Abstract
As the global energy policy gradually shifts from fossil energy to renewable energy, lithium batteries, as important energy storage devices, have a great advantage over other batteries and have attracted widespread attention. With the increasing energy density of lithium batteries, promotion of their safety is urgent. Thermal runaway is an inevitable safety problem in lithium battery research. Therefore, paying attention to the thermal hazards of lithium battery materials and taking corresponding preventive measures are of great significance. In this review, the heat source and thermal hazards of lithium batteries are discussed with an emphasis on the designs, modifications, and improvements to suppress thermal runaway based on the inherent structure of lithium batteries. According to the source of battery heat, we divide it into reversible heat and irreversible heat. Additionally, superfluous heat generation has profound effects, including thermal runaway, capacity loss, and electrical imbalance. Thereafter, we emphatically discuss the design and modification strategies for various battery components (anodes, cathodes, electrolytes, and separators) to suppress thermal runaway. Preparation of solid electrolyte interphase layers with excellent thermal stability and mechanical properties is the core of the modification strategy for anode materials. Additives, stable coatings, elemental substitution, and thermally responsive coating materials are commonly used to improve the safety of cathodes. Novel electrolyte additives, solid-state electrolytes, and thermally stable separators provide a good opportunity to solve the thermal runaway problem of next-generation high-performance electrochemical storage devices.
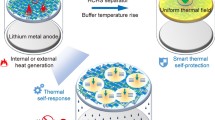
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Energy is essential for human survival and a key factor in the sustainable development of society. Unchecked misuse and exploitation of fossil fuels have led to climate change and an energy crisis. To mitigate the impact of the depletion of nonrenewable energy resources and the adverse effect of climate change arising from carbon dioxide emissions, developing alternative clean energy sources and new forms of energy utilization is essential. Researchers have developed a variety of electrochemical and photoelectric energy storage and conversion systems to satisfy the requirements of modern society, such as lithium batteries [1,2,3,4,5], electrocatalytic batteries [6], fuel cells [7], microbial batteries [8], metal–air batteries [9], and supercapacitors [10]. To date, lithium batteries have proven to be nearly the most important energy storage devices due to their ultrahigh energy and power densities, excellent cycling stability, and environmental friendliness [9]. Figure 1 shows the stages in the development of lithium batteries. Despite being the most widely used battery on the market, the performance of lithium batteries must be verified by practical application. In addition to being limited by the inherent electrical, thermodynamic, and electrochemical impedances, lithium batteries generate a certain amount of heat while supplying energy [10]. Furthermore, lithium batteries usually possess ultrahigh energy density, and studies have shown that a higher energy density results in poorer stability [11]. Under severe conditions such as extreme temperatures and overcharging/overdischarging, lithium batteries fail [12, 13]. In 1912, G. N. Lewis successfully developed the first batch of lithium batteries, and they were approved for manufacture and commercialization in the 1990s by SONY Corp. However, the initial product underwent a combustion event due to thermal escape. The failure mechanism was determined to be the formation of lithium dendrites on the electrodes, which then pierced the separator, resulting in a short circuit. Meanwhile, the increasing temperature melted the lithium metal, which eventually led to thermal runaway. Most typical battery components, such as separators, electrolytes, and packaging, are flammable and destroy the intrinsic stability of the battery under various adverse conditions. Researchers believe that a series of exothermic side effects induce the final thermal runaway [14,15,16,17,18,19,20], for example, solid electrolyte interphase (SEI) decomposition due to heat that results in a short circuit, electrolyte decomposition due to excessive charge and discharge, melting of polyethylene (PE) and polypropylene (PP) separators at high temperatures, formation of dendrites on lithium electrodes that puncture the separators, and accumulation of combustible substances in the sealed battery. These effects are successively triggered because of increasing temperature and continue to produce heat that cannot be released, which initiates a chain reaction inside the battery leading to thermal runaway. Eventually, catastrophic consequences may occur, such as severe combustion and explosion [21]. Figure 2 shows aviation/airport incidents involving lithium batteries on passenger airliners and cargo aircraft recorded from 2014 up to November 4, 2020 (these are recent events that the Federal Aviation Administration is aware of) [22]. From the statistics, we know that passenger aircraft that carry numerous people have a higher probability of incidents. Moreover, the number of aircraft incidents involving lithium batteries has dramatically increased since 2016, which emphasizes the urgent need to enhance the thermal stability of lithium batteries.
Aviation/airport incidents involving lithium batteries recorded from 2014 up to November 4, 2020. Data obtained from Ref. [22]
The four essential ingredients of a lithium battery are the cathode, the anode, the electrolyte, and the separator. As the lithium ions move between the anode and the cathode, the lithium battery and the external wire form a closed circuit, enabling work to be performed externally. To maximize the portability and high energy density advantages, broaden the market, and comprehensively mitigate the thermal hazards of lithium batteries, research has aimed to strengthen the inherent safety and improve the thermal management system of batteries to prevent thermal failure [23,24,25,26,27,28,29,30,31,32,33,34,35,36]. Strengthening the inherent safety involves improving the intrinsic properties of the battery, such as the fire-retardant characteristics of the battery components and the stability of the SEI and the cathode–electrolyte interface (CEI), and inhibiting the formation of lithium dendrites. The improvement in the thermal management system involves strategies such as incorporating thermally responsive smart materials to control the chemical reaction of the battery, a cooling system in usage, a thermal hazard warning, and a firefighting device in case of danger. Before the occurrence of thermal runaway, when the temperature of lithium batteries increases, a large amount of flammable gas is released, forming a bulge within the battery. Battery safety devices such as temperature sensors and gas sensors are employed to monitor and warn about hazards and to prevent further escalation of battery failures. These revolutionary and innovative modifications, combined with theoretical models, advanced characterization, and electrochemical analysis techniques, greatly facilitate a deeper comprehension of the safety matters of lithium batteries. In recent years, to expand the application range of lithium batteries and avoid catastrophic failures, extensive research has been conducted on techniques to inhibit thermal runaway and on the material design of lithium batteries. However, few comprehensive reviews have focused on modification of the material design of single lithium batteries and their components. In this review, we discuss the heat sources of lithium batteries and thermal hazards in lithium batteries based on their inherent structures, focusing on the design, optimization, and modification of the components of a single battery to inhibit thermal runaway. First, we present a summary of safety incidents resulting from lithium battery failure in recent years. Second, we reveal the source of battery heat in the inherent structure and elaborate on the thermal hazards caused by overheating. Thereafter, we focus on the design and modification strategies for various battery components to prevent thermal runaway, including the selection of electrode materials (cathodes and anodes) and optimization of other battery components (such as the electrolytes, separators, and collectors). Finally, we summarize the detailed review and discuss the further development of battery safety in future.
2 Safety Accidents Caused by Lithium Battery Failures
Table 1 lists accidents caused by lithium battery failure in recent years. Lithium batteries have numerous common applications, such as in airplanes, mobile phones, laptops, and electric buses. Airplane incidents with notorious social effects are often the most distressing and the most publicized. These accidents include failures attributed to pilot errors that result in structural damage of the airplane, short-circuiting of battery packs, and fires (Accident 1). Alternatively, the battery packs on an airplane can leak electrolytes due to collisions, resulting in large high-temperature fires (Accident 3). In another scenario, failure of the carried electronic equipment can cause the airplane battery pack to ignite, filling the cabin with dense fog. This impairs the pilot's field of vision, leading to diversion of the airplane's flight path (Accident 2). Battery pack failures in airplanes are generally the result of separator puncture, external impact, or pressure or temperature changes. These conditions result in electrolyte leakage and a short circuit in the battery, triggering a fire (Accident 4 and Accident 5). Accordingly, strict control measures should be taken to search passengers and goods transported by airplanes to ensure control over the sources and eliminate the hidden risk of fire. As portable electronic devices are used in many aspects of our lives, the failure of battery packs in mobile phones and laptops may cause harm to life and property, which has triggered great social concern. As shown in Table 1, mainstream mobile phone brands such as Samsung, Apple, and Huawei have not been able to prevent thermal runaway, even though these large companies lead the industry in quality control. Mobile phone and laptop accidents are often caused by overcharging (Accident 7 and Accident 9). The overcharging of electronic equipment generates a large amount of heat that is sufficient to damage the inherent structure of the lithium battery, causing short circuits and consequently fires. In addition, the natural characteristics of mobile devices during ordinary use are also worthy of attention (Accident 6 and Accident 8). In the pursuit of higher energy density and power density batteries, large mobile phone brands have reduced the weight of inherent equipment such as separators, thereby increasing the probability of accidents. In view of the hazard of thermal runaway in such portable devices, progressively more research has aimed to promote the energy density of batteries while simultaneously improving the inherent battery safety. Electric vehicles are driven by a high-power battery system that contains numerous parallel battery cells in series. Due to the long-term driving and unique working environments of electric vehicles, the battery pack system needs to operate at different vibration frequencies, at extreme temperatures and humidity, under water invasion, and at excessive discharge depths. These are all challenges for electric vehicles. Running electric vehicles may encounter problems such as vehicle collisions, overcharge and overdischarge, electrolyte leakage, and electrical system failure, which can lead to thermal runaway and spontaneous combustion (Accident 10 and Accident 11). Such failures will seriously hinder the development of the electric vehicle industry. A more detailed and comprehensive understanding is required to identify safety issues that result in lithium battery accidents to design safer and more reliable battery systems for electric vehicles.
An increasing number of countries and regions require lithium batteries to be granted approval through various standardized tests for social acceptance of widespread lithium battery applications in the market. The probability of accidents caused by lithium batteries can be greatly reduced if the test standards are adhered to. However, during the use of lithium batteries, external conditions (including pressure, extreme temperatures, and hard object collisions), battery abuse, and overcharge/overdischarge will still lead to accidents even if batteries on the market meet these standards. In addition, the battery itself may have defects from the manufacturing process. Moreover, electrical equipment aging occurs with repeated charging and discharging, which also results in failure. Hence, researchers are dedicated to studying the working principles of lithium batteries and the source of internal heat to reduce heat generation by modifying the cathode, the anode, and the contact surface. This will enable control of the thermal stability and safety of the battery pack after heat release by optimizing the composition of each inherent battery component (e.g., the separator and the electrolyte).
3 Heat Generation of Lithium Batteries
3.1 Intrinsic Properties of Lithium Batteries
Lithium is the lightest element in the alkali metal group and has the smallest radius. These characteristics impart an ultrahigh theoretical capacity and rapid conduction properties to lithium metal. When lithium metal, which has the highest negative potential of any electrode material, serves as the anode of a battery, it endows lithium metal batteries (LMBs) with high discharge voltages and high energy densities [48]. However, because of its high reactivity, lithium is rapidly oxidized in the presence of a minimum supply of water. Consequently, the lithium metal is unavoidably coated with lithium oxide (Li2O), lithium hydroxide (LiOH), lithium carbonate (Li2CO3), and lithium nitride (Li3N) [49]. The high reactivity and ultralow potential result in corrosion of the lithium metal by the electrolyte, which could hamper the opportunities and prospects for lithium batteries. In addition, the growth of lithium dendrites that puncture the separator results in various side reactions that consume the lithium metal and the electrolyte. This can lead to short-circuiting of lithium batteries, creating serious safety hazards [50]. To overcome these problems, lithium-ion batteries (LIBs) were developed. In LIBs, lithium ions with a small atomic radius are used to transfer charge. Under normal circumstances, the lithium metal in LIBs will not deposit in large quantities during charging, and thus, the problem of lithium dendrite formation will not occur [51]. The cathodes of LIBs are often paired with carbon-based anodes. The different types of LIBs are differentiated by the material used as the cathode. In LIBs, the most common electrolyte is a mixture of LiPF6, propylene carbonate, and ethyl carbonate [52]. LIBs are a promising technology, and considerable research has focused on the development of new LIBs with different anodes, cathodes, and electrolyte materials. Research has also aimed to improve the capacity, energy density, cycle life, and safety performance of LIBs [53]. However, regardless of whether an LMB or an LIB is used, the original stable structure can be damaged by abuse and other factors, leading to thermal runaway [54]. Improving the understanding of the working mechanism and principal heat sources of lithium batteries, selecting improved electrode materials, and optimizing the battery system are the main methods for avoiding thermal runaway in lithium batteries.
3.1.1 Working Principles of LMBs
LMBs are widely used in contemporary industry. Early on, people believed that batteries needed to discharge only once, which led to the invention of the lithium primary battery (LPB). LPBs have become the main power source for products with long-term and high-capacity energy requirements and are widely used in military and industrial applications [55]. LPBs usually comprise Li/CFx, Li/MnO2, Li/SO2, Li/SOCl2, and Li/CuF2 batteries, which are stable and discharge over a wide range of temperatures at a high theoretical discharge voltage of 3–4.5 V (vs. Li/Li+) and a high specific energy (> 700 Wh kg−1) [56]. The first five columns of Table 2 list the different types of common LPBs. Table 2 shows that there is always a voltage deviation between the actual plateau voltage and the theoretical discharge voltage of LPB cells. Taking a Li/CFx battery as an example, the theoretical voltage can reach 4.52 V (vs. Li/Li+), but the actual plateau voltage is much lower than the theoretical voltage. The CFx cathode prepared with the most common carbon source can only provide a plateau voltage of approximately 2.6 V (vs. Li/Li+) [57]. Researchers promoted the actual plateau voltage of Li/CFx by synthesizing and preparing various innovative carbon sources, such as hard carbon, graphene nanoribbons, and carbonized metal organic frameworks (MOFs). Predictably, owing to the poor electrical conductivity of fluorinated carbon, a large amount of thermal energy is generated by the Li/CFx battery [58]. Additionally, the energy associated with the deviation between the theoretical voltage and the actual plateau voltage is dissipated in the form of heat, as shown in Fig. 3. Because of this, the performance of Li/CFx batteries is unsatisfactory despite the high capacity and high voltage. In practical use, if thermal energy cannot be immediately released, then the battery will short-circuit, and the capacity will greatly attenuate, leading to thermal runaway with incalculable consequences.
With the development of society, numerous LPBs are being discarded after use. Moreover, due to the long-term continuous operation of equipment using LPBs, the inability to recycle them is becoming more apparent. To retain the negative potential of lithium (− 3.040 V vs. the standard hydrogen electrode), extra-high capacity (3860 mAh g−1), and low density (0.53 g cm−3), a rechargeable LMB system uses intercalation compounds as the cathode and lithium metals as the anode, aiming to overcome the shortcomings of LPBs. Therefore, a large number of new materials can be developed for rechargeable LMB systems. Excitingly, rechargeable LMB systems with innovative cathode materials composed of a multielectron system overcome the charge storage limitations of the inserted composite electrode material while displaying higher energy densities. From this viewpoint, Li-O2 and Li-S batteries possess extremely high theoretical capacities of up to 2567 Wh kg−1 and 3582 Wh kg−1, respectively [59,60,61,62,63]. The specific theoretical voltages and capacities of Li-S batteries are shown in Table 2. However, the large-scale application of metallic lithium is severely hampered by serious deficiencies such as high reactivity, instability of the SEI, volume change, dendrite growth, and the formation of dead lithium during electroplating/peeling processes [48, 59, 61]. The inferior cycling performance of LMBs is caused by continuous formation of dead lithium. Additionally, unrestricted dendrite growth will lead to puncture of the separator, causing catastrophic short-circuiting of the battery and leakage of the electrolyte, leading to thermal runaway. Based on the above failure mechanisms, to obtain a desirable safety performance of LIBs, breakthroughs are essential to control the electrochemical performance and deposition of lithium ions. Innovative designs include building an artificial anode/electrolyte interface and designing electrolytes and functional interlayers to suppress lithium dendrites, thereby achieving safe and stable lithium metal anodes.
3.1.2 Working Principles of LIBs
In 1991, SONY successfully commercialized LIBs with a cathode composed of lithium cobalt oxide. Because lithium ions alternate in the electrolyte, these LIBs are known as “rocking chair” batteries. The standard model for these batteries is “18650,” which is a columniform battery 65 mm in length and 18 mm in diameter. These LIBs have not only the characteristic of a high energy density but also no memory effect and a low maintenance cost [71]. After nearly 30 years of development, due to the innovation in electrode materials, the energy/power density and safety of LIBs have significantly improved [72]. Graphite is generally used as the anode in LIBs. LIBs are categorized according to the cathode material used: (i) cathodes based on metal dioxides, (ii) “spinel” cathodes, and (iii) cathodes containing transition-metal phosphates [73]. Various types of LIBs are shown in Table 3. LIBs utilize lithium ions as a “bridge” between the positive and negative poles that cycle regularly. Without a large amount of lithium metal in the LIB, the lithium dendrite formation problem will not occur. Compared with LMBs, LIBs have better cycling stability, which can prevent capacity loss and life shortening within the adaptive temperature range. However, in practical applications, LIBs become difficult to control. Their capacity decreases under abnormal conditions such as extreme temperatures, overcharge and overdischarge, and pressure caused by a hard material. As a result, the SEI and electrode materials decompose, promoting side reactions, the loss of the ability to work independently, and even short-circuiting, which eventually leads to thermal runaway [74]. In addition, long-term battery cycling will lead to anode lithium plating and poor rate discharge performance. Heat generation crucially affects the safe operation of LIBs; therefore, a thermal management system is required for LIBs to avoid heat escape and maintain stable performance in extreme cases. An appropriate thermal management system can extend the battery life, allowing the battery to achieve improved autonomous performance [75].
3.2 Lithium Battery Thermal Behavior
3.2.1 Principal Heat Sources of Lithium Batteries
The working foundation of batteries is chemical reactions. The amount of heat generated is closely related to the electrode material and the reaction equation. To create a thermal management system for lithium batteries, the principal heat sources of lithium batteries and the vertical parameters that influence heat generation must be understood. Viswanathan et al. highlighted that both reversible and irreversible heat generation must be considered in battery management systems [76]. Irreversible heat is released by the Joule effect (resistance resulting from charge transfer in a collector), while reversible heat is derived from electrochemical reactions. The reversible heat effect is significantly different for different lithium batteries due to the specific chemical reactions involved. For example, the reversible heat generated in graphite/LiCoO2 cells is 700% of the irreversible heat. Additionally, for charge and discharge at a depth of discharge (DOD) of 5%–40%, the difference in heat generation rates is 1.4 kW. However, for graphite/LFP batteries, the difference in heat generation rates is merely 50 W at a DOD of 2%–95%. The above values indicate better thermal stability of graphite/LFP batteries [76]. Therefore, graphite/LFP batteries are widely used in practical applications. In the following, we discuss the principal heat sources of lithium batteries in more detail [77].
Reversible heat (heat released by electrochemical reactions)
-
(i)
Materials of the cathode and the anode: The various battery materials determine how the different batteries work (i.e., the different chemical reactions). The choice of electrode material has a major effect on the amount of heat generated.
-
(ii)
Residual energy in the battery, the state of charge (SOC), energy released in a battery, and DOD: These parameters are related to the diffusion rate of lithium ions, which suggests that prevention of overcharge and overdischarge of the battery is a feasible approach to avoid thermal runaway.
-
(iii)
Temperature inside the battery: As the temperature inside the battery continues to rise, irreversible side reactions in the battery are excited, which generate a portion of the battery heat.
Irreversible heat (heat released by the Joule effect)
-
(i)
Charge and discharge currents: According to the formula Q = I2RT, the higher the discharge current is, the more the heat is generated by the Joule effect.
-
(ii)
Inherent resistance of the battery elements: This resistance includes those of the anode, the cathode, and electrolyte materials.
-
(iii)
Resistance associated with the diffusion of lithium ions through diverse components of the battery.
