Abstract
Metal oxide (–M–O–) linkages modify the entire characteristics of epoxy coatings significantly for different steel surface applications. To support this concept, a highly effective corrosion-resistant coating was developed by combining an epoxy resin, known as diglycidyl ether of bisphenol A (DGEBA), with a hardener in the presence of titanium tetra-acetoximate (TTA) [Ti(ON=C(CH3)2)4] (epoxy-TTA coating). The microstructure and surface morphology of the coatings were characterized by Fourier-transform infrared spectroscopy (FT-IR) and scanning electron microscopy (SEM) coupled with energy dispersive spectroscopy (EDS) which supports reduction in porosity of epoxy adhesive because of O–Ti–O and Ti–O–C linkages in epoxy framework. The adhesive strength of the coatings also increases in dry and wet conditions as examined through pull-off test. The anticorrosive nature of coatings was indicated by weight loss, electrochemical and salt spray tests, conducted in 3.5% NaCl aqueous solution. To reinforce the experimental results, reactivity of crosslinked epoxy and epoxy-TTA coatings with metallic surface was also investigated using density functional theory (DFT) and molecular dynamics (MD) methods. In electrochemical findings, increase in polarization resistance (bare steel: 2.55 KΩ cm2; epoxy-TTA coated steel: 52.42 × 103 KΩ cm2) and a drop in corrosion rate, from 4.58 mpy (bare steel) to 1.05 × 10−4 mpy (epoxy-TTA) strongly indicate epoxy-TTA hybrid composite coatings’ capacity to effectively protect against corrosion. DFT study demonstrated that epoxy and epoxy-TTA resins interacts firmly with metal surface utilizing donor–acceptor mechanism. Modification of epoxy with TTA enhances its interaction with steel surface in dry as well as in wet conditions as indicated by the adhesion energy calculated by MD simulations. These modeling outcomes, in line with the experimental findings proposed the superior epoxy adhesion in case of its modification with TTA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
MS (mild steel) is widely employed in the construction industries due to its strength and inexpensive cost, however, corrosion is an unavoidable cause of mild steel degradation (Xavier 2020). According to the report of International Zinc Association (IZA) 2021, Every year India loses its GDP (Gross Domestic Product) around 5–7% due to corrosion (Chopra et al. 2022) and NACE International reported global cost due to corrosion is nearly 3.4% of global GDP (Hossain and Ulčickas 2021).
Organic coatings are widely employed as a barrier to protect metal from corrosion. Epoxy-based coatings received top marks among organic coatings due to their strong cross-linking density, strong mechanical power, and great adhesion to metal surfaces. The publications published globally illustrate the meritorious character of epoxy coatings (Dagdag et al. 2020a). However, when exposed to corrosive media, organic coatings such as epoxies lose their corrosion protective capabilities because of surface micropores that allow the penetration or diffusion of corrosive initiative species like as H2O, Cl− ions, and others. Porosity can be minimized by adding chemicals to coating formulations that block surface micropores. Porosity can be minimized by adding chemicals to coating formulations that block surface micropores (Zhang et al. 2016). Numerous nanometal oxides, such as TiO2 (Chopra et al. 2022; Mostafa et al. 2019; Jaseela et al. 2020; Bach et al. 2019), SiO2 (Kirtay 2014), CeO2 (Ramaprakash et al. 2016), Al2O3 (Wang et al. 2006) and others, have been extensively used as reinforcing additives in polymer matrixes to improve their anticorrosive nature; however, homogeneous distribution of nanoparticles (NPs) in the epoxy matrix remains a difficult task due to uncontrollable agglomeration of nanoparticles due to their high surface energy (Liu et al. 2018; Li et al. 2023; Hsissou et al. 2022a).
Surface functionalization of NPs or in situ generation of nanoparticles are suggested to overcome this problem. Pour et al. (2018) reported that surface treatment of TiO2 NPs with macromolecular coupling agents boosted adhesion over steel which leads to improved anticorrosion properties. Dagdag et al. (2020b) also examined anticorrosive effect of TiO2 NPs in solution of 3% NaCl for carbon steel. He has investigated the effect of TiO2 in epoxy adhesion by experimentally and by using molecular dynamics simulations. “High performance anti-corrosive epoxy-titania hybrid nanocomposite coatings” was also investigated experimentally by Ghosal and Ahmad (2017) in which he has generated titania framework in situ using titanium iso-propoxide as a precursor. He has documented that in situ generation of titania reduced the porosity in epoxy adhesives by promoting the crosslinking between different chains of epoxy molecules which led to the increased anticorrosive property of the coatings. However, in situ generation of titania framework and its interaction with epoxy resin is still an unexplored area.
Therefore, in the present investigations we have used titanium tetra-acetoximate, [Ti(ON=C(CH3)2)4] (TTA) as a precursor to generate in situ titania framework during the crosslinking of epoxy with hardener.
The novelty of the current research has been investigated further by comparing the current result with the reported literature related to the subject of the present research (Table 1).
We have chosen acetoxime as a ligand in TTA because during hydrolysis TTA released oxime molecules which may add some merit in the anticorrosion nature of the hybrid composite coatings. It is proficiently accepted that “organic compounds containing oxygen and nitrogen atoms adsorbs on the metal through hetero- atoms along with aromatic rings or conjugated double bonds to employ their inhibition” and it is clearly reflected by the recent findings of Li et al. (2014) which presented corrosion inhibition effect of various oxime experimentally as well as computationally on aluminum surface. Acetoxime also contains functional group, OH–, C=N– with electronegative O, N atoms and double bonds at the same time which fulfills the requirement of an inhibitor.
Modeling approaches based on QM (quantum mechanics) and MD (molecular dynamics) have been discovered to be extremely useful tools for unraveling the interaction process underlying polymeric coating adherence to metal substrates. (Bahlakeh and Ramezanzadeh 2017). These methods functioning at atomic/electronic scales deliver a quantitative/qualitative view related to surface bonding behavior and interfacial adhesion of coatings. This has been confirmed that, by using quantum mechanics methods based on DFT (density functional theory), one can derive fundamental marks about the coating electronic features leading its adhesion manner with regards to metal oxide/metal substrate.
