeGregory KA, Morris AL, Ramsdale EE (2016) A comprehensive look at polypharmacy and medication screening tools for the older cancer patient. Oncologist 21:723–730. https://doi.org/10.1634/theoncologist.2015-0492
Bushardt RL, Massey EB, Simpson TW et al (2008) Polypharmacy: misleading, but manageable. Clin Interv Aging 3:383–389
Burt J, Elmore N, Campbell SM et al (2018) Developing a measure of polypharmacy appropriateness in primary care: systematic review and expert consensus study. BMC Med 16:91. https://doi.org/10.1186/s12916-018-1078-7
Hanlon JT, Schmader KE (2013) The medication appropriateness index at 20: where it started, where it has been, and where it may be going. Drugs Aging 30:893–900. https://doi.org/10.1007/s40266-013-0118-4
By the American Geriatrics Society 2015 Beers Criteria Update Expert Panel (2015) American geriatrics society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 63:2227–2246. https://doi.org/10.1111/jgs.13702
O’Mahony D, O’Sullivan D, Byrne S et al (2015) STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 44:213–218. https://doi.org/10.1093/ageing/afu145
Payne RA, Abel GA, Avery AJ et al (2014) Is polypharmacy always hazardous? A retrospective cohort analysis using linked electronic health records from primary and secondary care. Br J Clin Pharmacol 77:1073–1082. https://doi.org/10.1111/bcp.12292
Krentz HB, Cosman I, Lee K et al (2012) Pill burden in HIV infection: 20 years of experience. Antivir Ther (Lond) 17:833–840. https://doi.org/10.3851/IMP2076
Hasse B, Ledergerber B, Furrer H et al (2011) Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis 53:1130–1139. https://doi.org/10.1093/cid/cir626
Guaraldi G, Malagoli A, Calcagno A et al (2018) The increasing burden and complexity of multi-morbidity and polypharmacy in geriatric HIV patients: a cross sectional study of people aged 65–74 years and more than 75 years. BMC Geriatr 18:99. https://doi.org/10.1186/s12877-018-0789-0
Edelman EJ, Gordon KS, Glover J et al (2013) The next therapeutic challenge in HIV: polypharmacy. Drugs Aging 30:613–628. https://doi.org/10.1007/s40266-013-0093-9
Gimeno-Gracia M, Crusells-Canales MJ, Armesto-Gómez FJ et al (2016) Polypharmacy in older adults with human immunodeficiency virus infection compared with the general population. Clin Interv Aging 11:1149–1157. https://doi.org/10.2147/CIA.S108072
Ssonko M, Stanaway F, Mayanja HK et al (2018) Polypharmacy among HIV positive older adults on anti-retroviral therapy attending an urban clinic in Uganda. BMC Geriatr 18:125. https://doi.org/10.1186/s12877-018-0817-0
Liverpool HIV drug interactions. https://www.hiv-druginteractions.org/checker. Accessed 29 Sept 2018
U.S. Department of Health and Human Services (DHHS) Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv/0. Accessed 8 Oct 2018
Lepik KJ, Yip B, Ulloa AC et al (2018) Adverse drug reactions to integrase strand transfer inhibitors. AIDS 32:903–912. https://doi.org/10.1097/QAD.0000000000001781
Gilead Sciences (2018) Bictegravir/tenofovir alafenamide/emtricitabine [Package Insert]. https://www.gilead.com/~/media/files/pdfs/medicines/hiv/biktarvy/biktarvy_pi.pdf. Accessed 30 Sept 2018
Bedimo R, Maalouf NM, Zhang S et al (2012) Osteoporotic fracture risk associated with cumulative exposure to tenofovir and other antiretroviral agents. AIDS 26:825–831. https://doi.org/10.1097/QAD.0b013e32835192ae
Mills A, Arribas JR, Andrade-Villanueva J et al (2016) Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: a randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. Lancet Infect Dis 16:43–52. https://doi.org/10.1016/S1473-3099(15)00348-5
Mallal S, Phillips E, Carosi G et al (2008) HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med 358:568–579. https://doi.org/10.1056/NEJMoa0706135
Marcus JL, Neugebauer RS, Leyden WA et al (2016) Use of abacavir and risk of cardiovascular disease among HIV-infected individuals. J Acquir Immune Defic Syndr 71:413–419. https://doi.org/10.1097/QAI.0000000000000881
Mollan KR, Smurzynski M, Eron JJ et al (2014) Association between efavirenz as initial therapy for HIV-1 infection and increased risk for suicidal ideation or attempted or completed suicide: an analysis of trial data. Ann Intern Med 161:1–10. https://doi.org/10.7326/M14-0293