-
(iv)
Interfacial resistance to charge transfer at the interfaces between the electrolyte solution and insertion materials.
Note that (ii) is classified as an ohmic resistance, while (iii) and (iv) are classified as electrochemical resistances. When investigating the heat released by electrochemical batteries, these principal heat sources must be comprehensively considered.
3.2.2 Causes of Lithium Battery Failure
Generally, lithium batteries have a stable structure. During normal charging and discharging activities, lithium ions are transferred back and forth among the cathode and the anode. Unfortunately, various abuses may occur during use, resulting in destruction of the original structure of the lithium battery and eventual thermal runaway. Thermal runaway in lithium batteries generally has three stages [78,79,80]. First, when the temperature exceeds 80 °C, the SEI begins to decompose, while lithium formed on the anode starts to continuously consume the nearby electrolyte. Second, when the temperature exceeds 150 °C, the cathode material becomes active, and oxygen is rapidly released. Finally, if the temperature exceeds 190 °C, then both the cathode material and the electrolyte oxidize and decompose. This is a high-speed exothermic process with a temperature increase rate of up to 50 °C min−1. If it is not restrained in time, then accidents such as burning or explosions with open flames may occur. In addition, battery charging at extremely low temperatures, ultrahigh voltage, and ultralow voltage will have adverse consequences. We summarize the behavior of lithium batteries for various operating windows and incidental temperatures in Fig. 4. Thermal runaway is affected by various factors, in addition to abuse. Failure to stop it in time can result in smoke, flames, and even explosions (Fig. 5). The parameters that influence thermal runaway are as follows:
(i) Physical collision and hard penetration: When a lithium battery is subjected to a strong physical collision or hard penetration, the battery system is prone to deformation, resulting in tearing of the battery separator, internal short-circuiting, and even electrolyte leakage. The large amount of heat generated when the battery is continuously short-circuited can cause a fire. A study on the behavior of the battery system after physical collision and hard penetration requires multidimensional research from the basic component level to the battery level and the pack level. Sahraei et al. established a mechanical model that could predict short-circuiting inside a battery [81]. Their results showed that a lithium battery can withstand considerable deformation before short-circuiting but cannot maintain mechanical and electrothermal coupling. Zhang et al. improved the collision model from a purely mechanical model to one in which the mechanical, battery, and heat aspects were coupled [82]. To determine the reason for lithium battery damage during physical injury, more practical prototype tests should be designed. Coupling the mechanical, battery, and thermal aspects, as well as combining internal short-circuiting and thermal control, is also preferable.
(ii) Overcharge and overdischarge: Overcharge refers to the process during battery charging when the battery is connected to the power supply for a long time and excessive energy is pumped into the battery. Continuous charging causes the battery to generate excessive heat, which in turn causes the electrolyte to decompose and produce gas. Meanwhile, the pressure inside the battery increases, the battery deforms, and the battery performance sharply drops. Compared with normal charging, due to additional side effects and an increase in internal resistance, heating during overcharging is more damaging than overdischarging. Saito determined that the charging current was positively correlated with heat output, which indicated that the heat generated due to the internal resistance was a significant heat source during overcharging [83]. Mao et al. found that a large amount of gas was produced during overcharging of batteries [15]. At the anode, lithium metal was deposited that did not participate in the next charging cycle but instead reacted with the electrolyte to release more heat. Thus, restricting the plating of lithium metal and reducing the reaction heat were determined to be crucial for improving and ensuring the thermal safety of LIBs during overcharge cycling. Overdischarge is another type of battery abuse that occurs if the battery is discharged to below the cutoff voltage. Overdischarge causes excessive loss of lithium ions from the cathode, destruction of the stability of the cathode structure, and irreversible damage. At the same time, the production of gases such as CO and CO2 leads to swelling of the battery [84]. Ouyang et al. showed that overdischarging leads to capacity loss. Moreover, serious overdischarging may lead to battery failure [17].
(iii) Overheating: This is an event in which an overheated battery experiences thermal runaway. Thermal factors lead to separator melting, electrode and electrolyte decomposition, and numerous other side effects. Thermal runaway is rooted in thermal abuse. Zheng et al. studied the mechanism of thermal runaway and revealed the mechanism of thermal escape [85]. Studies have determined that significant weight loss arises when thermal runaway occurs. Thermal escape is closely related to internal short-circuiting because it releases additional Joule heat equivalent to the heat released by the chemical reaction. However, internal short-circuiting does not necessarily result in thermal escape. The relationship between the occurrence of internal short-circuiting and the characteristics of thermal escape is interesting and requires more research.
(iv) Short-circuiting: The process of short-circuiting can be classified as external short-circuiting and internal short-circuiting. An external short circuit occurs when the different electrodes are connected by a conductor. External short-circuiting is mostly caused by battery impact, water immersion, and contact with human skin. Protective electronic devices can be used to cut off the power supply in the event of a high current to reduce the occurrence of external short-circuiting. Fuses [86], positive thermal coefficient (PTC) devices [87], magnetic switches, and bimetallic thermostats [51] are the most frequently used. An internal short circuit occurs when the separator fails and the cathode and the anode come into direct contact. Once an internal short circuit is triggered, spontaneous chemical reactions occur, resulting in battery failure and the release of a large amount of heat to the extent that fires and explosions are inevitable. Internal short-circuiting is the most likely cause of thermal runaway, and almost all cases of thermal runaway will eventually be accompanied by internal short-circuiting. Internal short-circuiting is caused under numerous circumstances, for instance, physical collision and hard penetration contributing to tearing of the separator, a continuous increase in the internal temperature contributing to separator melting, and lithium dendrite puncture of the separator at the site of anode deposition. Mitigating internal short circuits is the most obvious method to prevent thermal runaway. Modification of the electrolyte, the separator, and the lithium anode will be discussed in more detail in the next chapter. The mechanism of internal short-circuiting is extremely complicated. However, as the main cause of battery failure, it is worthy of further research.
(v) Aging and defects: Aging and defects in the battery also affect the thermal performance of the battery and cause loss of battery capacity and power. Battery aging can be classified as calendar aging and active aging, which are due to battery storage at high temperatures and long-term use of the battery. Both types of aging transform the properties of the battery. Internal defects in the battery due to, for example, poor production quality, poor-quality separators, and material contamination can lead to battery failure and thermal runaway. Mohanty et al. conducted a systematic study on electrode defects, including agglomeration, pinholes, metal particle contamination, and nonuniformity [88].
3.3 Thermal Effects of Lithium Batteries
The electrical system, performance, the life cycle, and safety of lithium batteries are significantly affected by the storage and working temperatures [89]. Long-term operation at elevated temperatures (> 50 °C), even if the heating is not out of control, can negatively affect the charging and discharging characteristics of the battery, thereby shortening the service life of the battery [90]. At low temperatures, owing to the reduced activity of the electrode material, the diffusion rate of lithium ions in the electrolyte and the activity of the electrode materials decrease, causing the battery performance to dramatically decline. Jaguemont et al. conducted battery cycle tests at four different temperatures. The energy decreased from 293 to 215 Wh at the same discharge rate with decreasing temperature [91]. In this section, we focus on the influence of temperature on battery properties, namely, thermal runaway, capacity loss, and charge imbalance, as well as the effects of low temperature on battery performance.
3.3.1 Thermal Runaway
As described in Sect. 3.2, thermal runaway occurs when the temperature is out of control, bringing about separator melting, electrode and electrolyte decomposition, and numerous side reactions. Barkholt et al. used thermogravimetric analysis combined with differential scanning calorimetry and temperature-resolved X-ray diffraction to study the thermal stability of LIBs on a large scale [92]. Test data showed that the SEI and the anode reacted first. At approximately 100 °C, the SEI began to melt and decompose. Next, the anode lithium was exposed to the electrolyte, which led to lithium deactivation. Bugrynieca et al. studied the stability of LFP cathode cells and the severity of thermal runaway [93]. They found that with increasing SOC, the thermal runaway severity increased and the battery stability reduced. Furthermore, the battery displayed strong thermal stability and safety if the temperature of the battery system remained below a critical temperature (190–200 °C, at which the cathode material decomposed and the electrolyte/oxygen reacted). These results indicated that the LFP cell was more reliable than other cathode materials. When the temperature was 85–110 °C, the SEI layer began to thermally decompose at the anode. The released energy was absorbed by the electrolyte, leading to evaporation of the electrolyte. The SEI melted at 140 °C, causing further problems; for example, the organic electrolyte in the lithium battery vaporized and then mixed with oxygen, leading to combustion. Shortly afterward, the graphite electrode dissolved and released extra heat when the temperature reached 300 °C. Finally, when the temperature exceeded 660 °C, the aluminum current collector melted, which is perilous for the battery system. Koch et al. performed autoclave measurements on approximately 50 kinds of LIBs [94]. They subsequently proposed a method for measuring the volume of escaped gas during thermal runaway. Studies have shown that the battery capacity and energy density influence the volume of escaped gas and the loss of battery mass. Higher energy densities and capacities at the cell level lead to earlier and severer thermal runaway reactions. Therefore, these factors need to be considered in the selection of battery systems. Current trends indicate a preference for higher energy densities and capacities for batteries, which suggests that more effort is required to prevent additional gas formation and the associated increase in the severity of thermal runaway. In the pursuit of lithium batteries with a higher energy density, the disposal of escaped gas and thermal runaway should be seriously considered.
3.3.2 Capacity Loss
If the active substances inside a battery are converted into inactive substances, then the effective capacity will decay, resulting in capacity reduction at all discharge rates. In addition, the internal resistance of a battery increases after long-term cycling, resulting in a decrease in the operating voltage at all discharge rates. Forecasting the capacity and power loss in lithium batteries is difficult due to the various kinds of electrode materials and battery chemistries. The capacity loss problem of LFP batteries is the key obstacle for electric vehicles. Yang et al. studied the impact of changes in relative capacity, temperature, and electrolyte interphase film growth on the charge–discharge cycles of LFP batteries. The capacity loss was approximately 19.7% after 2000 cycles when the discharge rate increased from 0.5 to 2 C [95]. Simultaneously, the environmental temperature greatly aggravated the battery capacity loss. Moreover, a thicker anode is beneficial to the relative capacity of the battery, while the thickness of the separator has little effect on the capacity. Honkura et al. applied a mathematically advanced differential voltage analysis to LiCoO2/graphite batteries to study the mechanism of capacity degradation of LiCoO2/graphite cells during charge–discharge cycles [96]. The charge–discharge curves of the initial states of the batteries were constructed by fitting the charge–discharge curves of the anode and the cathode measured in advance. The anode is believed to begin to control the battery capacity through repeated charge–discharge cycles. To comprehend the key degradation mechanism of the cathode and the anode during the charge–discharge cycle, Lin et al. applied a physics-based side-reaction coupled electrochemical model to the capacity fading of a graphite/LiMn2O4 battery [97]. This study proposed three stages of capacity degradation. In the acceleration stage, capacity degradation was fundamentally due to the lithium loss caused by the increase in the SEI at the anode. In the stabilization stage, capacity loss was caused by the dissolution of the Mn cathode, which exceeded the circulating lithium loss due to the increased thickness and the slower growth rate of the anode SEI. The cathode intercalation was greater at the end of the discharge. In the saturation stage, further dissolution of the Mn cathode led to a capacity decrease that became the limiting factor. Because of the severe capacity loss of the cathode, cyclable lithium was transferred to the anode. The results showed that cyclable lithium loss and cathode capacity loss were the two main factors leading to battery capacity fade; moreover, their synergistic effect determined the battery capacity.
Capacity loss also occurs when batteries are stored for long periods. Wu et al. compared the storage capacity fading of commercial 18650 lithium batteries containing a graphite anode and a composite cathode comprised of LMO and NMC at various DODs and temperatures [98]. The capacity loss due to battery storage not only increased with storage temperature but also depended on the DOD. The battery stored at a DOD of 50% displayed the highest lithium loss and cathode material loss, implying that the battery can be stored for up to 12 months at 60 °C at maximum capacity. However, the battery stored at 0% DOD completely lost its capacity due to the cathode material loss over 12 months of storage. Watanabe et al. found that during long-term storage at high temperatures, the changes in the surface crystal/electronic structure and the cation mixing of lithium NCA cathode materials were far less than those of LCO cathode materials, indicating that NCA has excellent storage characteristics [99].
3.3.3 Electrical Imbalance
The single-cell voltage of a lithium battery is approximately 3–5 V, which is not adequate for use in large electronic devices such as laptop computers, electric vehicles, and aviation aircraft. Hence, to provide higher voltages, the batteries must be connected in series. Mismatched batteries in series can cause the battery pack to produce less energy than if all the cells were identical because the lowest energy cells limit the performance of the entire battery pack. Similarly, the weakest cells will be overcharged during charging. Therefore, each cell of the battery pack must be monitored separately to ensure safe operation of the entire battery pack. Many researchers have proposed solutions to ensure maximum energy delivery and recovery. Hoque et al. proposed a voltage-equalization control algorithm for individual battery monitoring and balancing in a series of LIBs [100]. The algorithm could control the monitoring, charging, and discharging processes. The charge-equalization model was applied to ten lithium batteries connected in series using the developed control algorithm. The charge–discharge performance and unit balance performance results showed that the control algorithm could quickly respond and the battery pack could reach a state in which the maximum charge difference among the batteries was 2.5%, thereby protecting the battery from abnormal conditions and providing the components with tolerable stability to operate at higher efficiency of 84.9%.
3.3.4 Low-Temperature Performance of Lithium Batteries
The electrochemical performance of lithium batteries varies with temperature. If the battery is working at an ultralow temperature for a long time, then the transfer of ions and charge at the SEI will be hampered. Fan et al. studied charge–discharge voltage profiles at low temperature via pulse charging protocols and conventional constant current-constant voltage charging [101]. The results revealed that the lithium deposited on the anode surface could rapidly diffuse into the graphite anode at room temperature, whereas it could not diffuse into the graphite anode at − 20 °C. This indicated that the diffusion of lithium ions into the graphite anode was the ultimate constraint affecting the charging rate of lithium batteries at low temperatures. In addition, the internal resistance and impedance vary greatly at low temperatures. The entire resistance (Rcell) of lithium batteries is primarily composed of the bulk resistance (Rb), solid-state interface resistance (Rsei), and charge transfer resistance (Rct). Zhang et al. observed the variation in these resistances with decreasing temperature [102] and discovered that the increase in Rct was the largest. At low temperatures, the Rcell of the lithium batteries was dominated by Rct. Rct was much higher at low temperatures, resulting in poor performance. Moreover, it was much higher at full discharge, making the charging process very laborious. Because Rct is closely related to battery reaction kinetics, the main limitation of the low-temperature performance of LIBs can be attributed to slow battery reaction kinetics.
4 Solution One: Modification of the Electrode Material
The electrode material is the generator of battery work; therefore, to improve the resistance of electrode materials to thermal runaway, many researchers have attempted to modify the electrode material layer of lithium batteries. Optimized selection of electrode materials can decrease the total heat released. Each type of coating or element modification can increase the thermal stability of electrode materials. Preventing thermal runaway is of practical significance. In this chapter, we will discuss the selection and modification of battery (LMB and LIB) materials, focusing on the anode and the cathode.
4.1 Modification of Anode Materials
In LMBs, as the name implies, lithium is used as the anode of the battery, while in LIBs, most commercial anodes are carbon-based materials such as graphite. However, these types of batteries have some common characteristics. During the first charge–discharge cycle, an SEI film is formed on the anode surface. The SEI layer does not affect the transport of lithium ions and prevents direct contact between the anode and the electrolyte and loss of the anode due to degradation. Unfortunately, the presence of the SEI layer affects the performance of lithium batteries to some extent; moreover, its thermal performance at high temperatures is poor. Therefore, the preparation of an “artificial” SEI layer with excellent thermal stability and mechanical properties instead of a metastable “real” SEI has always been at the core of anode material modification, thereby improving the cycling performance, whether the anode is lithium metal or a carbon-based material.
4.1.1 Strategies for Stabilizing Lithium Metal Anodes
The rise of a new battery concept that combines lithium metal as the negative electrode with a nonaqueous electrolyte brought about technological breakthroughs that significantly enhanced the specific energy and energy density of the battery [103]. In various nonaqueous electrolytes, the kinetic stability of lithium metal is advantageous; however, due to the thermodynamic instability of the electrolyte, the initial decomposition of the electrolyte on the anode surface leads to the formation of an electronically insulating passivation layer, that is, the SEI, which protects the electrolyte from further degradation and is ideally permeable to lithium ions [104, 105]. If LMBs are to be commercialized, then many challenges must be faced, such as dendrite formation and short circuits that lead to thermal runaway, as shown in Fig. 6. In recent years, various strategies have effectively inhibited dendrite formation for long periods and significantly improved the cycling and rate performance.
Scheme of dendrite formation on the lithium metal anode. Reprinted with permission from Ref. [48]. Copyright © 2017 American Chemical Society
(i) 3D porous hosts capturing molten lithium. The high current density of lithium metal anodes leads to uneven distribution of the lithium-ion flux on the surface of the electrode [106]. Therefore, the uneven nucleation of anode lithium results in uncontrolled dendrite growth and a large volume change. Porous matrix materials were applied to reduce the local current density of the lithium anode to control lithium deposition [107]. The 3D lithium/Al2O3 (LIA) skeleton anode is regarded as a substitute for a planar lithium anode. It achieves smooth and compact lithium-ion deposition, thereby improving the cycling stability in high-energy LIBs (Fig. 7a) [108]. The LIA anode is not only a promising lithium substrate with negligible volume change during stripping and plating but also can reduce the ion concentration gradient during cycling through the interaction of the Li–Al–O coating with lithium ions, resulting in almost no dendrite deposition. Symmetrical lithium cells assembled with these composite electrodes displayed smooth charging and discharging plateaus at 8 mA−2 and an overpotential of only 400 mV (Fig. 7b) [108]. Zeolitic imidazolate frameworks (ZIFs) are a representative MOF. They are composed of tetrahedrally coordinated transition-metal ions (such as Cu2+, Co2+, and Zn2+) connected by imidazolate linkers [109, 110]. After high-temperature annealing, ZIFs change into characteristic microporous carbon structures with perfect crystal metal clusters [111]. Based on the lithium affinity of the carbonized MOF structure (cMOF) with Zn clusters, Zhang et al. prepared homogeneous Li–cMOF hybrids via molten lithium infusion (Fig. 7c) [112]. The growth and nucleation of metallic lithium in the cMOFs were dominant due to the plentiful uniformly dispersed Zn clusters and the 3D conductive structure of the matrix. The high conductivity and porous structure of the cMOFs further homogenized the distribution of the Li-ion current and electric fields, efficaciously preventing the germination of lithium dendrites and rendering cMOFs ideal hosts for lithium metal anodes (Fig. 7d). The hybrid exhibited extremely low voltage hysteresis, a long cycle life, and superior electrochemical performance [112]. The lithium affinity enabled molten lithium to penetrate the 3D porous structure without any hindrance.