In this study, we modified epoxy using TTA, and the modified epoxy was evaluated by FT-IR, SEM-EDX, and TG analyses. Pull-off experiments in dry and wet circumstances were used to study the adhesive power of modified epoxy coatings on mild steel, which was then compared to epoxy coatings alone. Electrochemical, weight loss, and salt spray experiments were used to investigate corrosion in a 3.5% NaCl solution. To back up the experimental findings, the reactivity of epoxy/modified epoxy resins with metallic surfaces was examined computationally using DFT and MD methods.
2 Experimental
2.1 Materials and methods
Mild steel composition is reported in Table 2.
Titanium tetra-iso-propoxide, [Ti(Pri)4] was of grade-Sigma Aldrich (99%) while epoxy resin, Araldite GZ 7071 × 75 and hardener, Crayamid 115 were purchased by Singhal Chemical Corporation (Meerut, India). The used solvents [benzene (RANKEM, 98%) (B.Pt. 80 °C), acetone (RANKEM, 99%) (B.Pt. 56 °C) and toluene (RANKEM, 99%) (B.Pt. 110 °C)] and reagents [hydroxylamine hydrochloride (RANKEM 99%) and sodium bicarbonate (RANKEM 99%)] were distilled and dried using conventional procedures (Mishra and Kumar 2014). To handle health-hazard compounds, adequate protections were established, like benzene.
IR data (400–4000 cm−1) was obtained on Perkin Elmer, FT-IR Spectrum 2 and the Pull-off tests were conducted through PosiTest AT-A (DeFelsko) to check adhesion of coatings using ASTM D 4541. SEM and EDX-analysis images were observed on an EDX-coupled scanning electron microscope JEOL make JSM-7610FPlus. XRD patterns were achieved on a Riigaku make SMARTLAB diffractometer using Fe source at 1.937 Å wavelength. Salt spray tests were carried out on Envysis salt-spray chamber as per the ASTM G44. Coating thickness were checked by Fisher DUALSCOPE FMP40.
2.2 Synthesis of titanium tetra-acetoximate [Ti(OCH(CH3)2)4] (TTA)
TTA, [Ti(ON=C(CH3)2)4] was synthesized and characterized as reported earlier (Intellectual Property India 2022; Chaudhary et al. 2011).
A benzene solution (~ 70 ml) of Ti(OPri)4 (2.46 g, 8.65 mmol) was added in 2.53 g (34.61 mmol) of acetoxime (molar ratio 1:4). The contents were refluxed and the released isopropanol, which obtained azeotropically with benzene, was used to estimate the reaction completion. The resultant was dried under vacuum to yield a white solid (yield 98.0%), which was characterized as titanium(IV) tetra-acetoximate, [Ti{ONC(CH3)2}4].
2.3 Preparation and deposition of epoxy and epoxy-TTA coatings
Mild steel samples of 3 mm × 100 mm × 100 mm (for salt spray and pull off tests) and 3 mm × 30 mm × 20 mm (for other testing) were cleaned with emery paper of different grades, then water is used for washing and acetone is used for degreasing, and dried before coating deposition.
2.3.1 Epoxy coatings
10 g of DGEBA was dissolved in 10 g of toluene and mixed for half an hour at room temperature (30 °C). 5 g of hardener was mixed in 5 g of toluene and added to the DGEBA solution dropwise while constantly stirring. This solution was then sonicated for 30 min at 30 °C. Following sonication, the solution was sprayed with a spray gun over surface-prepared steel samples (pressure, 20 psi; flow rate, 0.2 ml/s). The coated samples were first kept at 30 °C for a day before being moved to 50 °C for another 24 h to ensure complete curing and drying.
The preparation of cross-linked epoxy was investigated by FT-IR analysis.
2.3.2 Epoxy-TTA coatings
10 g DGEBA was dissolved in 10 g toluene and stirred for 30 min. On continuous stirring at room temperature, varying weight percentages (1%, 3%, and 5%) of pre-hydrolyzed TTA solution were added separately to the DGEBA solution. 5 g of hardener was mixed in 5 g of toluene and dropwise added to the different DGEBA-TTA solutions while constantly stirring. All of the mixes were then sonicated for half an hour at room temperature. Following sonication, the solution was sprayed over surface-prepared steel samples, and the coated substrates were initially kept at 30 °C for 24 h and then at 50 °C for another 24 h, resulting in the formulation of epoxy-TTA1, epoxy-TTA3, and epoxy-TTA5 composite coatings (where suffix indicates the weight% of TTA).
FT-IR analysis confirms the preparation of cross-linked coating material.
The thickness of epoxy coatings was in the range 60 ± 10 µm, whereas that of epoxy-TTA coatings was 60 ± 5 µm.
2.4 Weight loss measurement
Surface prepared bare and coated mild steel samples were weighed with an accuracy of 0.1 mg before being immersed in a aqueous NaCl solution of 3.5% for weight loss testing. After 120 h, samples were pulled out, cleaned with a brush in running water and acetone, dried out with a hot air gun, and precisely weighed again. The method was repeated until 720 h of immersion at room temperature. The corrosion rate (ν) was calculated using the method (Nath Upadhyay 2004):
where W is the average weight loss of three parallel MS sheets, S is the surface area of each exposed sample, and t is immersion time.
The ν determined above was then used to compute the inhibition efficiency (IE) as follows (Fayomi et al. 2018):
where νo and ν are the corrosion rates in bare MS and coated MS, respectively.
2.5 Contact angle
To determine the hydrophilicity/hydrophobicity of the specimens, contact angle testing of the coupons were done using goniometer, OCA 15EC Dataphysics Instrument. To measure the water contact angle between bare MS and coated samples, deionized water was used according to the sessile drop method.
2.6 Thermal studies
The thermal decomposition of epoxy and epoxy-TTA5 coating were explored by thermogravimetric (TG) analyses on Shimadzu, DTG-60H, Japan instrument with a temperature range from 25 to 900 °C and 10 °C/min heating rate under inert environment.
2.7 Electrochemical measurements
At room temperature, electrochemical measurements were done on the Biologic SP-150 electrochemical workstation. Electrochemical measurements, open circuit potential (OCP) experiments, and polarization experiments were carried out in a three-electrode flat corrosion cell equipped with a reference electrode i.e., SCE (saturated calomel electrode), CE (counter electrode) i.e., platinum mesh and WE (working electrodes) i.e., bare/coated samples. The coupons’ exposed area was 1 cm2, and the corrosive media was a aqueous NaCl solution of 3.5%.