Hughes CA, Tseng A, Cooper R (2015) Managing drug interactions in HIV-infected adults with comorbid illness. Can Med Assoc J 187:36–43. https://doi.org/10.1503/cmaj.131626
Nan C, Shaefer M, Urbaityte R et al (2018) Abacavir use and risk for myocardial infarction and cardiovascular events: pooled analysis of data from clinical trials. Open Forum Infect Dis 5:ofy086. https://doi.org/10.1093/ofid/ofy086
Evans-Jones JG, Cottle LE, Back DJ et al (2010) Recognition of risk for clinically significant drug interactions among HIV-infected patients receiving antiretroviral therapy. Clin Infect Dis 50:1419–1421. https://doi.org/10.1086/652149
Greene M, Steinman MA, McNicholl IR, Valcour V (2014) Polypharmacy, drug–drug interactions, and potentially inappropriate medications in older adults with human immunodeficiency virus infection. J Am Geriatr Soc 62:447–453. https://doi.org/10.1111/jgs.12695
Holtzman C, Armon C, Tedaldi E et al (2013) Polypharmacy and risk of antiretroviral drug interactions among the aging HIV-infected population. J Gen Intern Med 28:1302–1310. https://doi.org/10.1007/s11606-013-2449-6
Foisy MM, Yakiwchuk EM, Hughes CA (2008) Induction effects of ritonavir: implications for drug interactions. Ann Pharmacother 42:1048–1059. https://doi.org/10.1345/aph.1K615
Gellad WF, Grenard JL, Marcum ZA (2011) A systematic review of barriers to medication adherence in the elderly: looking beyond cost and regimen complexity. Am J Geriatr Pharmacother 9:11–23. https://doi.org/10.1016/j.amjopharm.2011.02.004
Cantudo-Cuenca MR, Jiménez-Galán R, Almeida-Gonzalez CV, Morillo-Verdugo R (2014) Concurrent use of comedications reduces adherence to antiretroviral therapy among HIV-infected patients. J Manag Care Spec Pharm 20:844–850. https://doi.org/10.18553/jmcp.2014.20.8.844
Prosperi MCF, Fabbiani M, Fanti I et al (2012) Predictors of first-line antiretroviral therapy discontinuation due to drug-related adverse events in HIV-infected patients: a retrospective cohort study. BMC Infect Dis 12:296. https://doi.org/10.1186/1471-2334-12-296
Huang ES, Karter AJ, Danielson KK et al (2010) The association between the number of prescription medications and incident falls in a multi-ethnic population of adult type-2 diabetes patients: the diabetes and aging study. J Gen Intern Med 25:141–146. https://doi.org/10.1007/s11606-009-1179-2
Erlandson KM, Allshouse AA, Jankowski CM et al (2012) Risk factors for falls in HIV-infected persons. J Acquir Immune Defic Syndr 61:484–489. https://doi.org/10.1097/QAI.0b013e3182716e38
Jyrkkä J, Enlund H, Lavikainen P et al (2011) Association of polypharmacy with nutritional status, functional ability and cognitive capacity over a three-year period in an elderly population. Pharmacoepidemiol Drug Saf 20:514–522. https://doi.org/10.1002/pds.2116
Gandhi TK, Weingart SN, Borus J et al (2003) Adverse drug events in ambulatory care. N Engl J Med 348:1556–1564. https://doi.org/10.1056/NEJMsa020703
Schambelan M, Wilson PWF, Yarasheski KE et al (2008) Development of appropriate coronary heart disease risk prediction models in HIV-infected patients. Circulation 118:e48–e53. https://doi.org/10.1161/CIRCULATIONAHA.107.189627
Deeks SG, Phillips AN (2009) HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ 338:a3172. https://doi.org/10.1136/bmj.a3172
Neuhaus J, Jacobs DR, Baker JV et al (2010) Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis 201:1788–1795. https://doi.org/10.1086/652749
Freiberg MS, Leaf DA, Goulet JL et al (2009) The association between the receipt of lipid lowering therapy and HIV status among veterans who met NCEP/ATP III criteria for the receipt of lipid lowering medication. J Gen Intern Med 24:334–340. https://doi.org/10.1007/s11606-008-0891-7
Guaraldi G, Menozzi M, Zona S et al (2017) Impact of polypharmacy on antiretroviral prescription in people living with HIV. J Antimicrob Chemother 72:511–514. https://doi.org/10.1093/jac/dkw437
Portsmouth SD, Osorio J, McCormick K et al (2005) Better maintained adherence on switching from twice-daily to once-daily therapy for HIV: a 24-week randomized trial of treatment simplification using stavudine prolonged-release capsules. HIV Med 6:185–190. https://doi.org/10.1111/j.1468-1293.2005.00287.x