Various 3D porous hosts capturing molten lithium. (a) Scheme of Al2O3 particles, the Al2O3 skeleton with a Li–Al–O layer, and the 3D LIA electrode. Schematic of Li plating on the anode. (b) Symmetric cell cycling performance using a bare lithium electrode or a composite electrode. Reprinted with permission from Ref. [108]. Copyright © 2018 John Wiley and Sons. (c) Schematic representation of the fabrication of Li–cMOFs. (d) Schematic illustration and cross section of a lithium film deposited in a cMOF. Reprinted with permission from Ref. [112]. Copyright © 2018 John Wiley and Sons
A carbon cloth doped with nitrogen and phosphorus has exceptional lithiophilicity; thus, a stable and safe 3D lithium metal anode was prepared by loading such a cloth with molten lithium (Fig. 8a) [113]. The formation of dendrites was significantly inhibited on the 3D lithium electrode such that a stable voltage distribution was maintained for over 600 h at 3 mA cm−2. Theoretical calculations indicated that the codoping of N and P greatly improves the lithophilic nature of the carbon cloth, which results in well-distributed deposition of molten lithium and is also beneficial to the reversible stripping and electroplating of lithium (Fig. 8b) [113]. Density functional theory (DFT) calculations showed that the Gibbs free energy change (ΔG) of the reaction between alkali metals (Li, Na) and metal oxides (such as Co3O4, SnO2, and CuO) is exceedingly small, indicating that metal oxides are uniquely suitable for adjusting the wettability of 3D carbon structures by using molten lithium (Fig. 8c) [114]. Based on this, Li et al. designed a layered 3D CO3O4–CS framework that improved the wettability of alkali metal anodes by introducing Co3O4 nanofibers into a carbon substrate. The prepared framework had a multilayer structure. A 3D carbon sheet served as the main framework, providing sufficient lithium nucleation sites and sufficient electrolyte/electrode contact to achieve rapid charge transfer, while Co/Li2O nanofibers provided physical constraints on the deposition of lithium and further redistributed the lithium-ion flux on each carbon fiber (Fig. 8d). The symmetrical cells of Li–Co–CS displayed an exceptional lifespan and a low overpotential at a high current density. When this framework was paired with an LFP cathode, the Li–Co–CS battery retained 88.4% of its capacity after 200 cycles [114]. Currently, flexible batteries with a lithium metal anode and a sulfur or oxygen cathode are widely used [115, 116]. In these batteries, the lack of flexibility of lithium metal is a major concern due to the brittleness of the SEI and severe dendrite formation. The planar lithium anode may break when bent, resulting in short-circuiting of the battery and thermal runaway. Controlling the infusion of material so that the lithium metal can withstand repeated bending is essential. Materials that are sufficiently flexible to avoid surface cracking need to be designed, thereby resolving the problem of dendrite growth and enabling the battery anode to function well under a variety of bending conditions [117, 118]. To date, a variety of 3D skeleton structures have been fabricated to inhibit the growth of lithium dendrites, including copper nanowire networks [119], a 3D double-continuous porous copper foil [120], a copper array of a 3D submicron skeleton [121], a self-supporting carbon network structure [122], and a C-wood material coated with ZnO [123, 124]. The 3D skeleton structure had a negative effect on lithium dendrite formation, and the main aim was to improve the lithium affinity of the 3D skeleton structure.
Various 3D porous hosts capturing molten lithium. (a) Illustration of NPCC–Li. (b) Optimized structure of the differential charge density for lithium in a pure/N-doped/N-P-doped/P-doped graphene sheet. Reprinted with permission from Ref. [113]. Copyright © 2019 John Wiley and Sons. (c) Digital images of bare CS and a Co3O4–CS framework, and schematic illustration of the multilevel structure of a Co3O4–CS skeleton. (d) Gibbs free energy change of the reaction between Co3O4 and Li. Simulation of the Li-ion flux at the electrolyte/nanofiber interface. Reprinted with permission from Ref. [114]. Copyright © 2019 John Wiley and Sons
(ii) Construction of lithiophilic sites to regulate lithium deposition. Although significant progress has been made in designing advanced composite lithium anodes by infusing molten lithium into 3D porous substrates, controlling the spatial deposition of lithium and limiting dendrite formation remain a challenge. When lithium ions encounter electrons, lithium metal deposits irregularly all over the anode, causing the formation of dendrites and short-circuiting [125]. Owing to its strong adsorption energy, lithium metal preferentially nucleates at lithiophilic sites, regulating the nucleation behavior of lithium and inhibiting the growth of lithium dendrites. The above problem can be solved by doping a single-atom (SA) metal into lithium matrix materials to form active sites [126, 127]. The introduction of new transition metals into a carbon-based lithium metal matrix is supposed to effectively regulate the nucleation regions of lithium and optimize the deposition of lithium metal in the lithium electroplating matrix, which is urgently needed. Zhai et al. reported a stable host for lithium metal deposition comprised of an SA metal supported on a N-doped graphene substrate (SAM-natural graphite, where M = Ni, Pt, Cu). With the addition of an SA metal, the degree of lithiophilicity of the electrode surface was ameliorated, and more meaningfully, the stability of the atomic structure of the lithium matrix improved (Fig. 9a) [128]. Theoretical calculations demonstrated the transfer of electrons from lithium to the surface of pristine graphene (PG), N-doped graphene (NG), and atomically dispersed Ni on NG (SANi-NG) during adsorption (Fig. 9b). Ultimately, uniform lithium deposition was achieved, for which no dendrite morphology was observed, thereby greatly enhancing the Coulombic efficiency and extending the cycle life. The obtained electrode had an extremely low voltage hysteresis of 19 mV and stable lithium plating and stripping performances even at a high current density [128]. Ultrafine titanium nitride (TiN), a transition-metal nitride, possesses high chemical stability and excellent electrical conductivity. Furthermore, various studies have shown that TiN is a potential candidate for promoting rapid electron transport and inducing a pseudocapacitive effect that is important for uniform adsorption and desorption at high lithium plating/stripping rates [129, 130]. Consequently, Lin et al. chose to impregnate a carbon nanofiber (CNF) mat with TiN to prepare CNF–TiN nanoparticles (Fig. 9c) [131]. These nanoparticles provided active sites for the uniform deposition of lithium anode current collectors and host materials (Fig. 9d). Theoretical calculations showed that lithium tends to adsorb on TiN with a low diffusion barrier, forming controlled nucleation sites and dendrite-free lithium deposits. Simultaneously, the sophisticated structure, specific surface area and high electrical conductivity enhance the lithium-ion transfer dynamics to reduce the local current density and homogenize the distribution of the lithium-ion flux, thereby regulating the nucleation behavior. The CNF–TiN@Li anode displayed exceptional electrochemical capability, reaching 98.6% Coulombic efficiency after 200 cycles.
Lithiophilic sites constructed to regulate lithium deposition. (a) Schematics: lithium adsorption energy distribution and illustration of lithium nucleation/plating processes on SAM-natural graphite. (b) Charge density changes of PG, NG, and SANi-NG with one lithium atom. Reprinted with permission from Ref. [128]. Copyright © 2019 John Wiley and Sons. (c) Schematic of the synthesis of CNF–TiN. (d) Lithium nucleation/plating processes on CNF–TiN. (d1) Schematic diagram of nucleation/plating processes on CNF–TiN. (d2–d5) Scanning electron microscopy (SEM) images of CNF–TiN after plating or stripping at various current densities. Reprinted with permission from Ref. [131]. Copyright © 2019 John Wiley and Sons
Researchers continue to explore the synthesis of a variety of lithiophilic particles to clad CNFs [132], such as Mo2N [133] and MgZnO nanoparticles [134]. The modified lithium metal anodes displayed good cycling stability during high current discharge, while full cells with coupled cathodes (LFP, LiNi0.8Co0.1Mn0.1O2) had high capacity retention. Exciting new 2D materials called MXenes have emerged in recent years that provide more channels for ion movement and greatly improve the speed of ion movement [135,136,137]. A parallel MXene (Ti3C2Tx) layer effectively guides the nucleation and growth of lithium on the 2D MXene nanosheet surface, forming a horizontally growing lithium anode (Fig. 10a) [138]. Specific fluorine-containing groups of the MXene react with lithium metal to form lithium fluoride at the anode/electrolyte interface, which provides an SEI and effectively regulates the electromigration of lithium ions. The assembled symmetrical lithium battery has excellent cycling performance up to 35 mAh cm− 2 (Fig. 10b, c). In addition, a scalable press method to prepare self-exfoliated MXene stacks can be applied to form an ultrathin lithium metal electrode. The outstanding conductivity and large surface area of the 2D laminated structure minimize the local current density and regulate the growth of lithium, thus enabling simultaneous deposition/extraction of lithium and formation of a stable SEI [139]. A 3D-printed, vertically aligned lithium anode (3DP-VALi) can effectively guide lithium deposition through “nucleation within microchannel walls,” thus achieving a dendrite-free lithium anode [140] (Fig. 10d). Microchannels facilitate rapid diffusion of lithium ions and provide a large space for lithium ions to be contained during electroplating/stripping (Fig. 10e, f). The results indicated that the symmetric cells with 3DP-VALi displayed excellent electrochemical performance. To optimize the dendrite growth, volume change, stability, and safety of lithium metal anodes, a variety of lithiophilic nanostructures have been designed and explored [136,137,138,139,140,141,142,143]. The rapid development of nanotechnology may bring innovation to next-generation LMBs.
Lithiophilic sites constructed to regulate lithium deposition. (a) Illustration of lithium nucleation/plating processes on lithium and PA-MXene layers. (b) Symmetric cell cycling performance using PA-MXene–Li. (c) SEM images of PA-MXene–Li after plating with lithium. Reprinted with permission from Ref. [138]. Copyright © 2019 John Wiley and Sons. (d) Schematic of 3DP-VALi. (e) Illustration of lithium nucleation/plating processes on 3DP-VALi and bare Li. (f) SEM images of lithium deposition on bare lithium and 3DP-VALi at various current densities, (f1) 1 mAh cm−2, (f2) 3 mAh cm−2, and (f3) 5 mAh cm−2, and (f4) after one plating and stripping cycle at 5 mAh cm−2. Reprinted with permission from Ref. [140]. Copyright © 2020 John Wiley and Sons
(iii) Building an artificial SEI film between the lithium anode and the electrolyte. An artificial anode–electrolyte interface is formed on the outside of the anode to protect the lithium underneath. An artificial SEI film with stability, high density, and high ionic conductivity can prevent direct contact between the lithium metal and the organic liquid electrolyte [60, 61]. Hence, deterioration of the electrolyte and the electrode materials, dendrite formation, and heterogeneous deposition caused by the original SEI layer can be avoided to achieve a uniform Li+ flux. Jiang et al. designed and prepared an artificial SEI layer comprised of a polymer and an alloy via a simple chemical modification strategy [144]. The poly(tetramethylene ether glycol) (PTMEG)–Li/Sn alloy hybrid layer was proposed by utilizing ethylene oxide as a promoter of the tetrahydrofuran ring-opening reaction (Fig. 11a). Compared with the single Li/polymer and Li/Sn layers, the hybrid layer displayed rapid Li+ conductivity and excellent electrochemical cycling stability (Fig. 11b). In various electrochemical tests, including on lithium symmetrical batteries, Li-S batteries, and Li-LFP full batteries, the fabricated lithium batteries exhibited remarkable performance. Most notably, the treated lithium displayed high air stability even in high-humidity environments due to the hydrophobicity of PTMEG. Even when exposed to moist air, the treated lithium maintained good activity in Li-S, Li-LFP full cells. This raises the prospect that the new strategy could be extended to other related battery systems, such as Li-O2 cells, or other metal anodes, such as Na or Zn [145]. LiF is considered an effective artificial SEI film owing to its mechanical and thermal stability (Fig. 11c) [146]. The LiF host not only encourages lithium to fill its large pores and to deposit in flat scale morphology but also facilitates the corrosion reaction with the electrolyte, providing a chemically stable interface layer for the lithium anode. Yan et al. further modified artificial SEI films of LiF [147]. CuF2 was chosen as the precursor to form a uniformly deposited LiF/Cu mixed ion–electron conductor interphase (MCI) film on a lithium metal anode via a controlled displacement reaction (Fig. 11d). The obtained MCI film formed a protective layer on the lithium anode through its preferred lithium storage, high ionic conductivity, and high Young’s modulus. X-ray photoelectron spectroscopy (XPS) results showed that LiF, Cu, Li3N, and LiNxOy are key components in the stabilization of solid–liquid interfaces and the protection of lithium metal anodes (Fig. 11e). The protective MCI provides Li metal anodes with superior electrochemical cycling performance and a highly mosaic structure with efficiency and stability. In addition, hybridization of organic and inorganic layers can maximize the role of the artificial SEI layer. Lithium metal was immersed in a fluoroethylene carbonate (FEC) solvent to form a double film on a lithium metal anode [148] (Fig. 11f). This dual-layered membrane with organic compounds (ROCO2Li and ROLi) in the upper layer had good flexibility to avoid damage to the SEI membrane. The bottom layer was composed of inorganic components (Li2CO3 and LiF). They formed nucleation sites, which guided the transport of lithium ions in an orderly manner and inhibited the formation of lithium dendrites. The surface morphology of the protected lithium was compact and uniform with low interfacial polarization. Furthermore, a number of additives have been used to solve the stability and ionic conductivity problems of SEI layers, such as LiBr [149], LiNO3 [150], lithium oxalyldifluoroborate [151], polymers [152], toluene [153], and pyrrole [154]. Long-term extensive research has contributed to the increasing stability of artificial SEI layers. The ultimate goal of the artificial SEI layer is to control the irreversible capacity loss, prevent electrolyte decomposition, reduce the interfacial resistance, and inhibit dendrite formation, thereby ensuring a safe and high-performance lithium anode. Attempts to develop practical lithium metal anodes began more than 40 years ago. Primary batteries based on lithium metal anodes are already in relatively mature use, while rechargeable batteries have only been commercially available for a short time. Unfortunately, because of a series of explosive accidents caused by the growth of lithium dendrites, the use of lithium anodes had been terminated. Over the past decade, extensive exploration of clean and sustainable energy sources has revived research into lithium metal anodes. Assorted Li metal anode modification strategies, 3D porous hosts, nucleation site control, and artificial SEI manufacturing can enable control of the Li+ flux and generation of dendrite-free lithium. As mentioned above, although remarkable performance improvements have been made in lithium metal anodes, practical lithium metal anodes have not yet been realized. Therefore, continuous efforts and further innovative methods are required to completely resolve the relevant problems.
Building an artificial SEI film between a lithium anode and an electrolyte. (a) Mechanism of the formation of PTMEG–Li/Sn. (b) Illustration of lithium nucleation/plating processes of treated lithium of PTMEG–Li/Sn. Reprinted with permission from Ref. [144]. Copyright © 2019 John Wiley and Sons. (c) Illustration of lithium plating behavior of bare Li and LiF-rich Li. Reprinted with permission from Ref. [146]. Copyright © 2019 Elsevier. (d) Schematic of the formation of a protective MCI. (e) XPS patterns of the protective MCI layer before cycling and after the tenth cycle. Reprinted with permission from Ref. [147]. Copyright © 2018 John Wiley and Sons. (f) Schematic of the formation of a dual-layered film on a lithium anode via FEC treatments. Reprinted with permission from Ref. [148]. Copyright © 2018 John Wiley and Sons
4.1.2 Enhancement of the Stability of Carbon-Based Anodes
Carbon-based anodes are frequently used in LIBs. Compared with lithium anodes, carbon-based anodes have a lower capacity, but they have stabler properties, are safer, and are less inclined to produce lithium dendrites. Additionally, the commercialization of carbon-based anodes is more advanced than that of lithium metal anodes. To improve the thermal stability of carbon-based anodes, the properties of the SEI layer must be improved to prevent short-circuiting and thermal runaway. Furthermore, researchers have designed smart materials to automatically shut down the power supply in time to prevent disasters when thermal runaway occurs [155].
(i) Surface coating at the atomic level. Volume change and SEI layer thickening during cycling are the most serious issues affecting the long-term structural stability. These phenomena result in sizeable capacity loss in each cycle and pave the way for short-circuiting [156, 157]. A mixture of graphite, vapor-grown carbon fibers (VGCFs), and carbon nanohorns (CNHs) (C-graphite/VGCF/CNH) was heat-treated in an Ar atmosphere via the vapor deposition method and coated with a carbon film. Figure 12a shows that both the graphite VGCFs and CNHs were surrounded by the carbon film [156]. The carbon film not only reduced the internal resistance of the anode and the interfacial resistance between the electrode and the electrolyte but also reduced the heat and irreversible capacity loss generated during charging and discharging, thereby enhancing the high-rate discharge performance of the LIB. CNHs fill the narrow gaps between the graphite and VGCFs, acting as a buffer during volume change of the graphite. Kim et al. reported that the use of hard carbon and microcrystalline graphite core–shell composite materials as anode materials for LIBs improved the electrochemical performance [157]. The discharge rate and safety performance of the composite material were superior to those of hard carbon. In recent years, atomic layer deposition (ALD) has proven to be a more accurate and targeted approach for modifying electrodes. Using this method, an inactive metal oxide was grown as an ultrathin protective coating without interfering with the interparticle electronic pathway. The coating was used by Jung to modify a natural graphite electrode [158]. Graphite coated with Al2O3 prepared via ALD displayed better stability and a relatively slow decay in reversible capacity at 50 °C (Fig. 12b). Importantly, ALD directly on the electrode prevented the electrical path between the particles (necessary for conductivity) from being covered by an insulating layer, resulting in better Coulombic efficiency and high-temperature performance (Fig. 12c). In addition, the versatility of direct ALD on composite electrodes can be used to develop coatings for any advanced battery material. To address the issue of drastic volume change during lithiation, Fang et al. reported an effective method of dispersing Sn nanowires coated with Al2O3 into a carbon matrix via ball-milling for the battery anode (Fig. 12d) [159]. In this structure, Sn enhanced the battery capacity, Al2O3 maintained the structural integrity during charging and discharging, and the introduction of the carbon base enhanced the conductivity of the entire electrode. When the thickness of Al2O3 was increased to 25 nm, the electrochemical performance of the Sn–Al2O3–C electrode decreased due to the nonconductivity of Al2O3. The thickness of the Al2O3 coating is the key factor affecting the electrochemical performance of the electrode. Owing to its low price and stable properties, Al2O3 is frequently used as a coating material to improve the safety performance [160,161,162,163]. Feng et al. prepared a low-cost and easily degradable NG powder via the sol–gel method (Fig. 12e) [160]. The smooth Al2O3 coating can be viewed as a preformed SEI, thereby reducing SEI regeneration and Li-ion consumption in subsequent cycles. The rate performance and cycling stability varied with the Al2O3 coating thickness, particularly at relatively high current densities. The ideal thickness enabled the anode material to achieve optimal safety (Fig. 12f). Due to its appropriate bandgap and Li-ion conduction capability, the coating performed similar functions to the SEI, that is, preventing electrons from reaching the outer surface of the electrode and allowing Li-ion transfer. Cheng et al. used the microwave plasma-enhanced chemical vapor deposition technique to coat a novel nitrogen-incorporated ultrananocrystalline diamond (N-UNCD) with a natural graphite electrode material. The electrode displayed superior stability. The natural graphite anode with the N-UNCD coating displayed outstanding cyclability, rate capability, and conductivity [164]. A comparison of the properties of the natural graphite electrode and the N-UNCD-coated anode is shown in Fig. 12g. N-UNCD films provide good ionic conductivity, allowing lithium ions to easily diffuse into the NG particles. The film reduces the tensile stress of graphite during lithiation/desalination. Coating with various other materials, including AlF3 [165] and Fe3O4 [166, 167], can also promote the properties of carbon-based anodes. In future, simple and inexpensive coating methods should be considered to modify the surface of graphite anodes with high-quality presynthesized materials.