Prior to measurements, the working electrode was immersed in a NaCl solution of 3.5% for 1 h, and the OCP was measured until a steady state was obtained. The potentiodynamic polarization test was done after OCP stabilization at a sweep rate of 1 mV/s in the potential range of ± 250 mV with respect to the OCP. Each sample was repeated at least three times to confirm that the findings were relevant.
EC Lab 10.4 software was used for Tafel data calculation and fitting, and the Stern–Geary equation was employed to calculate polarization resistance (Rp) (Hamdy et al. 2007):
where ꞵc is cathodic Tafel slope, ꞵa is anodic Tafel slope and Icorr is corrosion current density.
Impedance spectra, obtained in the frequency range of 10 mHz–100 kHz was investigated at OCP using AC signals with an amplitude of 10 mV. The data of EIS (electrochemical impedance spectroscopy) were shown by Nyquist plots. The inhibition efficiency (η%) can be evaluated using Eq. (1):
where \(R_{{\text{p}}}^{{\text{o}}}\) and Rp are the polarization resistance in absence and presence of coatings. The polarization resistance is the summation of the charge transfer resistance (Rct) and film resistance (Rf) as shown in below equation:
The data of EIS (electrochemical impedance spectroscopy) were shown by Nyquist plots (Fig. S1) and its parameters are shown in Table S1.
Salt spray tests on bare and coated samples were performed in a salt spray chamber with 3.5% NaCl solution vapors at a humidity of 95% and a temperature of 35 °C (ASTM G44).
2.8 Computational studies
2.8.1 Density functional theory (DFT)
The optimized structure of crosslinked epoxy resin in absence and presence of TTA were made by following Fig. S2.
The obtained structure undergoes geometry optimization process, firstly by Hartree–Fock theory with basis set 6-31G(d,p) and then by DFT with B3LYP/6-311G(d,p) level to know the electronic properties related to adhesion characteristics between mild steel surface and coating molecules (Bahlakeh and Ramezanzadeh 2017). Gaussian 16 suite were used to perform all above calculations in the gas phase.
The optimized structures were further used to assess frontier molecular orbitals, electronic properties, partial atomic charges and Fukui indices.
2.8.2 Molecular dynamics (MD)
MD simulations were performed by software Material Studio 6 by using Forcite module. To know the adsorption on mild steel surface of TTA modified crosslinked epoxy resin, Fe(110) crystallographic plane of iron was taken for MD simulations according to reported literatures. The steel surface is represented by Fe(110) to make slab model, first unit cell of Fe was cleaved along plane (110) and then thickness of crystalline surface was put to be nearly 1.5 nm that was more than the cut-off distance used for non-bonded interactions (Hsissou et al. 2021, 2022a, b; Ta et al. 2015). After this surface Fe(110) was replicated periodically in y and x directions to make surfaces with appropriate area and to convincingly simulate the substrate and coating interface. A 3-nm thickness of vacuum slab was placed above substrate. The dimensions of Fe(110) slab was nearly 3.5 × 3.5 × 4.4 (nm)3 (Bahlakeh and Ramezanzadeh 2017).
DFT optimized structure of crosslinked epoxy-TTA/epoxy was placed above Fe(110) slab in a periodic box with vacuum to simulate the steel and coating adhesion. To know the adhesion in aq. NaCl solution of coating, a solvent layer having 20 Na+ ions, 20 Cl− ions and 600 H2O molecules was inserted in the last steel/coating structure obtained from MD calculations in dry conditions. The solvent layer is divided into two fragments and each fragment contains 300 H2O molecules + 10 Na+ and 10 Cl− ions. The molecules of lower solvent layer are unable to move without any restriction and dissolved the coating molecules whereas the upper layer molecules positions are fixed which acted as a stiff for the lower layer. Amorphous cell module and building layers tools of materials studio software were used to make the above systems.
Acetoxime and TTA, [Ti(ON=C(CH3)2)4], were synthesized as reported earlier (Chaudhary et al. 2011; Semon 1923).
3 Results and discussion
TTA was synthesized by reaction of titanium iso-propoxide, [Ti(OCH(CH3)2)4] with acetoxime in 1:4 molar ratio (Intellectual Property India 2022; Chaudhary et al. 2011):
The reaction was observed by estimating liberated isopropanol, collected azeotropically by oxidimetric method. This reaction was quite facile and quantitatively yielded a white colored solid which was characterized as titanium tetra-acetoximate, [Ti(ON=C(CH3)2)4].
3.1 Modification of epoxy with TTA
When 50 wt.% of hardener (PA) is added to DGEBA, an oxirane ring-opening reaction occurs, resulting in the creation of NH-CH2 and NH-CO linkages, which then undergo condensation reactions, resulting in the development of cross-linked coating materials (epoxy coating).
To improve the crosslinking of epoxy polymers, we modified the epoxy molecules using pre-hydrolyzed titanium tetra-acetoximate, [Ti(ONC(CH3)2)4] (TTA), by adding 1, 3, and 5 wt.% TTA to DGEBA, followed by hardener.
TTA is a moisture sensitive compound which tend to undergo hydrolysis process easily and releases acetoxime molecules. Therefore, on mixing of DGEBA with pre-hydrolyzed TTA and polyamide it is assumed that TTA contains Ti–OH groups and acetoxime which is further supported by the FT-IR studies and are in agreement with the findings documented by Ghosal and Ahmad (2017). He has used moisture sensitive titanium iso-propoxide as a precursor for titania framework which modified epoxy molecules by interacting hydrolyzed Ti–OH groups with hydroxy groups of epoxy molecules during curing process.
Based on the previous findings and FT-IR studies, the proposed interactions of TTA with DGEBA and PA are shown in Scheme 1.
TTA in the inorganic phase transforms to cross linkers with O–Ti–O, Ti–O–C connections through metal hydroxide conversion followed by Ti–OH contact with –OH in the organic phase (epoxy). The generation of titanium cross linkers efficiently improves the crosslinking between polymer chains, resulting in higher adhesive ability and better surface topography. TTA dispersion resulted in the formation of Ti–O–C and O–Ti–O connections in the polymer framework, which function as inorganic fillers and provide a dense hybrid coating structure.