Airoldi M, Zaccarelli M, Bisi L et al (2010) One-pill once-a-day HAART: a simplification strategy that improves adherence and quality of life of HIV-infected subjects. Patient Prefer Adherence 4:115–125
Fogarty L, Roter D, Larson S et al (2002) Patient adherence to HIV medication regimens: a review of published and abstract reports. Patient Educ Couns 46:93–108. https://doi.org/10.1016/S0738-3991(01)00219-1
Saag MS, Benson CA, Gandhi RT et al (2018) Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the international antiviral society-USA panel. JAMA 320:379–396. https://doi.org/10.1001/jama.2018.8431
Blanco JR, Caro AM, Pérez-Cachafeiro S et al (2010) HIV infection and aging. AIDS Rev 12:218–230
Zalma A, von Moltke LL, Granda BW et al (2000) In vitro metabolism of trazodone by CYP3A: inhibition by ketoconazole and human immunodeficiency viral protease inhibitors. Biol Psychiatry 47:655–661
Greenblatt DJ, von Moltke LL, Harmatz JS et al (2003) Short-term exposure to low-dose ritonavir impairs clearance and enhances adverse effects of trazodone. J Clin Pharmacol 43:414–422
Geraci MJ, McCoy SL, Crum PM, Patel RA (2010) Antipsychotic-induced priapism in an HIV patient: a cytochrome P450-mediated drug interaction. Int J Emerg Med 3:81–84. https://doi.org/10.1007/s12245-010-0175-y
Pollack TM, McCoy C, Stead W (2009) Clinically significant adverse events from a drug interaction between quetiapine and atazanavir–ritonavir in two patients. Pharmacotherapy 29:1386–1391. https://doi.org/10.1592/phco.29.11.1386
Hantson P, Di Fazio V, Wallemacq P (2010) Toxicokinetic interaction between quetiapine and antiretroviral therapy following quetiapine overdose. Drug Metab Lett 4:7–8
McNicholl IR, Gandhi M, Hare CB et al (2017) A pharmacist-led program to evaluate and reduce polypharmacy and potentially inappropriate prescribing in older HIV-positive patients. Pharmacotherapy 37:1498–1506. https://doi.org/10.1002/phar.2043
Janssen prezcobix (darunavir and cobicistat) [Package Insert]. http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/PREZCOBIXpi.pdf. Accessed 30 Sept 2018
SPRINT Research Group, Wright JT, Williamson JD et al (2015) A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 373:2103–2116. https://doi.org/10.1056/nejmoa1511939
American Diabetes Association (2018) 11. Older adults: standards of medical care in diabetes—2018. Diabetes Care 41:S119–S125. https://doi.org/10.2337/dc18-S011
Bruce RD, Merlin J, Lum PJ et al (2017) 2017 HIVMA of IDSA clinical practice guideline for the management of chronic pain in patients living with HIV. Clin Infect Dis 65:e1–e37. https://doi.org/10.1093/cid/cix636
Lawson E, Sabin C, Perry N et al (2015) Is HIV painful? An epidemiologic study of the prevalence and risk factors for pain in HIV-infected patients. Clin J Pain 31:813–819. https://doi.org/10.1097/AJP.0000000000000162
Canan CE, Chander G, Monroe AK et al (2018) High-risk prescription opioid use among people living with HIV. J Acquir Immune Defic Syndr 78:283–290. https://doi.org/10.1097/QAI.0000000000001690
Uebelacker LA, Weisberg RB, Herman DS et al (2015) Chronic pain in HIV-infected patients: relationship to depression, substance use, and mental health and pain treatment. Pain Med 16:1870–1881. https://doi.org/10.1111/pme.12799
Surratt HL, Kurtz SP, Levi-Minzi MA et al (2015) Pain treatment and antiretroviral medication adherence among vulnerable HIV-positive patients. AIDS Patient Care STDS 29:186–192. https://doi.org/10.1089/apc.2014.0104
CDC HIV/AIDS. https://www.cdc.gov/hiv/default.html. Accessed 29 Sept 2018
Cuzin L, Katlama C, Cotte L et al (2017) Ageing with HIV: do comorbidities and polymedication drive treatment optimization? HIV Med 18:395–401. https://doi.org/10.1111/hiv.12441
Gruta C, Goldschmidt R (2017) HIV/AIDS: implications for older adult patients. https://aging.arizona.edu/sites/aging/files/fact-sheet-pdfs/hiv_1.pdf. Accessed 29 Sept 2018
Burgess MJ, Zeuli JD, Kasten MJ (2015) Management of HIV/AIDS in older patients-drug/drug interactions and adherence to antiretroviral therapy. HIV AIDS (Auckl) 7:251–264. https://doi.org/10.2147/HIV.S39655
Silverberg MJ, Ray GT, Saunders K et al (2012) Prescription long-term opioid use in HIV-infected patients. Clin J Pain 28:39–46. https://doi.org/10.1097/AJP.0b013e3182201a0f