Carbon-based anodes. (a) SEM images showing that graphite VGCF CNHs were surrounded by a carbon film. Reprinted with permission from Ref. [156]. Copyright © 2014 Elsevier. (b) Cycling performance of natural graphite composite electrodes coated with Al2O3 via ALD at 50 °C. Schematic of electron transport in natural graphite composite electrodes prepared via ALD on powder and via direct ALD on the electrode. (c) Electrochemical performance of natural graphite composite electrodes coated with Al2O3 via ALD. Differential first charge voltage profiles. Coulombic efficiency after the first cycle. Voltage profiles at room temperature. Reprinted with permission from Ref. [158]. Copyright © 2010 John Wiley and Sons. (d) Schematic of the synthesis route for the Sn–Al2O3–C nanocomposite. Reprinted with permission from Ref. [159]. Copyright © 2016 John Wiley and Sons. (e) Schematic representation and SEM image of an Al2O3 coating on graphite. (f) Rate performance of Al2O3-coated graphite obtained at 15 to 480 mA g−1. Reprinted with permission from Ref. [160]. Copyright © 2016 American Chemical Society. (g) Failure mechanism of the natural graphite electrode and high stability of the N-UNCD-coated electrode. Reprinted with permission from Ref. [164]. Copyright © 2020 Elsevier
(ii) Autonomous shutdown to suppress battery failure. Batteries continuously generate heat during discharge. If a short circuit occurs, then the temperature sharply increases. The increasing temperature accelerates side reactions, which in turn produce more heat, creating a dangerous positive-feedback mechanism that leads to thermal runaway. Thermally induced shutdown of LIBs was achieved by incorporating thermally responsive polymer microspheres into the anode of the battery (Fig. 13a) [155]. When the internal environment of the battery reached a critical temperature, the microspheres melted and covered the anode with a nonconductive barrier, stopping Li-ion transfer and permanently shutting down the battery. Electron microscopy observation of the anode after shutdown indicated that a conformal PE film was formed in situ and that PE permeated the surface of the anode. Because the melting transition temperature of the encapsulated polymer determines the trigger temperature for battery shutdown, this highly customizable mechanism should be applicable to a variety of battery chemical reactions with unique shutdown requirements to prevent the occurrence of thermal runaway.
(a) Schematic representation of an autonomic shutdown anode to suppress battery failure. Reprinted with permission from Ref. [155]. Copyright © 2012 John Wiley and Sons. (b) Schematic microstructural illustration of a) and b) bare SiNWs and c) and d) TiO2-coated SiNWs. a) and c) are as-grown samples, while b) and d) are cycled samples. Reprinted with permission from Ref. [168]. Copyright © 2013 Royal Society of Chemistry. (c) Structure of Li4Ti5O12 and Li7Ti5O12, with no volume changes. Reprinted with permission from Ref. [171]. Copyright © 2010 John Wiley and Sons. (d) Schematic of the synthesis of a porous mooncake-shaped structure. Reprinted with permission from Ref. [172]. Copyright © 2020 John Wiley and Sons. (e) SEM images of mesoporous TiNb2O7. Reprinted with permission from Ref. [173]. Copyright © 2014 American Chemical Society
In summary, owing to its excellent volume expansion stability and high thermal resistance, graphite is still the stablest material in LIBs. To ensure advanced applications of LIBs in future, research and development is focused on improving the battery capacity, safety, and the lifespan. This requires the proposal of modification strategies to overcome the challenges of the poor discharge rate and poor performance at high temperatures.
4.1.3 Other Creative and Developing Anode Materials
To expand the application of LIBs, a great deal of research has been invested in the development of a wider variety of new-generation anode materials. Silicon is especially intriguing as an anode material for LIBs because of its abundance and extremely high capacity (3500 mAh g−1 at room temperature). Silicon nanowires (SiNWs) were coated with TiO2 via the ALD method, which significantly improved the safety performance and Coulombic efficiency of the silicon anode (Fig. 13b) [168]. The TiO2-coated SiNWs not only doubled the relative capacity retention rate at 0.1 C and more than tripled it at 5 C but also increased the Coulombic efficiency to 99%, which is the best performance reported for silicon-based electrodes to date. The activation of the TiO2-containing SEI effectively protected the electrode. TiO2 coatings perform best when applied to completely amorphous electrodes. For silicon-based anodes, ZnO and Al2O3 coating improves the stability of the SEI layer and enhances the safety performance of the anode [169, 170]. To fabricate a cathode–anode combination that meets the stringent transfer requirements, Amine et al. first studied Li4Ti5O12 (LTO) as the anode. Traditional carbon-based anode materials undergo volume changes during lithium ion embedding, and LTO anodes can not only accommodate up to three lithium ions in the spinel structure but also exhibit no volume changes (Fig. 13c) [171]. A porous mooncake-shaped LTO anode fabricated from nanoparticles was successfully synthesized by using a MOF (Mil-125) as a template. More importantly, SmF3 was used to modify the porous mooncake-shaped LTO material. After modification, numerous SmF3 nanoparticles were observed to adhere to the surface of the LTO material owing to the large specific surface area of its porous mooncake-like structure (Fig. 13d) [172]. The SmF3 modification can facilitate the transition from Ti4+ to Ti3+. Additionally, it can reduce the electrode polarization, Rct, and the SEI impedance (Rsei) while improving the Li-ion diffusion coefficient (DLi). The decrease in the internal resistance results in reduced heat generation from the Joule effect during discharge, which enhances the safety performance. In some Ti-based binary metal oxides, ordered mesoporous TiNb2O7 is applied as the anode of the LIB (Fig. 13e) [173]. The large pores (approximately 40 nm in diameter) lead to a shorter Li-ion diffusion length and faster penetration by the electrolyte. In addition, dozens of innovations have been made in nanostructured metal oxides, especially those with porous and hollow structures, which greatly improve the safety performance and electrochemical properties. These materials include V2O5 [174], Co3O4 [175], SnO2 [176], and ZnO [177]. The surface structures of these metal oxide anodes were modified by ALD, which not only maintained the high theoretical capacity but also improved the safety performance and rate properties during discharge. In addition to the above, various studies are underway; however, there is still much improvement to be made before commercialization of these anode materials is possible.
4.2 Modification of Cathode Materials
In addition to the notable developments in anode materials, cathode materials are similarly worth studying as a counterpart to the anode materials in lithium batteries. Cathode materials directly participate in or indirectly catalyze electrochemical reactions, which play a vital role in determining the performance of LIBs in terms of voltage, capacity, thermal stability, and potential. The cathode tends to be less conductive than the anode and generates more heat in operation. Under extreme conditions, the cathode material will decompose and collapse first [178]. Therefore, to improve the safety performance of the battery, modifying the cathode material is critical. The properties of cathodes in lithium batteries are affected by their composition, morphology, particle size, and structure [179]. In this section, we emphasize improvement in the thermal and electrochemical performance of these materials through surface and bulk treatments, including surface coating, lattice doping, and element substitution.
4.2.1 Improving the Stability of Lithium Intercalation Cathodes
Lithium coupled with intercalation-type cathodes has attracted wide interest in recent years. In general, there are three groups of intercalation-type cathode materials based on the structure, namely, spinel, olivine, and layered. These materials include LFP, LCO, LMO, NMC, and NCA. The corresponding anode material is usually graphite, and the commercialization of such batteries is very mature. The anode materials can be lithium metal, other carbon-based materials, or silicon-based materials. When intercalation-type cathodes are in an overheated or overcharged state, phase transitions occur in which reactive oxygen species are released and significant heat is generated. In the fully charged state, the cathode and the anode are in highly oxidized and reduced states, respectively, and the battery is the most dangerous [59]. Therefore, countermeasures should be taken to inhibit harmful internal activities and to improve battery safety. Three primary techniques are employed: use of additives and coating layers for protection, element substitution, and use of thermally responsive cathodes to control battery switch off.
(i) Additives and coating layers for protection
Protection of the intercalation cathode material is crucial to the stability of the battery. The required additives and coatings can be electrochemically oxidized before the electrolyte components form a passivation layer on the cathode. Thus, the incorporation of a protective layer reduces heat generation. LFP is widely used owing to its environmental friendliness and thermostability. Nevertheless, sluggish mass and charge transport kinetics lead to poor performance of the LFP cathode. Liu et al. successfully synthesized nanoscale LFP/carbon nanotube (CNT) composite materials by combining Fe2O3, POx, and Li2O subrings via ALD technology (Fig. 14a) [180]. After annealing, LFP/CNTs exhibited excellent battery performance as positive electrode materials, including excellent rate capability and safety performance and a long life. At a current density of 0.1 C, the LFP/CNT materials displayed a stable discharge capacity of approximately 167 mAh g−1 after 100 cycles (Fig. 14b). The remarkable performance of LFP/CNT cathode materials prepared via ALD can be attributed to several factors. The synthesized LFP/CNTs with unique and uniform nanostructures could enable rapid insertion and extraction of Li+. Electron transfer between the LFP and CNTs was enhanced, ion diffusion was rapid in the well-crystallized LFP, and the conductive Fe3P impurities improved the conductivity of the LFP/CNTs. LCO is the most commercially successful cathode material for LIBs. The discharge capacity of LIBs with LCO cathodes decreases exponentially over time. To increase the battery power density and reduce cathode degradation and other adverse side effects, the amount of electrolytes can be significantly increased; however, this results in thermal runaway. Coating the surface of an LCO cathode with a metal oxide appears to be a promising method to stabilize the thermal performance of the cathode and improve the cycling performance at high operating voltages. These metal oxides include ZnO [181], Al2O3 [182], TiO2 [183], LiNbO3 [184], Li3PO4 [185], and self-terminated hyperbranched oligomers [186]. In addition, LCO/multiwalled CNT (MWCNT) free-standing electrodes were prepared via a simple papermaking process to enhance the safety performance [187]. Tebbe et al. presented a detailed mechanism by which HF reacted with an alumina coating to form a partially fluorinated alumina surface instead of forming AlF3 and H2O. The alumina film reduced cathode degradation by purifying HF and avoiding the formation of H2O (Fig. 14c) [188]. The metal oxide coating reacted with the HF to form hydroxyl groups with H2O. Mn is frequently used as an electrode material because of its variable valence. Transition-metal (TM) oxides rich in Mn with a chemical formula of xLi2MnO3·(1 − x) LiMeO2 (Me = Mn, Ni, Co) have been viewed as cathode materials for next-generation high-safety lithium batteries. The evolution of Mn oxide in fresh recycled electrodes was studied via the finite-difference method for the near-edge structure code [189]. Sarkar et al. developed a multiphysics-based model that incorporated stress mechanics, lithium-ion diffusion, and the dissolution of TM ions from the spinel LiMn2O4 cathode to explain the principle of CeO2 thin-film-coated particles (Fig. 14d) [190]. The theoretical model along with the experimental results showed significantly improved Coulombic efficiency and safety performance of the electrode coated with a CeO2 thin film. Xiao et al. proposed a novel FePO4 coating on LiNi0.5Mn1.5O4 (LNMO) formed via ALD (Fig. 14e) [191]. X-ray absorption near-edge structure (XANES) analysis revealed that the ultrathin FePO4 layer inhibited reduction of the surface Mn4+ to Mn2+ through electrolyte reduction and Jahn–Teller distortion. A small amount of Mn2+ maintained the consistency of the surface without significant dissolution in the electrolyte. Other LNMO surface coatings can also increase the thermal stability [192,193,194]. High voltage cathode electrode materials, for example, NMC and NCA, are driving the commercialization of modern electric vehicle batteries. However, many obstacles need to be confronted and overcome, such as voltage loss and the deposition of impurities on the anode surface during cycling. Zou et al. utilized a sustainable biomass (sodium alginate) as a template to synthesize a series of multishelled, Ni-rich NMC hollow fibers (Fig. 14f, g) [195]. This new eggbox-structured alginate effectively immobilized TM cations (Ni2+, CO2+, Mn2+), which inhibited the mixing of cations in the subsequent calcination step of NMC synthesis and enhanced the stability of the cathode material. The excellent electrochemical performance was attributed to low cation mixing, the unique structure, the 1D morphology, and the porous multishelled hollow structure. Li et al. deposited a uniformly dense lithium-conductive LiTaO3 coating with a controllable thickness on the surface of an NMC electrode [196]. The cycling performance and safety performance were further improved by coating encapsulation. In other modification methods, an ultrathin polyacrylate surface coating was integrated with an NMC cathode [197], or a LiAlO2 coating was prepared via ALD on an NCA electrode [198]. Both methods had positive effects on the ternary electrodes.
Additives and coating layers for protection of the cathode. (a) Schematic of ALD of amorphous LiFePO4. (b) Cycling stability of annealed LiFePO4/CNTs at 1 C and 0.1 C. Reprinted with permission from Ref. [180]. Copyright © 2014 John Wiley and Sons. (c) Cathode degradation cycle via HF attack and water regeneration. Reprinted with permission from Ref. [188]. Copyright © 2015 American Chemical Society. (d) Schematic of CeO2-based ALD coating of a LiMn2O4 particle. Reprinted with permission from Ref. [190]. Copyright © 2017 American Chemical Society. (e) Schematic illustration of LNMO-n during cycling. Reprinted with permission from Ref. [191]. Copyright © 2015 John Wiley and Sons. (f) SEM image of LiNixCoyMnzO2 and corresponding energy-dispersive X-ray spectroscopy (EDS) mapping of the elements Ni, Co, and Mn. (g) Cross-sectional SEM images of multishelled LiNixCoyMnzO2 hollow fibers. Reprinted with permission from Ref. [195]. Copyright © 2017 John Wiley and Sons
(ii) Element substitution for improved safety performance
Intercalation-type cathodes in lithium batteries release oxygen during heating that reacts with the electrolyte, thereby generating more heat and resulting in thermal runaway. The oxygen release leads to microstructure defects, such as the formation of large pores inside the cell that have a significant impact on the cell [199]. This is a key problem in improving the safety performance of batteries. Element substitution can effectively improve the thermal properties of cathode materials by stabilizing the crystal structure. Using the unique electronic structure of environmentally friendly Fe, Hu et al. substituted Mn with Fe in high-energy-density LiNi0.5Mn1.5O4 to improve its thermal stability (Fig. 15a) [200]. Compared with the unsubstituted material, the optimized LiNi0.33Mn1.33Fe0.33O4 material displayed significantly improved thermal stability. Furthermore, O2 release was not observed at high temperatures up to 500 °C. Moreover, the capacity, cyclability, and rate capability (Fig. 15b, c) were well preserved. He et al. proposed a novel strategy for corrosion inhibition of batteries [201]. The researchers, using sodium dodecyl sulfate as a surfactant, designed Na+-doped Li1.2Ni0.13Co0.13Mn0.54O2 (LMR) with abundant stacking faults to ensure that Na+ was uniformly distributed deep in the crystal lattice and not just at the surface or in the coating. Al or Mg can be employed to partially substitute Ni or Mn to form Li1.05Mn1.95−zMzO4 (M = Al or Mg) to enhance the thermal performance [202].
(a) Schematic of the effect of Fe substitution on structural modification. (b) Charge and discharge profiles with the capacity retention of LiNi0.5−xMn1.5−xFe2xO4 (2x = 0, 0.33). Batteries were cycled at a current density of 0.11 mA cm−2. (c) Rate performance of LiNi0.5−xMn1.5−xFe2xO4 (2x = 0, 0.33). Reprinted with permission from Ref. [200]. Copyright © 2016 John Wiley and Sons. (d) Schematic of the mechanism of the thermally responsive LCO–PTC cathode. Reprinted with permission from Ref. [203]. Copyright © 2016 Royal Society of Chemistry
(iii) Thermally responsive materials to control battery switch off
When a battery reaches a high-risk temperature, use of a PTC electrode is an attractive safety strategy to promptly remove electrons or ions between the electrodes or internally in an electrode to interrupt the reaction of the battery. Moreover, PTC electrodes are reliable, cost-effective, and particularly easy to use. Ji et al. described a novel thermally responsive cathode coated with ultrathin poly(3-octyl thiazole) (P3OT) less than 1 mm thick to be combined into a Al/P3OT/LiCoO2 sandwich cathode (LCO–PTC) [203] (Fig. 15d). The test results demonstrated that the LCO–PTC cathode had the same electrochemical performance as a conventional LiCoO2 cathode but was able to automatically switch off the battery reaction through PTC behavior within a specific temperature range to prevent the battery from thermal runaway. A PTC compound was prepared from a carbon black/PE composite by Kise et al. [204]. In the overcharge experiment, the overcharge performance of the composite improved with increasing PTC material content. As the resistance of the PTC element increased, the charge current flowed through carbon black at increased temperatures. The high-temperature performance was effectively improved, and the battery could operate at 100–130 °C. Additionally, PTC materials based on ethylene vinyl acetate were introduced into LFP cathodes by direct mixing them with LFP powder [205]. When the temperature of the battery system was above 90 °C, the LFP/PTC composite electrode displayed a current-limiting effect.
In summary, as cathode materials are being commercialized and become ready for public use, the thermal problems of intercalation-type materials need to be urgently resolved. Modification of cathode materials can reduce the heat release from the source or promptly suspend the cell reactions in high-risk situations. This will improve the durability, stability, and the lifespan while enhancing the safety features of these intercalation-type cathode materials.
4.2.2 Exploration of Sulfur and Oxygen Cathodes
For long-distance electric vehicles, in addition to large energy storage systems to balance the power load, energy storage equipment with greater gravimetric/volumetric energy density is also needed. Therefore, researchers have turned their attention to the development of new cathode materials composed of multielectron systems with a higher energy density, as well as lithium-based batteries that overcome the charge storage limitations of lithium insertion into composite electrode materials. Surprisingly, sulfur and oxygen cathodes paired with lithium metal anodes are good choices for achieving these goals [59, 60] because the theoretical capacity of sulfur cathode materials is one order of magnitude higher than that of phosphate cathode materials. However, sulfur (S8), oxygen (O2), and their various discharge products (Li2S, Li2S2, and Li2O2) exhibit poor conductivity and ionic conductivity, which leads to large internal resistances of Li-S and Li-O2 cells, resulting in high voltage polarization and significant heating, which further reduce the Coulombic efficiency and energy efficiency of the cells. Klein et al. demonstrated that the cathodic reaction of novel salt-containing electrolyte additives with low conductivity Li2S could form a stable conductive encapsulation layer on the surface of Li2S and overcome the problem of material loss [206]. This resulted in improved material encapsulation, thermal stability enhancement, and improved capacity retention of the cathode. Electrolyte additives such as P2S5 [207], InI3 [208], and cobaltocene [209] activate commercially available bulk Li2S, thereby producing high-capacity, thermally stable cathodes with no initial charge barrier. Liu et al. proposed and synthesized a simple and effective lithium battery system based on Li2S that was realized by using a bifunctional electrolyte additive [210]. Li2S cathodes were loaded on carbonized wipes by solution infiltration. The researchers found that the unique indium triiodide (InI3) electrolyte additive acted as a cathode redox mediator to reduce the activation potential of the Li2S cathode. The prepared SnO2/Li2S battery had stable electrochemical properties. A graphene–sulfur composite surface coated with an ultrathin Al2O3 film as the cathode improved the performance of lithium–sulfur batteries [211]. In recent years, continuous research has been conducted on Li-O2 batteries. Johnson et al. described a unified mechanism that could explain O2 reduction across a range of solvents [212]. They found that the morphology of Li2O2 was related to the solvent donor number. Gao et al. reported that the introduction of the additive 2,5-ditert-butyl-1,4-benzoquinone (DBBQ) accelerated the formation of a solution phase of Li2O2 in low polarity and weakly solvating electrolyte solutions [213]. Additionally, the product suppressed direct reduction of Li2O2 on the cathode surface, thereby preventing the growth of a Li2O2 film and runaway of the battery. The DBBQ additive prevented the formation of active intermediate LiO2 in solution through a new mechanism. In summary, although Li-S, Li-O2 batteries point the way for future energy storage devices and exhibit attractive advances, the scientific and technological constraints must be determined before their practical commercialization.