On increasing the concentration of TTA in DGEBA, the increase in Ti–O–C and O–Ti–O linkages gives a increment in adhesive strength, and improved surface topography which is further supported by SEM images and pull-off tests.
It is worth noting that during TTA contact with epoxy, free oxime molecules are liberated in the polymer, which may enhance the corrosion resistance of crosslinked epoxy, since oxime molecules have been found to be strong corrosion inhibitors (Saini et al. 2022).
3.2 Structural characterization
3.2.1 FT-IR analysis
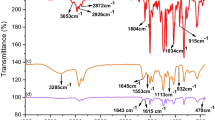
Figure 1 shows the FT-IR spectra of epoxy (DGEBA), cured epoxy (DGEBA-PA), and epoxy-TTA (DGEBA-PA-TTA).
Epoxide characteristic peaks (914 cm−1) (Allen and Sanderson 2006), the IR spectra of epoxy resin contains aromatic rings (3055 cm−1, 1605 cm−1, 770 cm−1), alkyl–aryl ether (1035 cm−1) and symmetrical and asymmetrical stretching vibrations (2925 cm−1 and 2875 cm−1) (Fig. 1a) (Chiniwalla et al. 2003).
When hardener (PA) was added to epoxy, a wide band centered at 3290 cm−1 formed, which may be due to N–H stretching (Ali et al. 2020) (Fig. 1b). In addition, a shifting of 16 cm−1 at higher wavenumber was found in the cured epoxy polymer’s epoxide peak, showing DGEBA interaction with PA. Additional peaks, C–N stretching (1115 cm−1), C=O stretching (1643 cm−1), and N–H bending (1550 cm−1) verified the condensation process between DGEBA’s epoxide group and PA’s –NH2 group. The emergence of additional stretching bands owing to Ti–O (435 cm−1, 870 cm−1), Ti–O–C (1180 cm−1 and 1010 cm−1) and Ti–O–Ti (684 cm−1) (Ghosal and Ahmad 2017) after the addition of TTA to the epoxy polymer during the curing process verified the hydrolysis and condensation of TTA and their interaction with epoxy resin.
3.2.2 Weight loss measurement
The weight loss of the MS and coated MS samples was investigated after immersing specimens in NaCl solution of 3.5% for 720 h. The computed corrosion rate (CR) and inhibition efficiency (IE) (Hegde and Mohana 2020) are shown in Fig. 2. The epoxy-TTA5 coating had a lower C.R. (2.25 g/m2 h) and higher IE (86.04%) when compared to epoxy coating (C.R. = 7.61 g/m2 h, IE = 52.82%). This further supports the development of strongly crosslinked structures in TTA modified epoxy, which reduces the diffusion of corrosive species through coating.
3.2.3 Contact angle
Sessile drop method was utilized to examine the sample surface’s wettability. It was discovered that the contact angle values for bare steel were 60°, epoxy coatings were 68.15°, and epoxy-TTA1, epoxy-TTA3, and epoxy-TTA5 were, respectively, 77.7°, 79.7°, and 87.15° refer Fig. S3. According to the findings, the inclusion of TTA increases the coatings’ hydrophobicity, which results in a reduction in the penetrability of corrosive ions at the coating- metal interface. The hydrophobicity of the coatings rises as TTA % does, which pushes aqueous corrosive ions away from the coatings’ surface.
3.2.4 Thermal studies
The thermal decomposition of epoxy and epoxy-TTA5 coating were explored by TG analysis and the obtained TG curves are presented in Fig. 3.
As indicated by the TG curves, initial weight loss of 11% and 13% was obtained in epoxy and epoxy-TTA5 coating, respectively, at temperature of ~ 275 °C which may be due to the loss of solvent molecules which are trapped. Further weight losses were obtained because of degradation of the ether and amido linkages along with the aromatic ring degradation in the systems. It is worthwhile to mention here that un-stability may generate in the epoxy resin on addition of TTA, which has been reflected by lowering (30 °C) of its onset decomposition temperature. However, in the TTA modified epoxy, the temperature (offset Temp.) at which the decomposition appears to be completed was higher as compared to the epoxy resin alone. The residue of 4.5% was also obtained in the TTA modified epoxy resin which further confirmed the formation of Ti–O–Ti frameworks in DGEBA at high temperature. The findings support that TTA interacted with the epoxy molecules and effects its thermal behavior.
3.2.5 Pull-off test
Pull-off tests were used to determine the adhesion strength of epoxy and epoxy-TTA coatings on MS surfaces in the absence and presence of a 3.5% NaCl solution. The obtained results are in Fig. 4. According to the images, adding TTA to epoxy coatings improved their adherence to steel surfaces, and the adhesion strength increased as the proportion of TTA in the epoxy raised. The outcomes provide more evidence that epoxy-TTA hybrid coatings promote coating/metal contact. Epoxy and epoxy-TTA1 coatings failed completely adhesively when 4.4 MPa and 6.3 MPa of force were applied, respectively, but epoxy-TTA3 and epoxy-TTA5 coatings failed cohesively with higher force. Additionally, it was discovered that the addition of 5% TTA had the lowest adhesion failure and the maximum adhesive strength following exposure to corrosive environment. These results demonstrated that as epoxy’s TTA percentage increased, adhesion was significantly promoted at the epoxy/steel contact. Water molecules diffused into the epoxy film/steel contact while it was wet, weakening the bonds and causing the loss of adhesion. However, epoxy-TTA coatings also shown higher adhesion quality in wet conditions as compared to epoxy coatings on steel, which further supports lower porosity in these coatings.
3.2.6 SEM/EDX
Figure 5 displays the micrographs of the epoxy and epoxy-TTA coatings. The fact that SEM images show that coating compactness improves with a decrease in porosity supports TTA’s claim that it provided the crosslinkers O–Ti–O and Ti–O–C, which boosted coating compactness by causing crosslinking in the polymer framework. Additionally, the compactness increases as the percentage of TTA increased. The EDX results show that titanium is present in epoxy-TTA5 coating (Fig. S4).