Moatti JP, Carrieri MP, Spire B et al (2000) Adherence to HAART in French HIV-infected injecting drug users: the contribution of buprenorphine drug maintenance treatment. The Manif 2000 study group. AIDS 14:151–155
Ruiz M, Armstrong M, Reske T et al (2014) Antiretroviral therapy at the end of life: the experience of an academic HIV clinic. Am J Hosp Palliat Care 31:475–479. https://doi.org/10.1177/1049909113494459
Goodkin K, Kompella S, Kendell SF (2018) End-of-life care and bereavement issues in human immunodeficiency virus—AIDS. Nurs Clin N Am 53:123–135. https://doi.org/10.1016/j.cnur.2017.10.010
Slomka J, Prince-Paul M, Webel A, Daly BJ (2016) Palliative care, hospice, and advance care planning: views of people living with hiv and other chronic conditions. J Assoc Nurses AIDS Care 27:476–484. https://doi.org/10.1016/j.jana.2016.02.003
Rhodes RL, Nazir F, Lopez S et al (2016) Use and predictors of end-of-life care among HIV patients in a safety net health system. J Pain Symptom Manag 51:120–125. https://doi.org/10.1016/j.jpainsymman.2015.08.010
Schwarcz L, Chen M-J, Vittinghoff E et al (2013) Declining incidence of AIDS-defining opportunistic illnesses: results from 16 years of population-based AIDS surveillance. AIDS 27:597–605. https://doi.org/10.1097/QAD.0b013e32835b0fa2
Lima VD, Lourenço L, Yip B et al (2015) AIDS incidence and AIDS-related mortality in British Columbia, Canada, between 1981 and 2013: a retrospective study. Lancet HIV 2:e92–e97. https://doi.org/10.1016/S2352-3018(15)00017-X
Stewart A, Chan Carusone S, To K et al (2012) Causes of death in HIV patients and the evolution of an AIDS hospice: 1988–2008. AIDS Res Treat 2012:390406. https://doi.org/10.1155/2012/390406
Wardlaw LA (1994) Sustaining informal caregivers for persons with AIDS. Fam Soc J Contemp Soc Serv 75:373–384. https://doi.org/10.1177/104438949407500606
Van Deventer C, Wright A (2017) The psychosocial impact of caregiving on the family caregivers of chronically ill AIDS and/or HIV patients in home-based care: a qualitative study in Zimbabwe. South Afr J HIV Med. https://doi.org/10.4102/sajhivmed.v18i1.718
Friedland GH, Williams A (1999) Attaining higher goals in HIV treatment: the central importance of adherence. AIDS 13(Suppl 1):S61–S72
Llibre JM, Hung C-C, Brinson C et al (2018) Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet 391:839–849. https://doi.org/10.1016/S0140-6736(17)33095-7
Aboud M, Orkin C, Podzamczer D, Boger J (2018) Durable suppression 2 years after switch to DTG +RPV 2-drug regimen: SWORD-1 and SWORD-2 studies, 22nd International AIDS Conference, Amsterdam, Netherlands, July 23–27, 2018
ViiV Healthcare (2016) An efficacy, safety, and tolerability study comparing dolutegravir plus lamivudine with dolutegravir plus tenofovir/emtricitabine in treatment naïve HIV infected subjects (Gemini 1). In: NCT02831673. https://clinicaltrials.gov/ct2/show/NCT02831673. Accessed 27 Sept 2018
ViiV Healthcare (2016) An efficacy, safety, and tolerability study comparing dolutegravir (DTG) plus lamivudine (3TC) with dolutegravir plus tenofovir/emtricitabine in treatment naïve HIV infected subjects (Gemini 2). In: NCT02831764. https://clinicaltrials.gov/ct2/show/NCT02831764. Accessed 28 Sept 2018
Acknowledgements
Dr. Johnston receives support from NIH TL1 TR000459 and NIAID T32AI007613. Dr. Siegler receives support from Gilead Sciences, Inc. for an investigator-initiated study IN-US-311-4182.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Siegler receives support from Gilead Sciences for an investigator-initiated study. Dr. Faragon is on the speakers’ bureaus of AbbVie, Gilead Sciences, Merck, and Janssen Pharmaceutical. Drs. Freedman, Johnston, and Del Carmen report no conflicts of interest.
Ethical approval
The preparation of this review did not involve human or animal participants.
Informed consent
No informed consent was required.
Rights and permissions
About this article
Cite this article
Freedman, S.F., Johnston, C., Faragon, J.J. et al. Older HIV-infected adults: complex patients (III)—polypharmacy. Eur Geriatr Med 10, 199–211 (2019). https://doi.org/10.1007/s41999-018-0139-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41999-018-0139-y