4.2.3 Innovation in LPB Cathodes
LPBs are still irreplaceable due to their long storage life, ultrahigh energy/power density, and wide working temperature range. Various cathode materials are in use, among which the CFx cathode has the highest energy density. However, because of its high internal resistance and poor conductivity, the CFx cathode generates substantial heat during discharge, especially in pouch batteries. Herein, we use a specific CFx cathode material as an example to review the solutions to the heat issue of LPBs. As mentioned in Sect. 3.2.1, two heat sources exist in the lithium battery, namely the electrochemical reaction and the Joule effect [77]. On the one hand, we need to improve the discharge voltage so that the actual discharge voltage of CFx (2.5 − 3.0 V vs. Li/Li+) is as close as possible to the theoretical discharge voltage (4.52 V vs. Li/Li+). The energy corresponding to the voltage gap is converted into heat energy that is released to the outside. On the other hand, to decrease the heat generated by the Joule effect, we should improve the conductivity of CFx and reduce its internal resistance.
(i) Decrease in the heat released by the electrochemical reaction
The C–F bond is the critical factor determining the discharge voltage of Li/CFx. Additionally, the high electronegativity of F atoms results in an assortment of C–F bond types: covalent bonds, semi-ionic bonds, and ionic bonds (Fig. 16a) [214]. Sato et al. confirmed for the first time the existence of a semi-ionic C–F bond in fluorine-graphite intercalation compounds [215]. The length of the C–F bond differs for different C–F bond types, which in turn affects the bond energy of the C–F bond. Dubecký found that the longer the C–F bond is, the lower the energy required to break it (Fig. 16b) [216]. To promote the plateau voltage of Li/CFx, reducing the energy required to break the C–F bond in CFx is essential. This has guided researchers to pursue a high proportion of C–F semi-ionic bonds in CFx materials. Through theoretical calculations, Nakajima et al. demonstrated that fluorinated fullerene consists of nearly ionic and semi-ionic bonds [217]. Zhang et al. confirmed that a decrease in the curvature of the material affects the strength of the C–F bond, leading to an increase in the covalence of the C–F bond and enhancement of the robustness of the C–F interaction in CFx [218]. Based on this principle, Peng et al. reacted a high-curvature calcined macadamia nutshell with F2 gas (F-cMNS) below 300 °C and prepared CFx with a high content of semi-ionic C–F bonds and a discharge voltage exceeding 3.0 V (vs. Li/Li+) (Fig. 16c) [55]. Additionally, a pouch-type Li/CFx cell with a capacity of 1 Ah utilizing F-cMNS as the cathode exhibited excellent electrochemical performance and safety performance (no bulging) (Fig. 16d). Structural defects and reduced F/C ratios also increase the proportion of semi-ionic C–F bonds [57, 219]. Lam et al. prepared sub-fluorinated CFx (0.33 < x < 0.63) from NG. They confirmed that the lower the fluorine content is (CFx, x = 0.33), the higher the voltage of the Li/CFx cell is. Sun et al. employed acetonitrile–chloroform and a low-boiling-point intercalation method to perform solvothermal exfoliation of fluorinated graphite to prepare defective fluorinated graphene [57]. Through XPS analysis of C1s and F1s, they demonstrated that the product contained many semi-ionic C–F bonds (Fig. 16e). The cell displayed a discharge voltage exceeding 2.7 V (vs. Li/Li+). Moreover, using pure F2 gas to unzip single-walled CNTs (SWCNTs) at high temperatures [70], Feng et al. prepared a single layer of fluorinated graphene for the first time (Fig. 16f), and it had an unprecedented energy density of 2738.45 Wh kg−1 and a discharge voltage exceeding 3.0 V (vs. Li/Li+). This was achieved through the abundant edges and defective periodic structure, respectively. DFT calculations showed that F atoms were added to the SWCNTs following a zigzag path. The reduction in Gibbs free energy confirmed the spontaneous unzipping of SWCNTs at high fluoridation temperatures.
Innovation in CFx cathodes. (a) Ionic/semi-ionic/covalent C–F bond energy from C1s XPS spectra. Reprinted with permission from Ref. [214]. Copyright © 2016 John Wiley and Sons. (b) Length of the C–F bond in fluorinated graphene and energy to remove a neutral F atom from fluorinated graphene. Reprinted with permission from Ref. [216]. Copyright © 2015 American Chemical Society (1 kcal mol−1 = 4.184 kJ mol−1). (c) Discharge profiles of F-cMNS at 10 mA g−1. (d) Discharge profiles of a pouch-type lithium battery fabricated by utilizing F-cMNS-280 with a capacity of 1 Ah at 0.01 C. The weight of the pouch-type battery is 5.75 g. Reprinted with permission from Ref. [55]. Copyright © 2019 Elsevier. (e) High-resolution XPS spectra of fluorinated graphite that underwent solvothermal exfoliation. Reprinted with permission from Ref. [57]. Copyright © 2014 Royal Society of Chemistry. (f) Schematic of the synthesis of fluorinated graphene nanoribbons using F2 at high temperature. Reprinted with permission from Ref. [70]. Copyright © 2020 Springer Nature
(ii) Decrease in the heat released by the Joule effect
Li/CFx batteries are primarily used in low-power applications owing to the slow kinetics at the CFx cathode, which is mainly attributed to the poor conductivity of CFx. Many strategies have been used to reduce the internal resistance, such as adding conductive carbon or oxide to improve the conductivity and enhancing the conduction of lithium atoms to improve the specific capacity through dopants, defects, and graphene grains. Movable fluorine acceptor species lead to increased conductivity, while increasing the content of covalent C–F bonds results in poor conductivity due to percolation [220]. Li et al. improved the discharge performance of Li/CFx, especially at higher rates, by replacing ordinary acetylene black with MWCNTs [221]. The 3D network formed by MWCNTs in the cathode is believed to expand the interfacial contact area, promote electron transfer, and accelerate the diffusion of lithium ions in the fluorinated layer and electron transfer in the cathode, thus improving the discharge performance of the Li/CFx batteries. Yang et al. reported a graphene/Au composite paper applied as a novel current collector for CFx cathodes to enhance the rate performance and safety without affecting their high capacity [222]. Their findings highlighted the significant role of the composite active layer/current collector interface and provided a new strategy for the design of CFx cathodes with high power density and high energy density. Hybrid cathodes composed of CFx and MnO2 with different arrangements displayed dissimilar electrochemical performances [223]. When CFx and MnO2 were in parallel, the electrochemical performance was excellent. Wang et al. applied in situ transmission electron microscopy (TEM) to investigate the lithiation and nanoscale electrochemical kinetics of CFx nanocrystals [224], which are essential for elucidation of the battery chemistry of CFx compounds and can provide guidance for the next step in Li/CFx development.
5 Solution Two: Optimization of Battery Components
In the battery system, in addition to the cathode and the anode, other components are integral parts that work with the electrode materials to form a stable circuit. When the battery is in operation, these components cooperate with each other to achieve orderly operation and to maximize the efficiency of their respective reactions. The battery will fail when a battery component malfunctions. Moreover, improper operation can even lead to thermal runaway. Therefore, enhancing the safety performance and thermal stability or optimizing the ability to autonomously switch off the reaction of each battery component will have a positive effect on thermal runaway inhibition. In this chapter, we discuss optimization of battery components, that is, electrolytes, separators, and other battery components (e.g., current collectors), to improve the safety.
5.1 Improved Safety Performance of Electrolytes
The electrolyte is the carrier of ions in the battery. In lithium battery systems, electrolytes are generally composed of a high-purity organic solvent, a lithium electrolyte salt, necessary additives, and other raw materials under specific conditions and in specific proportions. The electrolyte conducts ions between the cathode and the anode of lithium batteries, forms a circuit, and provides a reaction environment that ensures high voltage and specific energy in LIBs. Unfortunately, organic solvents are highly flammable and highly volatile, characteristics that pose serious safety concerns [225,226,227]. Since the nascence of lithium batteries, the use of safer solvents, additives, and solid electrolytes has been the main approach to enhance the safety of lithium batteries.
5.1.1 Additives that Stabilize Liquid Electrolytes
Currently, a mature electrolyte system comprises lithium salt (LiPF6) dissolved in a carbonate mixed solvent, which can be dimethyl carbonate (DMC), diethyl carbonate (DEC), ethylene carbonate (EC), PC, ethyl methyl carbonate (EMC), 1,2-dimethoxyethane (DME), or 1,3-dioxolane (DOL) [228]. The properties of these solvents are summarized in Fig. 17. Because of its effect on the battery performance, adjusting the composition of the electrolyte is the first step in electrolyte optimization.
Various additives are used to protect the interfaces between the anode, the cathode materials, and the electrolyte, which affects the operating temperature range of the device. Zheng et al. demonstrated that the optimal dosage of LiPF6 (0.05 M, 1 M = 1 mol L−1) as a LiTFSI–LiBOB double salt/carbonate solvent electrolyte additive led to promotion of the charging capacity and cycling stability of LMBs (Fig. 18a) [229]. Stable circulation was achieved at a current density of 1.75 mA cm−2 at 60 °C (Fig. 18b). LiPF6 additives play a vital role as stable current collectors and, more importantly, accelerate the formation of an indurative and conductive SEI layer, which can effectively inhibit the formation of dead lithium. Room-temperature ionic liquids are safe and inherently nonflammable. A novel nonflammable ionic liquid electrolyte formed from 1-ethyl-3-methylimidazolium cations and high-concentration bis(fluorosulfonyl)imide anions, with sodium bis(trifluoromethanesulfonyl)imide (NaTFSI) as an essential additive, was synthesized by Sun et al. (Fig. 18c) [230]. Na ions accelerate the formation of a mixed passivating interphase, contributing to dendrite-free lithium formation through morphological and chemical changes. Symmetrical Li/Li cells demonstrated cycling stability during long cycling in this novel electrolyte (Fig. 18d). The high LiCoO2 mass load of 12–16 mg cm−2 demonstrated impressive cycling performance for up to 1200 cycles. Furthermore, an SEI rich in LiF with 2% tris(2,2,2-trifluoroethyl)borate in the electrolyte enhanced the migration of lithium ions and uniform lithium deposition by regulating the growth of lithium dendrites [231]. Wang et al. proposed lithium iodide as a functional additive for dendrite-free lithium deposition in ether-based electrolytes to guide in situ electrolyte polymerization and generate an SEI layer rich in elastic oligomers on the lithium surface [232]. Long-range stabilization of the SEI and the CEI formed on the anode and the cathode is critical in retarding electrode interface degradation and improving the cycle life of lithium batteries. PF5 and HF reactions generated during the decomposition of the commonly used LiPF6 electrolyte inevitably destroy the SEI and CEI layers. Han et al. synthesized a cyclic carbonate electrolyte additive comprised of 3-(1-ethoxyethoxy)-1,2-propylene carbonate and 3-trimethoxysilylpropyloxy-1,2-propylene carbonate to maintain the integrity of the SEI and the CEI in lithium batteries [233]. The additive had high resistance to Lewis acids (PF5) along with a HF scavenging capability (Fig. 18e, f), thereby improving the rate and safety performance of a LiNi0.6Mn0.2Co0.2O2/graphite cell, indicating significant potential for use in high-energy–density LIBs. The use of malonic-acid-decorated fullerene with active superoxide dismutase as an electrolyte additive deactivated PF5 by water scavenging and played a positive role in stabilizing the SEI and the CEI [234]. Pham et al. studied the effect of a multifunctional electrolyte additive, methoxytriethyleneoxypropyltrimethoxysilane (MTE-TMS), on the protection of the cathode and the anode (Fig. 18g) [235]. MTE-TMS formed a stable SEI layer on the graphite anode, which stabilized the graphite structure and prevented the SEI from thickening. The use of electrolyte additives tremendously improved the performance of batteries, achieving capacity retention rates up to 84% and Coulombic efficiency up to 99.8% after 100 cycles (Fig. 18h). Han et al. revealed an electrolyte additive with a strong electron donor capability and an ability to scavenge HF and PF5. It was composed of (trimethylsilyl)isothiocyanate based on amino silane [236]. The electrolyte additive could effectively passivate reactive species and thus maintained the safety performance of the SEI and the CEI layers cladding the anode and the cathode in the LIPF6-based electrolyte.
Additives that stabilize liquid electrolytes. (a) Schematic of a LiPF6 additive dissolved in the LiTFSI–LiBOB electrolyte to promote the safety of the lithium anode. (b) Li-NMC full battery electrochemical performances with various electrolytes in an EC–EMC solvent at a current density of 1.75 mA cm−2 at 60 °C. Reprinted with permission from Ref. [229]. Copyright © 2017 Nature Publishing Group. (c) Schematic of the composition of the EM–5Li–Na IL electrolyte in the battery. (d) Symmetrical Li battery with the EM–5Li–Na IL electrolyte at various cycles. Reprinted with permission from Ref. [230]. Copyright © 2020 John Wiley and Sons. (e) Schematic of PF5 stabilization. (f) Schematic illustration of HF scavenging by the TMSPC additive. Reprinted with permission from Ref. [233]. Copyright © 2020 American Chemical Society. (g) Schematic of the failure mechanism occurring in the graphite/NCM851005 battery a) without and b) with an MTE-TMS additive. (h) Cycling performances and Coulombic efficiency without and with an MTE-TMS additive. Reprinted with permission from Ref. [235]. Copyright © 2016 Elsevier
To eliminate potential thermal hazards, the thermal stability of lithium battery electrolytes should be further improved. Consequently, numerous types of additives have emerged. The use of triphenyl phosphate- and silicon-containing additives as flame retardants can increase the initial temperature for electrolyte thermal runaway, which improves the cycling stability, rate performance, and safety [237]. Zeng et al. reported the fabrication of a safe battery electrolyte using nonflammable phosphonate. Electrolytes have good prospects for broader applications [238]. In addition, a novel additive, 1,10-sulfonyldiimidazole (SDM), has the capacity to promote the cycling performance of LiNi0.5Mn1.5O4 at high voltages and high temperatures (55 °C) [239]. p-Toluenesulfonyl isocyanate (PTSI) can be used as an electrolyte additive to produce a stable SEI film on a graphite surface that can inhibit electrolyte decomposition and electrode corrosion [240]. Some additives can enhance the electrochemical performance of batteries at low temperatures. Ignatova et al. applied a 15-crown-5 additive in primary Li/CFx cells at low temperatures [241]. 15-Crown-5 added to 1 M LiPF6 in EC/DMC/EMC was functional between − 50 °C and − 45 °C, while the corresponding capacities ranged from 140 to 110 mAh g−1. The absorption of the additive on the electrode surface to form a conductive layer was subsequently proven via theoretical calculations. Thermally responsive smart additives can suspend chemical reactions in time to prevent thermal runaway [242] in batteries. Janssen et al. recently synthesized overcharge-protection shutdown additives in electrolytes that were applied in LMBs and LIBs. Impressively, 1,3-dimethylimidazolidin combined with various ligands (–BF3, –PF5, and –PF4CF3) at the molecular level could stop the reaction at different cutoff potentials [243]. Additionally, analysis and tests showed that the electrolyte additive had no effect on the battery performance. Noelle et al. developed an electrolyte additive consisting of diol and diamine that increased the internal resistance of lithium cells upon short-circuiting, thereby preventing generation of uncontrolled Joule heat [244]. Xia et al. investigated a thermally polymerizable monomer as an electrolyte additive to protect batteries from thermal runaway [245]. The mechanism involved polymerization of the additive at 110 °C to rapidly solidify the electrolyte and significantly block ion transfer between the electrodes, thereby causing thermal shutdown of the electrode reaction to enhance the safety of the lithium battery. Moreover, an improved self-extinguishing ability to inhibit electrolyte combustion was presented [246]. Gas chromatography and mass spectrometry confirmed the presence of a large amount of the additive after 501 cycles, thus ensuring the safety of the electrolyte. Conventional lithium batteries have temperature operating windows of − 20 to 50 °C and voltage operating windows of 0.0 to 4.3 V. They are limited by the high affinity between solvents and ions and the high flammability of carbonate electrolytes. Fan et al. weakened the force between the solvent and lithium ions by adding fluorinated electrolytes to highly fluorinated nonpolar solvents [247]. The modified electrolyte expanded the voltage (0.0 to 5.6 V) and temperature (− 125 to 70 ℃) windows while displaying high ionic conductivity. The Coulombic efficiency of NCA as the cathode cell was 99.9% at temperatures ranging from − 95 to 70 °C. Even at − 85 °C, the NCA/lithium battery retained approximately 50% of its room-temperature capacity. In other words, electrolyte additives present inspiring ways to broaden the safe operating temperature range of lithium batteries. However, more innovation and research are required.
5.1.2 Novel and Safe Solid-State Electrolytes
Solid-state electrolytes (SSEs) with high safety are an excellent choice for next-generation electrochemical energy storage devices. Inherently nonflammable solid electrolytes that replace combustible organic liquid electrolytes can overcome explosion problems in extreme conditions and have several advantages, such as high mechanical strength, exceptional electrochemical stability, a wide operating temperature range, and a large ion transference number [248]. The challenges associated with SSEs coexist with these advantages and impede the practical application of all-solid-state batteries, especially at room temperature. The interfacial resistance between the solid electrolyte and the electrode is the key factor affecting the performance of lithium batteries in which solid electrolytes are employed. Consequently, as in the case of improving the safety performance of batteries, increasing attention has been paid to the interfacial resistance [249]. Recent research indicates that solid garnet batteries have broad application prospects [250,251,252,253]. Jiang et al. prepared a 3D Li6.75La3Zr1.75Ta0.25O12 (LLZTO) self-supporting skeleton connected by a polytetrafluoroethylene (PTFE) binder via a simple grinding method without using any solvent. Subsequently, a composite all-SSE based on garnet was obtained by filling the flexible 3D LLZTO framework with solid succinonitrile (Fig. 19a) [250]. This method not only avoided a complex preparation process, high-cost toxic solvents, and high-temperature sintering but also decreased the surface blockage by active LLZTO powder and binder molecules. Additionally, the LLZTO skeleton connected by PTFE had good thermal stability due to its nonflammability. Experimental results showed that a wide electrochemical operating window of 4.8 V (vs. Li/Li+) was achieved with the obtained substance as the electrolyte along with an ion transference number of 0.53. Li-LFP and Li-NMC batteries prepared by using the ultrathin composite electrolyte exhibited discharge specific capacities of 153 and 158 mAh g−1, respectively (Fig. 19b), with good cycling stability at room temperature. Huo et al. studied the effect of ceramic-in-polymer (CIP) and polymer-in-ceramic (PIC) composite electrolytes on dendrite inhibition using various garnet particle sizes. The CIP with smaller LLZTO particles displayed superior ionic conductivity, while the PIC with larger LLZTO particles displayed excellent mechanical strength. Based on the results, a sandwich-type composite electrolyte (SCE) with multisized LLZTO particles was prepared to inhibit lithium dendrite formation and enhance the safety performance (Fig. 19c) [252]. The assembled LFP/SCE/Li cells exhibited outstanding rate performance (Fig. 19d). The pairing of sulfide electrolytes with appropriate cathodes is essential for the development of stable all-solid lithium batteries. The thioantimonate ionic conductor Li6.6Ge0.6SB0.4S5I has excellent ionic conductivity up to 1.6 m s−1. Moreover, it can be coupled as a solid electrolyte with an iron sulfide (FeS2) cathode, with a theoretical capacity of up to 894 mAh g−1 without additional interface engineering [253].