3.2.7 Salt spray test
In the salt spray test, all coated MS samples were exposed to a hard corrosive environment (NaCl solution of 3.5%) for time period of 500 h. Figure 6 shows pictures of the samples after 500 h of exposure to salt fog. The findings show that epoxy coatings cause the deposition of corrosion products, but epoxy-TTA coatings make samples to appear less rusted. The coatings’ corrosion resistance improves as the amount of TTA increase. Blisters and delamination were eliminated from the epoxy-TTA5 coating. These findings confirm that the inclusion of TTA creates a stronger barrier layer protection at the coating-to-metal contact, improving corrosion protection effectiveness in highly corrosive environments.
3.2.8 Electrochemical test
3.2.8.1 Tafel polarization
To quantitatively analyze the anticorrosive performance of bare steel, epoxy, and Epoxy-TTA5 coated steel samples, the electrochemical tests were carried out in 3.5 wt.% NaCl aqueous solution. The findings are shown in Fig. 7 and Table 3. The Icorr value was significantly reduced from bare steel (9.89 µA/cm2) to epoxy-TTA5 coated steel (2.28 × 10−4 µA/cm2), as shown. Corrosion potential, which rises as corrosion current density declines, is a strong indicator of the epoxy-TTA5 hybrid composite coatings’ capacity to effectively protect against corrosion (Liu et al. 2017). This may be due to the development of compact, hydrophobic, highly cross-linked Ti–O–C and Ti–O–Ti composite coatings. By strengthening the interaction between the coating and the steel, these linkages enhance reasonable adhesion and enhance the blocking actions against corrosive species.
The above findings are further supported by an increase in polarization resistance (bare steel: 2.55 KΩ cm2; epoxy-TTA5 coated steel: 52.42 × 103 KΩ cm2) and a drop in corrosion rate, from 4.58 mpy (bare steel) to 1.05 × 10−4 mpy (epoxy-TTA5).
When compared to earlier similar results, it is worth noting that epoxy-TTA5 coating seems to represent a possible corrosion barrier in 3.5% NaCl (Table 3).
3.2.8.2 Electrochemical impedance spectroscopy
EIS is a potent tool to investigate nature of coatings on the surface of metal exposed to the harsh environment. The steel samples were dipped in 3.5% NaCl for varied immersion time and the observed Nyquist plots are presented in Fig. S1.
As per the Nyquist plots, addition of TTA in epoxy has a positive impact on the corrosion resistivity of MS in the solution. The appearance of semicircles with larger diameter in the Nyquist plots in epoxy-TTA5 suggested great efficiency of the coating. In bare steel, one time constant was observed, however, in the coated samples second time constant was appeared at higher frequencies.
The electrical circuits for one and two time constants are given in Fig. 8.
In Fig. 8, Rct is the charge transfer resistance, Rs is the solution resistance, Rf is the film resistance and CPEdl and CPEf are the constant phase element of double layer and film, respectively. The best fitting parameters are presented in Table S1.
The inhibition efficiencies of the coated steel samples were calculated using Eq. (1) and it was found to be higher (99.99%) for epoxyTTA5 coated samples as compared to epoxy coated samples (97%) after 24 h immersion in NaCl solution. The same trend was further obtained after 7 days immersion, i.e., 94.03 and 99.60 respectively, for epoxy and epoxy-TTA5 coated samples.
3.3 Computational studies
3.3.1 DFT (density functional theory)
DFT calculations were made to investigate the adsorption mode of crosslinked epoxy and epoxy-TTA molecules over steel surface. The resultant DFT optimized structures and their frontier molecular orbitals (FMO) of both the molecules are shown in Fig. 9 and their quantum parameters are summarized in Table 4 (Intellectual Property India 2022).
The highest occupied molecular orbital (HOMO) of crosslinked epoxy, as depicted in Fig. 9, is located mainly on ether O atoms and its adjacent benzene rings in one bisphenol A fragment of the molecule, whereas the LUMO (lowest unoccupied molecular orbital), which is dispersed on aromatic rings in the other bisphenol A fragment, which is consistent with the study of a similar work described by Bahlakeh et al. (2016).While the LUMO of TTA-epoxy is primarily located over the –O–Ti(OH3) part of the epoxy molecule, indicating its electron acceptor nature during the epoxy-metal interactions, the HOMO of the epoxy-TTA molecule is mainly dispersed over aromatic rings in one bisphenol A fragment and its adjoining ether O atoms, which is linked with high electron density (Ghosal and Ahmad 2017). The quantum parameters displayed in Table 4 further demonstrated that the most of the reported DFT indices supported the experimental findings.
Low value of ΔE (ELUMO − EHOMO), which is connected with high reactivity of the coatings, facilitates donor acceptor interactions between an organic coating and metal surface. This is because low value of ELUMO and high value of EHOMO. Epoxy-TTA formulation’s strong interaction with the metallic surface is indicated by the fact that its ΔE (4.46 eV) value is lower than epoxy’s (5.248 eV). Additionally, increased softness (0.447 eV) and reduced hardness (2.23 eV) values promote the epoxy-TTA’s reactivity, which can accept and transfer electrons during epoxy-metal interactions (Dagdag et al. 2020a). Both the epoxy (0.306 eV) and TTA-epoxy (0.275 eV) optimized structures have a positive and less than 3.6 ΔN (Dagdag et al. 2020b) value, indicating that both inhibitor molecules can readily interact with metallic surfaces by donating their electrons into the vacant orbitals of the Fe metal. The remaining quantum parameters are in good accordance with the experimental findings, with epoxy-TTA having greater inhibitory efficacy than epoxy (Dagdag et al. 2020b).
The Mulliken atomic charge distribution on the epoxy and epoxy-TTA atoms is depicted in Fig. 10. The atoms of nitrogen, oxygen, and certain carbon atoms in epoxy and epoxy-TTA all possessed negative charges and were assumed to be the cause of the nucleophilic attack on the metal surface based on the evaluation made from Fig. 10 (Dagdag et al. 2020a). The titanium center in epoxy-TTA, however, had a positive charge that allowed it to receive electrons from the steel surface and promote the donor–acceptor interaction with the metal surface. The acceptor–donor interactions of the local reactive regions in epoxy and epoxy-TTA resin with the metal surface were examined further using the Fukui indices (FI). Fukui functions were calculated using the DMol3 code, which generalized-gradient approximation (GGA) using Perdew–Burke–Ernzerhof (PBE) scheme was used to was used to compute electronic exchange correlation term through double numeric polarization (DNP) basis set (Perdew et al. 1996). The first derivative of the electron density (ρ(\(\vec{r}\))) with respect to the total number of electrons (N) under constant potential (ν(\(\vec{r}\))) yields the Fukui function (f(\(\vec{r}\))). Using Hirshfeld population analysis and the finite difference (FD) approximation, Fukui functions connected to nucleophilic (\(f^{ + } (\vec{r}\))) and electrophilic (\(f^{ - } (\vec{r}\))) centers were calculated and are shown in F3. of the supporting information (Parr et al. 2005). According to the evidence (Fig. S5), the distribution of HOMO and point charges in epoxy resin is consistent with the Fukui f− function observed on benzene rings in one bisphenol A fragment, as well as its nearest aminoamide nitrogen atoms and ether oxygen atoms, which show that these locations act as reactive electrophilic centers and lose their electrons during their reaction with metal surface.