Safe SSEs. (a) Schematic of Li–FEC/LiFePO4 solid-state cells. (b) Voltage profiles at various rates for Li–FEC/LiFePO4 batteries and Li–FEC/LiNi0.5Mn0.3Co0.2O2 batteries. Reprinted with permission from Ref. [250]. Copyright © 2020 John Wiley and Sons. (c) Schematic of PIC, CIP, and sandwich-type electrolytes. (d) Rate performances of LFP/SCE/Li and LFP/PIC/Li batteries at various current rates. Reprinted with permission from Ref. [252]. Copyright © 2019 John Wiley and Sons. (e) Ex situ and in situ synthesis of SPEs. Reprinted with permission from Ref. [255]. Copyright © 2019 Nature Publishing Group. (f) SEM images of PEO–LCO, CMC–LCO, and Na-alginate–LCO electrodes. Schematic of the binding capability of PEO and CRP binders. (g) Comparison of adsorption energy of PEO, PVDF, CMC monomer, and Na-alginate monomer on LiCoO2 (001). Reprinted with permission from Ref. [256]. Copyright © 2020 John Wiley and Sons
Solid-state polymer electrolytes (SPEs) have attracted the attention of researchers due to their outstanding mechanical properties, low cost, light weight, and mass production processes [254]. Generally, satisfactory SPEs should display at least two of the above characteristics. Furthermore, SPEs should be constantly mechanically stable and chemically inert during charging/discharging. SPEs are characterized by high ionic conductivity and fast interfacial transfer of lithium ions between the battery electrodes. Various strategies to enhance the electrochemical and mechanical properties of SPEs have been reported. Zhao et al. synthesized a novel SPE using cationic aluminum species to initiate ring-opening polymerization of molecular ether. This enabled contact to be maintained with the concomitant interfaces of all battery components [255]. In situ SPE formation via liquid precursor polymerization was more advantageous than ex situ SPE formation (Fig. 19e). The SPE formed in situ displayed high ionic conductivity (> 1 mS cm−1) and low interfacial resistance at room temperature. Moreover, it enhanced uniform lithium deposition. The application of the SPE in Li-S, Li-LFP, and Li-NMC cells demonstrated that the design achieved high Coulombic efficiency (99%) and a long lifespan (700 cycles). SPEs based on polyethylene oxide (PEO) are not compatible with some cathodes because of the limited electrochemical oxidation window of PEO. Liang et al. studied the application of common binders such as PEO, polyvinylidene fluoride (PVDF), and carboxyl-rich polymers (CRPs) [including sodium alginate (Na-alginate) and sodium carboxymethyl cellulose (CMC)] in all-solid-state polymer batteries [256] (Fig. 19f). Experimental results showed that batteries with LCO electrodes and CRP binders demonstrated excellent cycling performance up to 1000 cycles. To understand the advantages of CRP binders, the interface properties between the CRP binder and LCO were studied by using DFT (Fig. 19g). The adsorption energy of the CMC monomer and Na-alginate was 2.96 eV and 3.38 eV, respectively, which was much higher than that of the PEO and PVDF dimers. The greater binding and adsorption energy between the polymers and the surface determined the robustness of the solid electrolyte. The application of a combination of single-ion conduction and hyperbranched structures in SPEs showed promise [257]. Bouchet et al. designed a single-ion conductor based on block copolymers comprised of polystyrene segments with a unique TFSI anion and a structure capable of achieving the important delocalization of negative charges [258]. The design overcame numerous limitations of solid electrolytes and delivered nearly uniform Li-ion transport, superb mechanical properties, and a robust electrochemical operating window spanning 5 V versus Li+/Li. Cao et al. prepared a novel single-ion SPE with a high Li-ion transference number [259]. The produced single-ion SPE membrane exhibited excellent ionic conductivity. Li et al. prepared a single-ion SPE using carbon quantum dots (CQDs) through calcination of poly(lithium 4-styrene sulfonate) and citric acid [260]. The large size of the CQD anions and the hydrogen bonds prevented anions from migrating in the polyoxyethylene matrix, resulting in a high Li+ transport number. In addition, thermally responsive SPEs can achieve thermoinduced autonomous shutdown of lithium batteries at unsafe temperatures [261]. Owing to the high safety and stability of solid electrolytes, the belief that solid electrolytes will undergo focused research that contributes to solving the thermal runaway problem of next-generation high-performance electrochemical energy storage devices is reasonable.
5.2 Separators Based on Thermal Stability
The battery separator is an electrically insulating film between the anode and the cathode with a complex 3D porous structure allowing rapid transfer of lithium ions back and forth when the battery is filled with the liquid electrolyte, which is an indispensable condition for the battery reaction. The cathode is physically separated from the anode; otherwise, an internal short circuit would occur. Although separators are not active components of batteries and do not normally participate in battery chemical reactions, they play a key role in battery safety [262, 263]. Most commercial lithium battery separators are polyolefin films such as PE or PP. On this basis, researchers have made continuous extensions and modifications [264,265,266]. Polyimide (PI) nanofibers with an average diameter of 300 nm were prepared by electrospinning a polyamide acid solution with excellent electrolyte wettability and thermal stability. The large pores of PI nanofibers can accelerate Li-ion transfer but increase the battery impedance [264, 265]. To overcome this problem, Lee et al. dip-coated both sides of the PI separator with a thin Al2O3 layer [264]. Shrinkage was assessed by placing the separator in an oven and heating it for 30 min at different temperatures between 150 and 200 °C. The common commercial PP separator visibly shrank above 150 °C, which may cause direct contact of the cathode and the anode at high temperatures. Surprisingly, PI displayed thermal stability up to 200 °C. Neither the PI separator nor the Al2O3–PI separator deformed (Fig. 20a). The experimental results showed that the contact angles of the PP, PI, and Al2O3–PI separators were 27.86°, 22.09°, and 18.41°, respectively (Fig. 20b). Due to the excellent properties of Al2O3–PI, the cycling performance, rate performance, and high-temperature performance of the battery with the Al2O3–PI separator were significantly enhanced. Liu et al. prepared a specially designed polyphenylene sulfide (PPS) separator from a binary diluent to enhance the thermal stability of lithium batteries [266]. Diphenyl sulfone and diphenyl ketone formed the first diluent, and polyether sulfone was used as the other diluent, which were labeled PPS1 and PPS2, respectively. The concentration of PPS was maintained at 25%. The PPS separator exhibited a curved and highly porous structure, endowing the separator with outstanding ionic conductivity, excellent electrolyte wettability and a high lithium-ion transference number. Benefiting from excellent thermal dimensional stability, PPS displayed excellent high-temperature performance and ultrahigh strength. Moreover, it did not shrink until 280 °C (Fig. 20c). The Li-LFP battery with PPS displayed excellent rate performance (Fig. 20d). Polyethylene terephthalate coated with cubic alumina nanoparticles and a macroporous separator composed of a polymer with intrinsic microporosity significantly improved the properties of the battery separator [267, 268]. Generally, for safety and good cycling performance, the separator should have excellent mechanical strength and thermal stability and good wettability. Concurrently meeting all these requirements is a daunting task [269,270,271,272,273,274,275,276].
Separators based on thermal stability. (a) Digital images of shrinkage of PP, PI, and Al2O3–PI separators at various temperatures. (b) Digital images of the liquid electrolyte infiltration behavior of PP, PI, and Al2O3–PI separators. Digital images of the liquid electrolyte contact angles of the separators. Reprinted with permission from Ref. [264]. Copyright © 2014 Elsevier. (c) Digital images of PE, PPS1-81, and PPS2-81 separators after heat treatment at various temperatures. (d) Rate performance of batteries with PE, PPS1-81, and PPS2-81 separators. Reprinted with permission from Ref. [266]. Copyright © 2019 Electrochemical Society. (e) Schematic of a “smart” electrospun separator with thermally triggered flame-retardant properties for batteries. Reprinted with permission from Ref. [277]. Copyright © 2017 American Association for the Advancement of Science. (f) Schematic of the coaxial-fiber separator shutdown concept for LIBs. Reprinted with permission from Ref. [278]. Copyright © 2017 Royal Society of Chemistry. (g) Schematic of a thermally responsive microsphere-coated separator. Reprinted with permission from Ref. [279]. Copyright © 2015 Royal Society of Chemistry. (h) Schematics of a multifunctional electrospun separator with thermally triggered flame-retardant properties for batteries. (i) Schematics of Li2Sx on APP and binding energy of APP and Li-S. Reprinted with permission from Ref. [281]. Copyright © 2018 John Wiley and Sons
Another effective strategy for improving battery separators involves shutting down the battery in time during overheating, which can prevent thermal runaway. Cui et al. manufactured a new type of “smart” nonwoven electrostatic separator with a thermal trigger and flame-retardant properties for LIBs (Fig. 20e) [277]. During thermal runaway of the lithium battery, the temperature rises to a critical level, and the protective polymer shell melts, releasing the flame retardant that effectively inhibits combustion of the combustible electrolyte. This type of smart separators is expected to be used in other high-energy storage devices that may have safety issues associated with thermal runaway. Jiang et al. successfully fabricated a thermoinduced shutdown separator from a polylactic acid and polybutylene succinate blend (PLA@PBS) via a facile coaxial electrostatic spinning process (Fig. 20f) [278]. The PLA@PBS electrostatically spun separator was characterized by high thermal sensitivity, high thermal stability, and outstanding liquid electrolyte wettability. Furthermore, batteries assembled with the dual-function PLA@PBS separator exhibited better cycling performance than those containing commercial separators. A coating of assorted microspheres on the separator can control thermal runaway of the battery [279, 280]. In the normal state, the microspheres do not affect the chemical reaction. Once a critical temperature is reached, the microspheres melt and prevent the reaction. Ji et al. developed a thermoplastic coating comprised of EVA copolymer microspheres that could automatically shut down the chemical reaction at 90 °C to protect the lithium battery [279]. The working mechanism involved melting of the EVA coating and formation of a nonconductive partition covering the separator once the critical temperature was reached. Li-ion conduction between the electrodes was quickly cut off, terminating the battery reaction and protecting the battery from thermal runaway (Fig. 20g). In addition, electrochemical experiments showed that this type of separators had no negative effect on the normal battery performance, thus providing an internal self-protection mechanism to serve as a safety control system in lithium batteries. Lithium–sulfur batteries in practical applications are significantly impacted by the polysulfide solution and the safety hazards caused by flammable sulfur and polymer separators. Lei et al. reported that an incombustible multifunctional separator could effectively inhibit polysulfide dissolution and enhance the high-temperature performance of lithium batteries. The separator was electrospun by using polyacrylonitrile and ammonium phosphate (PAN@APP) [281]. Because APP is rich in amines and phosphate radicals, the PAN@APP separator had a strong binding effect on polysulfide, generating a strong charge repulsion. The specific implementation effect is shown in Fig. 20h. Under working conditions, PAN@APP effectively limited the migration of polysulfide. When the battery temperature rises uncontrollably, the APP melts and covers the battery surface, thereby isolating air and heat (Fig. 20i). The capacity retention rate of the Li-S battery utilizing the PAN@APP separator was 83% after 800 cycles. Liao et al. designed a new type of dual-functional coating prepared from a polystyrene poly(butyl acrylate) copolymer with silicon nanoparticles as a cladding material for coating of a PP separator. The chemical reaction was shut off above 80 °C [282]. Notably, separators with high thermal stability, high mechanical strength, low cost, and excellent electrochemical performance have great value in high-power LIBs.
5.3 Other Optimization Strategies for Battery Systems
In practical applications, lithium batteries are required to operate in harsh ambient environments. In addition to the electrolytes and separators, configuring other components of battery systems to efficiently control the battery temperature and prevent thermal runaway is extremely important.
5.3.1 High-Safety Current Collectors
High-density foil current collectors are typically an integral part of lithium batteries that may pose a fire safety hazard yet provide no additional capacity. Cui et al. prepared an ultralight PI current collector from a high-performance PI based on triphenyl phosphate flame retardants sandwiched between two ultrathin copper layers, thereby minimizing the “dead weight” inside the battery and improving fire safety [283]. Compared with lithium batteries assembled with the thinnest commercial foil current collectors, batteries furnished with this collector achieved a 16% to 26% increase in specific energy and displayed rapid self-extinguishing under extreme conditions such as short circuits and out-of-control heating. Wang et al. illustrated a functional current collector that isolated the internal short circuit from the undamaged area of the battery during the failure of a fully charged battery. The heat generated by the internal short circuit was dramatically reduced to a negligible level [284]. The design strategy of a high-safety current collector holds tremendous promise for practical battery applications, providing safer lithium batteries with high specific energy.
5.3.2 Phase-Change Material Cooling Systems
Phase-change materials (PCMs) are functional materials that can change their state without changes in their temperature. The stored energy can be released into the environment upon a phase change from a liquid to a solid as the temperature drops. Conversely, as the temperature rises, these materials absorb thermal energy in the form of latent heat [285]. Commonly, they fall into three categories: organic, inorganic, and eutectic. Thermoregulating functional PCMs have proven to be effective in the design of thermal management systems for lithium batteries [73]. Lv et al. investigated the performance of a compound PCM coupled with low fins as a battery thermal management system [286]. The PCM comprised low-density PE, expanded graphite, and paraffin. The phase-change temperature was between 44.5 and 50.2 °C to maintain the battery temperature below 50 °C. Jiang et al. studied the thermal optimization of PCM composites comprised of a PCM (paraffin RT44HC) and expanded graphite for thermal management of lithium batteries [287]. The introduction of expanded graphite improved the thermal conductivity of the PCM. Experimental results showed that the PCM composite reduced the maximum temperature of the battery by 10 °C compared with PCMs without expanded graphite. PCMs can be used as semi-passive thermal management systems that are more intelligent, more compact, and more efficient than active cooling systems. Many studies have been performed on the thermal management of batteries using PCMs [288,289,290,291,292]. PCMs have been tested as thermal management strategies and compared to conventional forced-convection cooling via air or coolants. They led to a lower maximum temperature within the cell and a more uniform temperature distribution.
5.3.3 Early Warning and Firefighting
To solve the battery safety problem, early warning and firefighting are the two most practical approaches. Early warning refers to real-time monitoring of voltage, current, resistance, and other data before the occurrence of a thermal hazard. An alarm is triggered when an abnormality is detected. This approach includes monitoring electrical performance parameter changes, temperature changes, and the escape of gases. In addition, firefighting is critical. The National Technical Information Service in the USA has assessed the potential of various battery fires and provides guidelines for firefighting strategies.
6 Summary and Prospects
As the global energy policy is slowly shifting from fossil energy to renewable energy, lithium batteries have a great advantage over other batteries as a vital method of storing energy and have subsequently attracted substantial attention. Safety concerns are a major obstacle to the large-scale application of lithium batteries. Thermal runaway is an inevitable subject of lithium battery safety research. Because of the rapid spread of information today, fires and explosions due to lithium batteries in applications ranging from mobile phones to electric cars and airplanes are often reported. These far-reaching events greatly endanger the life and property safety of consumers while damaging people's confidence, thus restricting the use of lithium batteries. Therefore, attaching importance to the thermal hazards of lithium battery materials and taking corresponding preventive measures are of great importance.
This review of the working mechanism of lithium batteries explains the different heat sources and various heat hazards of lithium batteries during charging and discharging. The review then focuses on the methods applied to enhance the safety performance of lithium batteries. These methods include modification of battery electrode materials (the anode and the cathode) and other important battery components (the electrolyte, the separator, and the collector), as well as development of battery management systems in recent years. Lithium batteries widely researched at present have been divided into two main categories based on their anode material, namely LMBs and LIBs. Whether dealing with an LMB or an LIB, the most basic working mechanism and main heat sources are identical. Abuse and inappropriate use will destroy the intrinsic properties of lithium batteries, leading to thermal runaway. In general, the heat sources of lithium batteries are categorized as reversible heat and irreversible heat. The main component of reversible heat is the heat released by the electrochemical reaction, while heat released by the Joule effect is a crucial part of irreversible heat. Once the battery experiences extreme conditions (physical damage, overcharging and overdischarging, overheating, and short-circuiting), it will inevitably fail and generate significant heat. Superfluous heat generation has profound effects, including thermal runaway, capacity loss, and electrical imbalance. Both the selection of electrode materials and optimization of the battery structure can enhance the safety performance of lithium batteries and inhibit thermal runaway. The modification methods discussed in this paper are as follows (Fig. 21):
-
(i)
Anode materials. The anode materials of lithium batteries include lithium metal, carbon-based materials, and silicon-based materials. An SEI layer will form on the surface of the anode. The preparation of an SEI layer with excellent thermal stability and mechanical properties is the core of any anode material modification strategy to improve the safety and cycling stability. Additionally, the disordered growth of lithium dendrites can result in dendrites piercing the separator, which leads to short-circuiting. For LMBs, a host material with high lithiophilicity can guide the deposition of lithium metal and inhibit the formation of lithium dendrites. Surface coating at the atomic level can improve the thermal stability and safety of carbon-based and Si-based anodes. In addition, a smart anode material can stop the battery chemical reaction in time to prevent tragedy.
-
(ii)
Cathode materials. During the reaction inside the battery, the cathode is less conductive than the anode and generates more heat. Under extreme conditions, the cathode material will decompose and collapse first. Taking the CFx cathode as an example, the heat output of Li/CFx batteries can be reduced by reducing the gap between the theoretical and actual voltages or by improving the electrical conductivity of the cathode. The use of additives or coating layers and element substitution are effective methods frequently employed in widely used intercalation-type cathodes. Moreover, coating the cathode with thermally responsive materials can improve battery safety.
-
(iii)
Electrolyte materials. Electrolytes are carriers of Li ions. However, unsafe liquid electrolytes are the most combustible components of batteries. Triphenyl phosphate, silicon-containing, and fluorine-containing additives can enhance the thermal stability of liquid electrolytes. In addition, highly active additives can inhibit the formation of PF5 and HF reactive species generated by conventional LiPF6 electrolyte decomposition, which inevitably destroy the SEI and CEI layers. With the deepening of the understanding of electrolytes, safe solid electrolytes have broad application prospects. Among them, solid garnet and SPEs have attracted the attention of researchers. Solid electrolytes provide a great opportunity to solve the thermal runaway problem of next-generation high-performance electrochemical memory devices.
-
(iv)
Separator materials. Battery separators interrupt the contact between the battery cathode and the anode. The commonly used separators for lithium batteries are polyolefin films (PP or PE) with poor thermal stability. The use of PI and PPS materials with improved thermal stability as separators, combined with modification and coating, can greatly improve the stability of the separator material. Additionally, the use of thermally responsive materials can achieve timely shutdown of the battery during overheating and prevent thermal runaway.
Overview of various approaches to solve the thermal problems of lithium batteries Reprinted with permission from Ref. [140, 155, 173, 180, 200, 203, 233, 250, 252, 264, 277]. Copyright © 2020 John Wiley and Sons, Copyright © 2012 John Wiley and Sons, Copyright © 2014 American Chemical Society, Copyright © 2014 John Wiley and Sons, Copyright © 2016 John Wiley and Sons, Copyright © 2016 Royal Society of Chemistry, Copyright © 2020 American Chemical Society, Copyright © 2020 John Wiley and Sons, Copyright © 2019 John Wiley and Sons, Copyright © 2014 Elsevier, Copyright © 2017 American Association for the Advancement of Science
Nevertheless, many opportunities remain to be explored via both theoretical analysis and material design. By using revolutionary and innovative modification methods, we propose developing a new theoretical model and using advanced characterization methods and electrochemical analysis technology to reduce the thermal hazards of lithium batteries. In addition, for an advanced lithium battery with practicality, tests conducted on pouch batteries are a priority given the proven safety of coin batteries. There is a vast gap in the current density distribution between the coin battery and the pouch battery. Therefore, despite the many breakthroughs, high-energy-density lithium batteries still face many challenges. Many questions need to be considered in depth.
-
(i)
Theoretical models of lithium batteries with the action of independent variables have been frequently proposed. However, the coupling of mechanical, thermal, and electrical aspects requires more research and development. The construction of a theoretical model that more closely matches actual systems to put forward a more targeted theory is urgently needed.
-
(ii)
The SEI and CEI layers are critical to the safety of lithium batteries. The fundamental understanding of the formation mechanism, structure, and composition of SEIs and CEIs is still relatively shallow; therefore, accurately enhancing the understanding of the role and composition of the SEI and the CEI and regulating their modification are necessary. In addition, in situ observations of changes in hierarchical structures during the formation of SEIs and CEIs are expected to be reported.
-
(iii)
Innovative anodes with high capacity and high safety must be exploited. At present, only a few anode materials can be commercialized. Carbon-based graphite has been commercialized owing to its low volume expansion rate (10%). However, the capacity of the graphite material is inferior, which prevents it from meeting the requirements of rapidly developing science and technology.
-
(iv)
Anisotropic volume changes in highly delithiated states during continuous cycling cause mechanical degradation and further capacity fading of cathode materials. Novel coatings can penetrate the cathode material to improve ion flow, thereby reducing the internal resistance and increasing the current-carrying capacity. Additionally, enhancing the thermal stability to prevent the release of oxygen from the cathode can greatly improve the safety.
-
(v)
Solid electrolytes are considered attractive for overcoming several safety concerns associated with nonaqueous electrolytes, including leakage, poor chemical stability, and flammability. However, owing to their low ionic conductivity, poor Li+ affinity, and the poor interface between the electrolyte and lithium metal, their practical application in lithium batteries requires considerable development.
-
(vi)
The design of the wiring and current collectors in high-energy-density pouch batteries is rarely considered. Although they seem insignificant, the wiring and current collectors have a strong effect on the battery current and heat distribution. Ingenious designs can greatly enhance the thermal stability of pouch batteries.
From the perspective of enhancing the safety, capacity, voltage and cycling stability, Coulombic efficiency, and storage performance of lithium batteries, the sustainable development of lithium batteries needs to be advanced. Many improvements are not fully optimized for the commercial application of lithium batteries. However, they can contribute to the development of lithium batteries in future.
References
Croce, F., Appetecchi, G.B., Persi, L., et al.: Nanocomposite polymer electrolytes for lithium batteries. Nature 394, 456–458 (1998). https://doi.org/10.1038/28818
Wang, H.S., Liu, Y.Y., Li, Y.Z., et al.: Lithium metal anode materials design: interphase and host. Electrochem. Energy Rev. 2, 509–517 (2019). https://doi.org/10.1007/s41918-019-00054-2
Zhao, W.J., Yi, J., He, P., et al.: Solid-state electrolytes for lithium-ion batteries: fundamentals, challenges and perspectives. Electrochem. Energy Rev. 2, 574–605 (2019). https://doi.org/10.1007/s41918-019-00048-0
Poizot, P., Laruelle, S., Grugeon, S., et al.: Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 407, 496–499 (2000). https://doi.org/10.1038/35035045
Gu, S., Sun, C.Z., Xu, D., et al.: Recent progress in liquid electrolyte-based Li-S batteries: shuttle problem and solutions. Electrochem. Energy Rev. 1, 599–624 (2018). https://doi.org/10.1007/s41918-018-0021-0
Li, Y.H., Li, Q.Y., Wang, H.Q., et al.: Recent progresses in oxygen reduction reaction electrocatalysts for electrochemical energy applications. Electrochem. Energy Rev. 2, 518–538 (2019). https://doi.org/10.1007/s41918-019-00052-4
Wu, D., Peng, C., Yin, C., et al.: Review of system integration and control of proton exchange membrane fuel cells. Electrochem. Energy Rev. 3, 466–505 (2020). https://doi.org/10.1007/s41918-020-00068-1
Zou, L., Qiao, Y., Li, C.M.: Boosting microbial electrocatalytic kinetics for high power density: insights into synthetic biology and advanced nanoscience. Electrochem. Energy Rev. 1, 567–598 (2018). https://doi.org/10.1007/s41918-018-0020-1
Mori, R.: Recent developments for aluminum-air batteries. Electrochem. Energy Rev. 3, 344–369 (2020). https://doi.org/10.1007/s41918-020-00065-4
Lokhande, P.E., Chavan, U.S., Pandey, A.: Materials and fabrication methods for electrochemical supercapacitors: overview. Electrochem. Energy Rev. 3, 155–186 (2020). https://doi.org/10.1007/s41918-019-00057-z
Whittingham, M.S.: Lithium batteries and cathode materials. Chem. Rev. 104, 4271–4302 (2004). https://doi.org/10.1021/cr020731c
Maleki, H., Howard, J.N.: Effects of overdischarge on performance and thermal stability of a Li-ion cell. J. Power Sources 160, 1395–1402 (2006). https://doi.org/10.1016/j.jpowsour.2006.03.043
Noh, H.J., Youn, S., Yoon, C.S., et al.: Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J. Power Sources 233, 121–130 (2013). https://doi.org/10.1016/j.jpowsour.2013.01.063
Feng, X.N., Fang, M., He, X.M., et al.: Thermal runaway features of large format prismatic lithium ion battery using extended volume accelerating rate calorimetry. J. Power Sources 255, 294–301 (2014). https://doi.org/10.1016/j.jpowsour.2014.01.005
Mao, N., Wang, Z.R., Chung, Y.H., et al.: Overcharge cycling effect on the thermal behavior, structure, and material of lithium-ion batteries. Appl. Therm. Eng. 163, 114147 (2019). https://doi.org/10.1016/j.applthermaleng.2019.114147
Ali, Y., Iqbal, N., Lee, S.: Role of SEI layer growth in fracture probability in lithium-ion battery electrodes. Int. J. Energy Res. 45, 5293–5308 (2021). https://doi.org/10.1002/er.6150
Ouyang, D.X., Chen, M.Y., Liu, J.H., et al.: Investigation of a commercial lithium-ion battery under overcharge/over-discharge failure conditions. RSC Adv. 8, 33414–33424 (2018). https://doi.org/10.1039/c8ra05564e
Liu, X., Wu, Z.B., Stoliarov, S.I., et al.: Heat release during thermally-induced failure of a lithium ion battery: impact of cathode composition. Fire Saf. J. 85, 10–22 (2016). https://doi.org/10.1016/j.firesaf.2016.08.001
Li, Z., Huang, J., Yann Liaw, B., et al.: A review of lithium deposition in lithium-ion and lithium metal secondary batteries. J. Power Sources 254, 168–182 (2014). https://doi.org/10.1016/j.jpowsour.2013.12.099
Li, Y.L., Feng, X.N., Ren, D.S., et al.: Thermal runaway triggered by plated lithium on the anode after fast charging. ACS Appl. Mater. Interfaces 11, 46839–46850 (2019). https://doi.org/10.1021/acsami.9b16589
Belov, D., Yang, M.H.: Failure mechanism of Li-ion battery at overcharge conditions. J. Solid State Electrochem. 12, 885–894 (2008). https://doi.org/10.1007/s10008-007-0449-3
Events with smoke, fire, extreme heat or explosion involving lithium batteries. Federal Aviation Administration Publishing. (2021). https://www.faa.gov/hazmat/resources/lithium_batteries/media/Battery_incident_chart.pdf. Accessed 31 May 2021
Yang, G., Song, Y.D., Wang, Q., et al.: Review of ionic liquids containing, polymer/inorganic hybrid electrolytes for lithium metal batteries. Mater. Des. 190, 108563 (2020). https://doi.org/10.1016/j.matdes.2020.108563
Jiang, F.W., Liu, K., Wang, Z.R., et al.: Theoretical analysis of lithium-ion battery failure characteristics under different states of charge. Fire Mater. 42, 680–686 (2018). https://doi.org/10.1002/fam.2522
Liu, N., Lu, Z.D., Zhao, J., et al.: A pomegranate-inspired nanoscale design for large-volume-change lithium battery anodes. Nat. Nanotechnol. 9, 187–192 (2014). https://doi.org/10.1038/nnano.2014.6
Shen, X.W., Li, Y.T., Qian, T., et al.: Lithium anode stable in air for low-cost fabrication of a dendrite-free lithium battery. Nat. Commun. 10, 1–9 (2019). https://doi.org/10.1038/s41467-019-08767-0
Cheng, X.B., Zhao, C.Z., Yao, Y.X., et al.: Recent advances in energy chemistry between solid-state electrolyte and safe lithium-metal anodes. Chem 5, 74–96 (2019). https://doi.org/10.1016/j.chempr.2018.12.002
Aurbach, D.: A short review of failure mechanisms of lithium metal and lithiated graphite anodes in liquid electrolyte solutions. Solid State Ion. 148, 405–416 (2002). https://doi.org/10.1016/s0167-2738(02)00080-2
Wang, Q.S., Mao, B.B., Stoliarov, S.I., et al.: A review of lithium-ion battery failure mechanisms and fire prevention strategies. Prog. Energy Combust. Sci. 73, 95–131 (2019). https://doi.org/10.1016/j.pecs.2019.03.002
Liu, X., Ren, D.S., Hsu, H., et al.: Thermal runaway of lithium-ion batteries without internal short circuit. Joule 2, 2047–2064 (2018). https://doi.org/10.1016/j.joule.2018.06.015
Woo, J.J., Zhang, Z.C., Amine, K.: Separator/electrode assembly based on thermally stable polymer for safe lithium-ion batteries. Adv. Energy Mater. 4, 1301208 (2014). https://doi.org/10.1002/aenm.201301208
Liang, J., Chen, Q.Y., Liao, X.B., et al.: A nano-shield design for separators to resist dendrite formation in lithium-metal batteries. Angew. Chem. Int. Ed. 59, 6561–6566 (2020). https://doi.org/10.1002/anie.201915440
Chombo, P.V., Laoonual, Y.: A review of safety strategies of a Li-ion battery. J. Power Sources 478, 228649 (2020). https://doi.org/10.1016/j.jpowsour.2020.228649
Chen, W., Lei, T., Wu, C.Y., et al.: Designing safe electrolyte systems for a high-stability lithium-sulfur battery. Adv. Energy Mater. 8, 1702348 (2018). https://doi.org/10.1002/aenm.201702348
Wen, L.Y., Zhou, M., Wang, C.L., et al.: Energy storage: nanoengineering energy conversion and storage devices via atomic layer deposition (adv. energy mater. 23/2016). Adv. Energy Mater. 6, 201670132 (2016). https://doi.org/10.1002/aenm.201670132
Lei, Y.K., Ni, J., Hu, Z.J., et al.: Surface modification of Li-rich Mn-based layered oxide cathodes: challenges, materials, methods, and characterization. Adv. Energy Mater. 10, 2002506 (2020). https://doi.org/10.1002/aenm.202002506
The CNN Wire Staff.: Jet breaks apart during landing in Colombia. CNN Publishing. https://en.wikipedia.org/wiki/AIRES_Flight_8250. Accessed 17 Aug 2010
UPS cargo plane crashes in Dubai, killing two. BBC Publishing. https://www.bbc.co.uk/news/world-middle-east-11183476. Accessed 3 Sept 2010
In-flight uncontained engine failure Airbus A380-842, VH-OQA, overhead Batam Island, Indonesia, 4 November 2010. ATSB Publishing. https://www.atsb.gov.au/publications/investigation_reports/2010/AAIR/AO-2010-089.aspx. Accessed 27 June 2013
Fresh faults with Boeing dreamliner planes (2013). BBC Publishing. https://www.bbc.com/news/business-20950287. Accessed 9 January 2013
Cooper, A.: Fire aboard empty 787 Dreamliner prompts investigation. CNN Publishing. https://edition.cnn.com/2013/01/07/travel/dreamliner-fire/index.html. Accessed 27 June 2013
British Broadcasting Corporation: ‘Fixed Samsung Galaxy Note 7’ catches fire on plane. BBC Publishing. https://www.bbc.com/news/technology-37570100. Accessed 5 Oct 2016
Firefighters respond to another Apple Store due to iPhone battery explosion. 9to5Mac Publishing. https://9to5mac.com/2018/01/10/firefighters-iphone-explosion. Accessed 10 Jan 2018
The truth of the Huawei phone explosion has surfaced. Netizen: silently love Huawei for a minute. Zhihu Publishing. https://zhuanlan.zhihu.com/p/61994348. Accessed 10 Apr 2019
Scared! The laptop burst into flames while charging. Sohu Publishing. https://www.sohu.com/a/288000639_356100. Accessed 10 Jan 2019
Tesla Model S catches fire in Norway. Thedrive Publishing. https://www.thedrive.com/new-cars/1492/tesla-model-s-catches-fire-in-norway. Accessed 1 Jan 2016
Tesla says car fire started in battery. Pingwest Publishing. https://wheels.blogs.nytimes.com/2013/10/02/highway-fire-of-tesla-model-s-included-its-lithium-battery/. Accessed 2 Oct 2013
Cheng, X.B., Zhang, R., Zhao, C.Z., et al.: Toward safe lithium metal anode in rechargeable batteries: a review. Chem. Rev. 117, 10403–10473 (2017). https://doi.org/10.1021/acs.chemrev.7b00115
Jeppson, D.W., Ballif, J.L., Yuan, W.W., et al.: Lithium literature review: lithium’s properties and interactions. Plasma Phys. Control. Fusion 1, 6885395 (1978). https://doi.org/10.2172/6885395
Wood, K.N., Kazyak, E., Chadwick, A.F., et al.: Dendrites and pits: untangling the complex behavior of lithium metal anodes through operando video microscopy. ACS Cent. Sci. 2, 790–801 (2016). https://doi.org/10.1021/acscentsci.6b00260
Balakrishnan, P.G., Ramesh, R., Prem Kumar, T.: Safety mechanisms in lithium-ion batteries. J. Power Sources 155, 401–414 (2006). https://doi.org/10.1016/j.jpowsour.2005.12.002
Eddahech A.: Modélisation du vieillissement et détermination de l’état de santé de batteries lithium-ion pour application véhicule électrique et hybride. Université Sciences et Technologies-Bordeaux I. (2014)
Fergus, J.W.: Recent developments in cathode materials for lithium-ion batteries. J. Power Sources 195, 939–954 (2010). https://doi.org/10.1016/j.jpowsour.2009.08.089
Ouyang, D.X., Chen, M.Y., Huang, Q., et al.: A review on the thermal hazards of the lithium-ion battery and the corresponding countermeasures. Appl. Sci. 9, 2483 (2019). https://doi.org/10.3390/app9122483
Peng, C., Li, Y., Yao, F.N., et al.: Ultrahigh-energy-density fluorinated calcinated macadamia nut shell cathodes for lithium/fluorinated carbon batteries. Carbon 153, 783–791 (2019). https://doi.org/10.1016/j.carbon.2019.07.065
Krause, F.C., Jones, J.P., Jones, S.C., et al.: High specific energy lithium primary batteries as power sources for deep space exploration. J. Electrochem. Soc. 165, A2312–A2320 (2018). https://doi.org/10.1149/2.1061810jes
Sun, C., Feng, Y., Li, Y., et al.: Solvothermally exfoliated fluorographene for high-performance lithium primary batteries. Nanoscale 6, 2634–2641 (2014). https://doi.org/10.1039/c3nr04609e
Zhang, S.S., Foster, D., Wolfenstine, J., et al.: Electrochemical characteristic and discharge mechanism of a primary Li/CFx cell. J. Power Sources 187, 233–237 (2009). https://doi.org/10.1016/j.jpowsour.2008.10.076
Zhang, H., Eshetu, G.G., Judez, X., et al.: Electrolyte additives for lithium metal anodes and rechargeable lithium metal batteries: progress and perspectives. Angew. Chem. Int. Ed. 57, 15002–15027 (2018). https://doi.org/10.1002/anie.201712702
Guo, Y.P., Li, H.Q., Zhai, T.Y.: Reviving lithium-metal anodes for next-generation high-energy batteries. Adv. Mater. 29, 1700007 (2017). https://doi.org/10.1002/adma.201700007
Ghazi, Z.A., Sun, Z.H., Sun, C.G., et al.: Key aspects of lithium metal anodes for lithium metal batteries. Small 15, 1900687 (2019). https://doi.org/10.1002/smll.201900687
Liu, J., Yuan, H., Cheng, X.B., et al.: A review of naturally derived nanostructured materials for safe lithium metal batteries. Mater. Today Nano 8, 100049 (2019). https://doi.org/10.1016/j.mtnano.2019.100049
Rahman, M.A., Wang, X.J., Wen, C.E.: A review of high energy density lithium-air battery technology. J. Appl. Electrochem. 44, 5–22 (2014). https://doi.org/10.1007/s10800-013-0620-8
Zhou, R.X., Li, Y., Feng, Y.Y., et al.: The electrochemical performances of fluorinated hard carbon as the cathode of lithium primary batteries. Compos. Commun. 21, 100396 (2020). https://doi.org/10.1016/j.coco.2020.100396
Watanabe, N., Nakajima, T., Hagiwara, R.: Discharge reaction and overpotential of the graphite fluoride cathode in a nonaqueous lithium cell. J. Power Sources 20, 87–92 (1987). https://doi.org/10.1016/0378-7753(87)80095-2
Fulvio, P.F., Veith, G.M., Adcock, J.L., et al.: Fluorination of “brick and mortar” soft-templated graphitic ordered mesoporous carbons for high power lithium-ion battery. J. Mater. Chem. A 1, 9414 (2013). https://doi.org/10.1039/c3ta10710h
Li, Y.Y., Wu, X.Z., Liu, C., et al.: Fluorinated multi-walled carbon nanotubes as cathode materials of lithium and sodium primary batteries: effect of graphitization of carbon nanotubes. J. Mater. Chem. A 7, 7128–7137 (2019). https://doi.org/10.1039/c8ta12074a
Li, X.T., Zhang, H.C., Liu, C., et al.: A MOF-derived multifunctional nano-porous fluorinated carbon for high performance lithium/fluorinated carbon primary batteries. Microporous Mesoporous Mat. 310, 110650 (2021). https://doi.org/10.1016/j.micromeso.2020.110650
Wang, L., Li, Y.Y., Wang, S., et al.: Fluorinated nanographite as a cathode material for lithium primary batteries. ChemElectroChem 6, 2201–2207 (2019). https://doi.org/10.1002/celc.201900194
Peng, C., Kong, L.C., Li, Y., et al.: Fluorinated graphene nanoribbons from unzipped single-walled carbon nanotubes for ultrahigh energy density lithium-fluorinated carbon batteries. Sci. China-Mater. 64, 1367–1377 (2021). https://doi.org/10.1007/s40843-020-1551-x