According to the evidence, the distribution of HOMO and point charges in epoxy resin is consistent with the Fukui f− function observed on benzene rings in one bisphenol A fragment, as well as its nearest aminoamide nitrogen atoms and ether oxygen atoms, which show that these locations act as reactive electrophilic centers and lose their electrons during their reaction with metal surface. While on the second bisphenol A fragment, the reactive sites for nucleophilic performance (i.e., f+) were found on two benzene rings, which are closely congruent with the LUMO region and are in good accord with the results reported by Bahlakeh et al. (2016).
In the scenario of epoxy-TTA, Fukui f+ was only found over the titanium center, which is in good accord with the LUMO region, and Fukui f− function existed over aromatic rings in one bisphenol A fragment, as well as its surrounding aminoamide nitrogen atoms and ether oxygen atoms. This is further supported by the values for second order Fukui functions (Δf), dual local softness (Δσ) and the dual philicity (Δω) for epoxy and epoxy-TTA (Fig. S6).
The experimental data provide complete support for the calculated DFT results. The FT-IR data show that TTA modification of epoxy molecules interacts with epoxy’s –OH groups to produce the O–Ti–O and Ti–O–C connections. The pull-off test showed that these bonds increased epoxy’s adherence to metal surfaces. The presence of the Ti center provides vacant orbitals that can accept electrons from the metallic surface and can facilitate interactions between epoxy and metal, according to DFT parameters, which also showed that adhesion of epoxy-TTA molecules over metal surfaces is enhanced because of increased donor–acceptor interaction.
3.3.2 Molecular dynamic (MD) simulation
Following 500 iterations of smart minimization, dynamics calculations were performed on all of the slab models for crosslinked epoxy resin and its TTA form over Fe(110).
The Anderson thermostat at 298 K and the NVT ensemble for 250 ps with a time step of 1.0 fs were used to continue the MD on the systems that had minimized their energy (Sun 1998). All of the potential energy parameters necessary for inter/intramolecular interactions at the metal and coating contact were generated using the COMPASS force field. Atom-based cut-off and Ewald approaches were used to represent the columbic and van der Waals interactions, respectively. All of the iron slab atoms’ locations were kept in a frozen state during simulations.
Figure 11 displays the low-energy orientations and the most stable of epoxy and epoxy-TTA on metal surfaces. Following simulation, the interaction of epoxy and epoxy-TTA with metallic surfaces via aromatic rings and heteroatoms showed their flat orientation. In epoxy-TTA, the –Ti(OH)3 group is also quite close to the iron surface, which suggests an important turn in increasing the system’s overall adsorption power. The hetero-oxime atoms are also moving toward the steel surface, which improved the system’s overall adsorption strength and led to the development of a powerful barrier. The following evaluations of the adsorption energy (Eads) in both dry and wet circumstances were made, and the results are shown in Table 5.
In dry condition,
In wet condition,
The Eads values for the epoxy and epoxy-TTA were − 422 and − 617 kcal/mol, respectively, while the obtained values for NaCl solutions were − 373 and − 583 kcal/mol. Negative Eads values show that epoxy and epoxy-TTA adsorption is a spontaneous event (Dagdag et al. 2020c). Moreover, as evidenced by the larger negative Eads in the epoxy-TTA case, treatment of epoxy with TTA enhances its reactivity with metal surface. The Eads values show that the interaction of coatings with metal surfaces reduces in aqueous NaCl solution due to corrosive species.
The results from the electrochemical test and salt spray methods are consistent with the comparative efficacy of the two anticorrosive materials, epoxy and epoxy-TTA.
4 Conclusions
In the present investigations, an epoxy resin was modified with titanium tetra-acetoximate, [Ti(ON=C(CH3)2)4] and the modified epoxy resin was estimated as anticorrosive coatings for mild steel in aqueous NaCl solution. The findings of the study are:
-
1.
Inhibition efficacy of the epoxy resin is enhanced in the TTA modified resin due to insertion of Ti–O–C and O–Ti–O linkages which shows blocking effect and obstruct the diffusion of corrosive species.
-
2.
Electrochemical and salt spray tests indicate that on modification of epoxy with TTA, their anticorrosive nature increases.
-
3.
According to DFT studies, epoxy resin has a number of active sites made up of aromatic rings and heteroatoms. These active sites are enhanced by TTA modification, which enabled epoxy resin to firmly adsorb on metal surfaces, enhancing adhesion.
-
4.
MD simulations furthermore suggest that the epoxy-TTA resin, as compared to epoxy resin, results in improved coating/metal interaction. Epoxy-TTA had a higher negative value of Eads than epoxy resin, indicating a high level of adsorption on metal surfaces.
Data availability
All the required data included in the present research is available in the manuscript and supplymentary file.
References
Ali F, Ali N, Altaf M, Said A, Shah SS, Bilal M (2020) Epoxy polyamide composites reinforced with silica nanorods: fabrication, thermal and morphological investigations. J Inorg Org Polym Mater 30(10):3869–3877. https://doi.org/10.1007/S10904-020-01518-5
Allen RO, Sanderson P (2006) Characterization of epoxy glues with FTIR. Appl Spectrosc Rev 24:175–187. https://doi.org/10.1080/05704928808060457
Bach LX, Van Thuan D, Thu VTH, Phan TB, Vu NSH, Nam ND (2019) An investigation on titania multilayer coatings for enhanced corrosion resistance of carbon steel in simulated seawater by sol–gel dip coating. J Mark Res 8:6400–6406. https://doi.org/10.1016/J.JMRT.2019.09.061
Bahlakeh G, Ramezanzadeh B (2017) A detailed molecular dynamics simulation and experimental investigation on the interfacial bonding mechanism of an epoxy adhesive on carbon steel sheets decorated with a novel cerium-lanthanum nanofilm. ACS Appl Mater Interfaces 9:17536–17551. https://doi.org/10.1021/ACSAMI.7B00644
Bahlakeh G, Ghaffari M, Saeb MR, Ramezanzadeh B, de Proft F, Terryn H (2016) A close-up of the effect of iron oxide type on the interfacial interaction between epoxy and carbon steel: combined molecular dynamics simulations and quantum mechanics. J Phys Chem C 120:11014–11026. https://doi.org/10.1021/ACS.JPCC.6B03133/ASSET/IMAGES/MEDIUM/JP-2016-03133Y_0015.GIF
Chaudhary A, Dhayal V, Nagar M, Bohra R, Mobin SM, Mathur P (2011) Chemically modified oximato complexes of titanium(IV) isopropoxide as new precursors for the sol–gel preparation of nano-sized titania: crystal and molecular structure of [Ti{ONC10H16}4·2CH2Cl2]. Polyhedron 30:821–831. https://doi.org/10.1016/J.POLY.2010.12.025
Chiniwalla P, Bai Y, Elce E, Shick R, Christopher McDougall W, Bidstrup Allen SA, Kohl PA (2003) Crosslinking and decomposition reactions of epoxide functionalized polynorbornene. Part I. FTIR and thermogravimetric analysis. J Appl Polym Sci 89:568–577. https://doi.org/10.1002/APP.12234
Chopra I, Ola SK, Priyanka, Dhayal V, Shekhawat DS (2022) Recent advances in epoxy coatings for corrosion protection of steel: experimental and modelling approach—a review. Mater Today Proc 62:1658–1663. https://doi.org/10.1016/J.MATPR.2022.04.659
Dagdag O, Berisha A, Safi Z, Dagdag S, Berrani M, Jodeh S, Verma C, Ebenso EE, Wazzan N, El Harfi A (2020a) Highly durable macromolecular epoxy resin as anticorrosive coating material for carbon steel in 3% NaCl: computational supported experimental studies. J Appl Polym Sci 137:49003. https://doi.org/10.1002/APP.49003
Dagdag O, Guo L, Safi Z, Verma C, Ebenso EE, Wazzan N, Masroor S, Haldhar R, Jodeh S, El Gouri M (2020b) Epoxy resin and TiO2 composite as anticorrosive material for carbon steel in 3% NaCl medium: experimental and computational studies. J Mol Liq 317:114249. https://doi.org/10.1016/J.MOLLIQ.2020.114249
Dagdag O, Safi Z, Erramli H, Wazzan N, Guo L, Verma C, Ebenso EE, Kaya S, El Harfi A (2020c) Epoxy prepolymer as a novel anti-corrosive material for carbon steel in acidic solution: electrochemical, surface and computational studies. Mater Today Commun 22:100800. https://doi.org/10.1016/J.MTCOMM.2019.100800
Fayomi OSI, Akande IG, Oluwole OO, Daramola D (2018) Effect of water-soluble chitosan on the electrochemical corrosion behaviour of mild steel. Chem Data Collect 17–18:321–326. https://doi.org/10.1016/J.CDC.2018.10.006
Ghosal A, Ahmad S (2017) High performance anti-corrosive epoxy–titania hybrid nanocomposite coatings. New J Chem 41:4599–4610. https://doi.org/10.1039/C6NJ03906E
Hamdy AS, Saeh AG, Shoeib MA, Barakat Y (2007) Evaluation of corrosion and erosion–corrosion resistances of mild steel in sulfide-containing NaCl aerated solutions. Electrochim Acta 52:7068–7074. https://doi.org/10.1016/J.ELECTACTA.2007.05.034
Hegde MB, Mohana KN (2020) A Sustainable and eco-friendly polymer based graphene oxide nanocomposite anti-corrosion coating on mild steel. ChemistrySelect 5:1506–1515. https://doi.org/10.1002/SLCT.201904534
Hossain F, Ulčickas V-A (2021) Analysis and prevention of environmental- and corrosion-related failures, failure analysis and prevention, pp 459–478. https://doi.org/10.31399/ASM.HB.V11.A0006782
Hsissou R, Benhiba F, Echihi S, Benzidia B, Cherrouf S, Haldhar R, Ahmad Alvi P, Kaya S, Serdaroğlu G, Zarrouk A (2021) Performance of curing epoxy resin as potential anticorrosive coating for carbon steel in 3.5% NaCl medium: combining experimental and computational approaches. Chem Phys Lett 783:139081. https://doi.org/10.1016/J.CPLETT.2021.139081
Hsissou R, Azogagh M, Benhiba F, Echihi S, Galai M, Shaim A, Bahaj H, Briche S, Kaya S, Serdaroğlu G, Zarrouk A, Ebn Touhami M, Rafik M (2022a) Insight of development of two cured epoxy polymer composite coatings as highly protective efficiency for carbon steel in sodium chloride solution: DFT, RDF, FFV and MD approaches. J Mol Liq 360:119406. https://doi.org/10.1016/J.MOLLIQ.2022.119406
Hsissou R, Benhiba F, El Aboubi M, Abbout S, Benzekri Z, Safi Z, Rafik M, Bahaj H, Kaba M, Galai M, Wazzan N, Briche S, Boukhris S, Zarrouk A, EbnTouhami M, Rafik M (2022b) Synthesis and performance of two ecofriendly epoxy resins as a highly efficient corrosion inhibition for carbon steel in 1 M HCl solution: DFT, RDF, FFV and MD approaches. Chem Phys Lett 806:139995. https://doi.org/10.1016/J.CPLETT.2022.139995
Intellectual Property India (2022) https://ipindiaservices.gov.in/PatentSearch/PatentSearch/ViewApplicationStatus (accessed 23 Dec 2022)
Jaseela PK, Kuruvilla M, Williams L, Jacob C, Shamsheera KO, Joseph A (2020) Excellent protection of mild steel in sodium chloride solution for a substantial period of time using a hybrid nanocoating of poly vinyl alcohol and Titania. Arab J Chem 13:6921–6930. https://doi.org/10.1016/J.ARABJC.2020.07.005
Kirtay S (2014) Characterization of SiO2–TiO2 hybrid corrosion protective coatings on mild steel. J Mater Eng Perform 23(12):4309–4315. https://doi.org/10.1007/S11665-014-1218-Y
Li X, Deng S, Xie X (2014) Experimental and theoretical study on corrosion inhibition of oxime compounds for aluminium in HCl solution. Corros Sci 81:162–175. https://doi.org/10.1016/J.CORSCI.2013.12.021
Li Z, Bi H, Weinell CE, Ravenni G, Benedini L, Dam-Johansen K (2023) Investigation of zinc epoxy coatings modified with pyrolyzed and gasified biochar nanoparticles for corrosion protection. Prog Org Coat 178:107477. https://doi.org/10.1016/J.PORGCOAT.2023.107477
Liu J, Zhang L, Mu X, Zhang P (2017) Studies of electrochemical corrosion of low alloy steel under epoxy coating exposed to natural seawater using the WBE and EIS techniques. Prog Org Coat 111:315–321. https://doi.org/10.1016/J.PORGCOAT.2017.06.012
Liu J, Yu Q, Yu M, Li S, Zhao K, Xue B, Zu H (2018) Silane modification of titanium dioxide-decorated graphene oxide nanocomposite for enhancing anticorrosion performance of epoxy coatings on AA-2024. J Alloys Compd 744:728–739. https://doi.org/10.1016/J.JALLCOM.2018.01.267
Mishra P, Kumar P (2014) Enhancement of dielectric properties of 0.2[BZT-BCT]–0.8[(1–x)epoxy–xCCTO] (x = 0.02, 0.04, 0.06, 0.08 and 0.1) composites for embedded capacitor and energy harvesting applications. J Alloys Compd 617:899–904. https://doi.org/10.1016/J.JALLCOM.2014.08.029
Mostafa KE, El-Sayed K, Hamid ZA, Salah Eldin TA, Khalil MW, Hassan HB (2019) Anti-corrosion nickel/reduced graphene oxide-titanium dioxide coating for mild steel in organic acids. J Mater Environ Sci 10:141–162. http://www.jmaterenvironsci.com (accessed 20 Dec 2022)
Nath Upadhyay S (2004) The inhibition of corrosion of mild steel by some fluoroquinolones in sodium chloride solution. Production of Biobutanol from Cyanobacterial Biomass View Project Waste Management View Project. Trans Indian Inst Met 57:297–306. https://www.researchgate.net/publication/43526805 (accessed 22 Dec 2022)
Parr RG, Ayers PW, Nalewajski RF (2005) What is an atom in a molecule? J Phys Chem A 109:3957–3959. https://doi.org/10.1021/JP0404596
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865. https://doi.org/10.1103/PhysRevLett.77.3865
Pour ZS, Ghaemy M, Bordbar S, Karimi-Maleh H (2018) Effects of surface treatment of TiO2 nanoparticles on the adhesion and anticorrosion properties of the epoxy coating on mild steel using electrochemical technique. Prog Org Coat 119:99–108. https://doi.org/10.1016/J.PORGCOAT.2018.02.019
Ramaprakash M, Sreedhar G, Mohan S, Panda SK (2016) Corrosion protection studies of CeO2–TiO2 nanocomposite coatings on mild steel. Trans IMF 94:254–258. https://doi.org/10.1080/00202967.2016.1209892
Saini A, Singh D, Dhayal V (2022) Structural and optical properties of titania nanostructures obtained from oxime-modified titanium (IV) precursor. Mater Res Innov 26:276–284. https://doi.org/10.1080/14328917.2021.1963577
Semon WL (1923) The preparation of hydroxylamine hydrochloride and acetoxime. J Am Chem Soc 45:188–190. https://doi.org/10.1021/JA01654A028/ASSET/JA01654A028.FP.PNG_V03
Sun H (1998) COMPASS: an ab initio force-field optimized for condensed-phase applications overview with details on alkane and benzene compounds. J Phys Chem B 102:7338–7364. https://doi.org/10.1021/JP980939V
Ta TD, Tieu AK, Zhu H, Kosasih B (2015) Adsorption of normal-alkanes on Fe(110), FeO(110), and Fe2O3(0001): influence of iron oxide surfaces. J Phys Chem C 119:12999–13010. https://doi.org/10.1021/ACS.JPCC.5B01847/ASSET/IMAGES/MEDIUM/JP-2015-01847J_0004.GIF
Wang Y, Lim S, Luo JL, Xu ZH (2006) Tribological and corrosion behaviors of Al2O3/polymer nanocomposite coatings. Wear 260:976–983. https://doi.org/10.1016/J.WEAR.2005.06.013
Xavier JR (2020) Effect of surface modified WO3 nanoparticle on the epoxy coatings for the adhesive and anticorrosion properties of mild steel. J Appl Polym Sci 137:48323. https://doi.org/10.1002/APP.48323
Zhang SD, Wu J, Qi WB, Wang JQ (2016) Effect of porosity defects on the long-term corrosion behaviour of Fe-based amorphous alloy coated mild steel. Corros Sci 110:57–70. https://doi.org/10.1016/J.CORSCI.2016.04.021
Acknowledgements
Veena Dhayal is grateful to the Department of Automobile Engineering, IISc Bangalore, as the computational work were done there. Veena Dhayal is thankful to SAIF and CAF labs, Manipal University Jaipur to carry out all the test.
Funding
Veena Dhayal is appreciative to SERB, New Delhi, for financing this project (TAR/2020/000233).
Author information
Authors and Affiliations
Contributions
Saraswati K Ola- Wrote the rough draft (experimental work) Ishita Chopra - Wrote the rough draft (computational work) Veena Dhayal- reviewed the manuscript S. Goplakrishana- reviewed the manuscript (computational).
Corresponding author
Ethics declarations
Conflict of interest
The authors of this article have stated categorically that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ola, S.K., Chopra, I., Gopalakrishnan, S. et al. Synergistic anticorrosive properties of titanium tetra-acetoximate modified epoxy hybrid coatings: experimental and computational approaches. Multiscale and Multidiscip. Model. Exp. and Des. 7, 459–475 (2024). https://doi.org/10.1007/s41939-023-00215-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41939-023-00215-3