Duan, J., Tang, X., Dai, H.F., et al.: Building safe lithium-ion batteries for electric vehicles: a review. Electrochem. Energy Rev. 3, 1–42 (2020). https://doi.org/10.1007/s41918-019-00060-4
Lu, Y., Zhang, Q., Chen, J.: Recent progress on lithium-ion batteries with high electrochemical performance. Sci. China-Chem. 62, 533–548 (2019). https://doi.org/10.1007/s11426-018-9410-0
Ianniciello, L., Biwolé, P.H., Achard, P.: Electric vehicles batteries thermal management systems employing phase change materials. J. Power Sources 378, 383–403 (2018). https://doi.org/10.1016/j.jpowsour.2017.12.071
Zhang, T.S., Gao, C., Gao, Q., et al.: Status and development of electric vehicle integrated thermal management from BTM to HVAC. Appl. Therm. Eng. 88, 398–409 (2015). https://doi.org/10.1016/j.applthermaleng.2015.02.001
Lisbona, D., Snee, T.: A review of hazards associated with primary lithium and lithium-ion batteries. Process Saf. Environ. Protect. 89, 434–442 (2011). https://doi.org/10.1016/j.psep.2011.06.022
Viswanathan, V.V., Choi, D., Wang, D.H., et al.: Effect of entropy change of lithium intercalation in cathodes and anodes on Li-ion battery thermal management. J. Power Sources 195, 3720–3729 (2010). https://doi.org/10.1016/j.jpowsour.2009.11.103
Hémery, C.V.: Etudes des phénomènes thermiques dans les batteries Li-ion. Université de Grenoble, Autre (2013)
Wang, Q.S., Ping, P., Zhao, X.J., et al.: Thermal runaway caused fire and explosion of lithium ion battery. J. Power Sources 208, 210–224 (2012). https://doi.org/10.1016/j.jpowsour.2012.02.038
Abraham, D.P., Roth, E.P., Kostecki, R., et al.: Diagnostic examination of thermally abused high-power lithium-ion cells. J. Power Sources 161, 648–657 (2006). https://doi.org/10.1016/j.jpowsour.2006.04.088
Selman, J.R., Al Hallaj, S., Uchida, I., et al.: Cooperative research on safety fundamentals of lithium batteries. J. Power Sources 97, 726–732 (2001). https://doi.org/10.1016/S0378-7753(01)00732-7
Sahraei, E., Meier, J., Wierzbicki, T.: Characterizing and modeling mechanical properties and onset of short circuit for three types of lithium-ion pouch cells. J. Power Sources 247, 503–516 (2014). https://doi.org/10.1016/j.jpowsour.2013.08.056
Zhang, C., Santhanagopalan, S., Sprague, M.A., et al.: Coupled mechanical-electrical-thermal modeling for short-circuit prediction in a lithium-ion cell under mechanical abuse. J. Power Sources 290, 102–113 (2015). https://doi.org/10.1016/j.jpowsour.2015.04.162
Saito, Y., Takano, K., Negishi, A.: Thermal behaviors of lithium-ion cells during overcharge. J. Power Sources 97, 693–696 (2001). https://doi.org/10.1016/S0378-7753(01)00703-0
Li, H.F., Gao, J.K., Zhang, S.L.: Effect of overdischarge on swelling and recharge performance of lithium ion cells. Chin. J. Chem. 26, 1585–1588 (2008). https://doi.org/10.1002/cjoc.200890286
Zheng, S.Q., Wang, L., Feng, X.N., et al.: Probing the heat sources during thermal runaway process by thermal analysis of different battery chemistries. J. Power Sources 378, 527–536 (2018). https://doi.org/10.1016/j.jpowsour.2017.12.050
Kitoh, K., Nemoto, H.: 100 Wh Large size Li-ion batteries and safety tests. J. Power Sources 81, 887–890 (1999). https://doi.org/10.1016/S0378-7753(99)00125-1
Smith, K., Kim, G.H., Darcy, E., et al.: Thermal/electrical modeling for abuse-tolerant design of lithium ion modules. Int. J. Energy Res. 34, 204–215 (2010). https://doi.org/10.1002/er.1666
Mohanty, D., Hockaday, E., Li, J., et al.: Effect of electrode manufacturing defects on electrochemical performance of lithium-ion batteries: cognizance of the battery failure sources. J. Power Sources 312, 70–79 (2016). https://doi.org/10.1016/j.jpowsour.2016.02.007
Bandhauer, T.M., Garimella, S., Fuller, T.F.: A critical review of thermal issues in lithium-ion batteries. J. Electrochem. Soc. 158, R1 (2011). https://doi.org/10.1149/1.3515880
An, Z.J., Jia, L., Ding, Y., et al.: A review on lithium-ion power battery thermal management technologies and thermal safety. Int. J. Therm. Sci. 26, 391–412 (2017). https://doi.org/10.1007/s11630-017-0955-2
Jaguemont, J., Boulon, L., Dube, Y., et al.: Low temperature discharge cycle tests for a lithium ion cell. In: 2014 IEEE Vehicle Power and Propulsion Conference (VPPC). Coimbra, Portugal. IEEE, 1–6 (2014). https://doi.org/10.1109/VPPC.2014.7007097
Barkholtz, H.M., Preger, Y., Ivanov, S., et al.: Multi-scale thermal stability study of commercial lithium-ion batteries as a function of cathode chemistry and state-of-charge. J. Power Sources 435, 226777 (2019). https://doi.org/10.1016/j.jpowsour.2019.226777
Bugryniec, P.J., Davidson, J.N., Cumming, D.J., et al.: Pursuing safer batteries: thermal abuse of LiFePO4 cells. J. Power Sources 414, 557–568 (2019). https://doi.org/10.1016/j.jpowsour.2019.01.013
Koch, S., Fill, A., Birke, K.P.: Comprehensive gas analysis on large scale automotive lithium-ion cells in thermal runaway. J. Power Sources 398, 106–112 (2018). https://doi.org/10.1016/j.jpowsour.2018.07.051
Yang, Y., Chen, L., Yang, L.J., et al.: Capacity fade characteristics of lithium iron phosphate cell during dynamic cycle. Energy 206, 118155 (2020). https://doi.org/10.1016/j.energy.2020.118155
Honkura, K., Horiba, T.: Study of the deterioration mechanism of LiCoO2/graphite cells in charge/discharge cycles using the discharge curve analysis. J. Power Sources 264, 140–146 (2014). https://doi.org/10.1016/j.jpowsour.2014.04.036
Lin, X.K., Park, J., Liu, L., et al.: A comprehensive capacity fade model and analysis for Li-ion batteries. J. Electrochem. Soc. 160, A1701–A1710 (2013). https://doi.org/10.1149/2.040310jes
Wu, S.H., Lee, P.H.: Storage fading of a commercial 18650 cell comprised with NMC/LMO cathode and graphite anode. J. Power Sources 349, 27–36 (2017). https://doi.org/10.1016/j.jpowsour.2017.03.002
Watanabe, S., Kinoshita, M., Nakura, K.: Capacity fade of LiNi(1–x−y)CoxAlyO2 cathode for lithium-ion batteries during accelerated calendar and cycle life test. I. Comparison analysis between LiNi(1–x−y)CoxAlyO2 and LiCoO2 cathodes in cylindrical lithium-ion cells during long term storage test. J. Power Sources 247, 412–422 (2014). https://doi.org/10.1016/j.jpowsour.2013.08.079
Hoque, M.M., Hannan, M.A., Mohamed, A.: Voltage equalization control algorithm for monitoring and balancing of series connected lithium-ion battery. J. Renew. Sustain. Energy 8, 025703 (2016). https://doi.org/10.1063/1.4944961
Fan, J., Tan, S.: Studies on charging lithium-ion cells at low temperatures. J. Electrochem. Soc. 153, A1081 (2006). https://doi.org/10.1149/1.2190029
Zhang, S.S., Xu, K., Jow, T.R.: Electrochemical impedance study on the low temperature of Li-ion batteries. Electrochim. Acta 49, 1057–1061 (2004). https://doi.org/10.1016/j.electacta.2003.10.016
Placke, T., Kloepsch, R., Dühnen, S., et al.: Lithium ion, lithium metal, and alternative rechargeable battery technologies: the odyssey for high energy density. J. Solid State Electrochem. 21, 1939–1964 (2017). https://doi.org/10.1007/s10008-017-3610-7
Besenhard, J.O., Winter, M., Yang, J., et al.: Filming mechanism of lithium-carbon anodes in organic and inorganic electrolytes. J. Power Sources 54, 228–231 (1995). https://doi.org/10.1016/0378-7753(94)02073-C
Peled, E.: The electrochemical behavior of alkali and alkaline earth metals in nonaqueous battery systems: the solid electrolyte interphase model. J. Electrochem. Soc. 126, 2047–2051 (1979). https://doi.org/10.1149/1.2128859
Zhang, Y., Liu, B.Y., Hitz, E., et al.: A carbon-based 3D current collector with surface protection for Li metal anode. Nano Res. 10, 1356–1365 (2017). https://doi.org/10.1007/s12274-017-1461-2
Yang, C.P., Zhang, L., Liu, B.Y., et al.: Continuous plating/stripping behavior of solid-state lithium metal anode in a 3D ion-conductive framework. Proc. Natl. Acad. Sci. USA 115, 3770–3775 (2018). https://doi.org/10.1073/Proc.Natl.Acad.Sci.U.S.A.1719758115
Fan, L., Li, S., Liu, L., et al.: Enabling stable lithium metal anode via 3D inorganic skeleton with superlithiophilic interphase. Adv. Enengy Mater. (2018). https://doi.org/10.1002/aenm.201802350
Ramirez, J.R., Yang, H.Y., Kane, C.M., et al.: Reproducible synthesis and high porosity of mer-Zn(Im)2 (ZIF-10): exploitation of an apparent double-eight ring template. J. Am. Chem. Soc. 138, 12017–12020 (2016). https://doi.org/10.1021/jacs.6b06375
Xiang, L., Sheng, L.Q., Wang, C.Q., et al.: Amino-functionalized ZIF-7 nanocrystals: improved intrinsic separation ability and interfacial compatibility in mixed-matrix membranes for CO2 /CH4 separation. Adv. Mater. 29, 1606999 (2017). https://doi.org/10.1002/adma.201606999
Li, Y., Fan, J., Zhang, J., et al.: A honeycomb-like Co@N-C composite for ultrahigh sulfur loading Li-S batteries. ACS Nano 11, 11417–11424 (2017). https://doi.org/10.1021/acsnano.7b06061
Zhu, M.Q., Li, B., Li, S.M., et al.: Dendrite-free metallic lithium in lithiophilic carbonized metal-organic frameworks. Adv. Energy Mater. 8, 1703505 (2018). https://doi.org/10.1002/aenm.201703505
Li, K., Hu, Z.Y., Ma, J.Z., et al.: A 3D and stable lithium anode for high-performance lithium-iodine batteries. Adv. Mater. 31, 1902399 (2019). https://doi.org/10.1002/adma.201902399
Li, S.Y., Liu, Q.L., Zhou, J.J., et al.: Hierarchical Co3O4 nanofiber-carbon sheet skeleton with superior Na/Li-philic property enabling highly stable alkali metal batteries. Adv. Funct. Mater. 29, 1808847 (2019). https://doi.org/10.1002/adfm.201808847
Hu, Y.H., Sun, X.L.: Flexible rechargeable lithium ion batteries: Advances and challenges in materials and process technologies. J. Mater. Chem. A 2, 10712–10738 (2014). https://doi.org/10.1039/c4ta00716f
Peng, H.J., Huang, J.Q., Zhang, Q.: A review of flexible lithium-sulfur and analogous alkali metal-chalcogen rechargeable batteries. Chem. Soc. Rev. 46, 5237–5288 (2017). https://doi.org/10.1039/c7cs00139h
Wang, A., Tang, S., Kong, D., et al.: Bending-tolerant anodes for lithium-metal batteries. Adv. Mater. (2018). https://doi.org/10.1002/adma.201703891
Huang, C., Xiao, J., Shao, Y.Y., et al.: Manipulating surface reactions in lithium-sulphur batteries using hybrid anode structures. Nat. Commun. 5, 1–8 (2014). https://doi.org/10.1038/ncomms4015
Lu, L.L., Ge, J., Yang, J.N., et al.: Free-standing copper nanowire network current collector for improving lithium anode performance. Nano Lett. 16, 4431–4437 (2016). https://doi.org/10.1021/acs.nanolett.6b01581
Yun, Q.B., He, Y.B., Lv, W., et al.: Chemical dealloying derived 3D porous current collector for Li metal anodes. Adv. Mater. 28, 6932–6939 (2016). https://doi.org/10.1002/adma.201601409
Yang, C.P., Yin, Y.X., Zhang, S.F., et al.: Accommodating lithium into 3D current collectors with a submicron skeleton towards long-life lithium metal anodes. Nat. Commun. 6, 1–9 (2015). https://doi.org/10.1038/ncomms9058
Jin, S., Xin, S., Wang, L.J., et al.: Carbon nanostructures: covalently connected carbon nanostructures for current collectors in both the cathode and anode of Li-S batteries. Adv. Mater. 28, 9016 (2016). https://doi.org/10.1002/adma.201670287
Zhang, Y., Luo, W., Wang, C.W., et al.: High-capacity, low-tortuosity, and channel-guided lithium metal anode. Proc. Natl. Acad. Sci. USA 114, 3584–3589 (2017). https://doi.org/10.1073/pnas.1618871114
Deng, W., Liang, S.S., Zhou, X.F., et al.: Depressing the irreversible reactions on a three-dimensional interface towards a high-areal capacity lithium metal anode. J. Mater. Chem. A 7, 6267–6274 (2019). https://doi.org/10.1039/c9ta00143c
Lu, D.P., Shao, Y.Y., Lozano, T., et al.: Failure mechanism for fast-charged lithium metal batteries with liquid electrolytes. Adv. Energy Mater. 5, 1400993 (2015). https://doi.org/10.1002/aenm.201400993
Li, Y.Z., Li, Y.B., Sun, Y.M., et al.: Revealing nanoscale passivation and corrosion mechanisms of reactive battery materials in gas environments. Nano Lett. 17, 5171–5178 (2017). https://doi.org/10.1021/acs.nanolett.7b02630
Zhu, M.Q., Li, S.M., Li, B., et al.: Homogeneous guiding deposition of sodium through main group II metals toward dendrite-free sodium anodes. Sci. Adv. 5, eaau6264 (2019). https://doi.org/10.1126/sciadv.aau6264
Zhai, P.B., Wang, T.S., Yang, W.W., et al.: Uniform lithium deposition assisted by single-atom doping toward high-performance lithium metal anodes. Adv. Energy Mater. 9, 1804019 (2019). https://doi.org/10.1002/aenm.201804019
Shang, C.Q., Dong, S.M., Wang, S., et al.: Coaxial Ni(x)Co(2x)(OH)(6x)/TiN nanotube arrays as supercapacitor electrodes. ACS Nano 7, 5430–5436 (2013). https://doi.org/10.1021/nn401402a
Ren, F.H., Lu, Z.Y., Zhang, H., et al.: Pseudocapacitance induced uniform plating/stripping of Li metal anode in vertical graphene nanowalls. Adv. Funct. Mater. 28, 1805638 (2018). https://doi.org/10.1002/adfm.201805638
Lin, K., Qin, X.Y., Liu, M., et al.: Ultrafine titanium nitride sheath decorated carbon nanofiber network enabling stable lithium metal anodes. Adv. Funct. Mater. 29, 1903229 (2019). https://doi.org/10.1002/adfm.201903229
Chen, X.R., Zhao, B.C., Yan, C., et al.: Review on Li deposition in working batteries: from nucleation to early growth. Adv. Mater. 33, 2004128 (2021). https://doi.org/10.1002/adma.202004128
Luo, L., Li, J.Y., Yaghoobnejad Asl, H., et al.: A 3D lithiophilic Mo2 N-modified carbon nanofiber architecture for dendrite-free lithium-metal anodes in a full cell. Adv. Mater. 31, 1904537 (2019). https://doi.org/10.1002/adma.201904537
Le, T., Liang, Q.H., Chen, M., et al.: A triple-gradient host for long cycling lithium metal anodes at ultrahigh current density. Small 16, 2001992 (2020). https://doi.org/10.1002/smll.202001992
Naguib, M., Kurtoglu, M., Presser, V., et al.: Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 23, 4248–4253 (2011). https://doi.org/10.1002/adma.201102306
Naguib, M., Come, J., Dyatkin, B., et al.: MXene: a promising transition metal carbide anode for lithium-ion batteries. Electrochem. Commun. 16, 61–64 (2012). https://doi.org/10.1016/j.elecom.2012.01.002
Naguib, M., Mashtalir, O., Carle, J., et al.: Two-dimensional transition metal carbides. ACS Nano 6, 1322–1331 (2012). https://doi.org/10.1021/nn204153h
Zhang, D., Wang, S., Li, B., et al.: Horizontal growth of lithium on parallelly aligned MXene layers towards dendrite-free metallic lithium anodes. Adv. Mater. 31, 1901820 (2019). https://doi.org/10.1002/adma.201901820
Chen, X., Shang, M.W., Niu, J.J.: Inter-layer-calated thin Li metal electrode with improved battery capacity retention and dendrite suppression. Nano Lett. 20, 2639–2646 (2020). https://doi.org/10.1021/acs.nanolett.0c00201
Gao, X.J., Yang, X.F., Adair, K., et al.: 3D vertically aligned Li metal anodes with ultrahigh cycling currents and capacities of 10 mA cm–2/20 mAh cm–2 realized by selective nucleation within microchannel walls. Adv. Energy Mater. 10, 1903753 (2020). https://doi.org/10.1002/aenm.201903753
An, Y.L., Fei, H.F., Zeng, G.F., et al.: Vacuum distillation derived 3D porous current collector for stable lithium-metal batteries. Nano Energy 47, 503–511 (2018). https://doi.org/10.1016/j.nanoen.2018.03.036
Liu, L., Yin, Y.X., Li, J.Y., et al.: Ladderlike carbon nanoarrays on 3D conducting skeletons enable uniform lithium nucleation for stable lithium metal anodes. Chem. Commun. 54, 5330–5333 (2018). https://doi.org/10.1039/c8cc02672f
Wang, S.H., Yin, Y.X., Zuo, T.T., et al.: Stable Li metal anodes via regulating lithium plating/stripping in vertically aligned microchannels. Adv. Mater. 29, 1703729 (2017). https://doi.org/10.1002/adma.201703729
Jiang, Z.P., Jin, L., Han, Z.L., et al.: Facile generation of polymer–alloy hybrid layers for dendrite-free lithium-metal anodes with improved moisture stability. Angew. Chem. Int. Ed. 58, 11374–11378 (2019). https://doi.org/10.1002/anie.201905712
Hong, X.D., Mei, J., Wen, L., et al.: Nonlithium metal-sulfur batteries: Steps toward a leap. Adv. Mater. 31, 1802822 (2019). https://doi.org/10.1002/adma.201802822
Yuan, Y., Wu, F., Bai, Y., et al.: Regulating Li deposition by constructing LiF-rich host for dendrite-free lithium metal anode. Energy Storage Mater. 16, 411–418 (2019). https://doi.org/10.1016/j.ensm.2018.06.022
Yan, C., Cheng, X.B., Yao, Y.X., et al.: An armored mixed conductor interphase on a dendrite-free lithium-metal anode. Adv. Mater. 30, 1804461 (2018). https://doi.org/10.1002/adma.201804461
Yan, C., Cheng, X.B., Tian, Y., et al.: Dual-layered film protected lithium metal anode to enable dendrite-free lithium deposition. Adv. Mater. 30, e1707629 (2018). https://doi.org/10.1002/adma.201707629
Lu, Y., Tu, Z., Shu, J., et al.: Stable lithium electrodeposition in salt-reinforced electrolytes. J. Power Sources 279, 413–418 (2015). https://doi.org/10.1016/j.jpowsour.2015.01.030
Zhang, S.S.: A new finding on the role of LiNO3 in lithium-sulfur battery. J. Power Sources 322, 99–105 (2016). https://doi.org/10.1016/j.jpowsour.2016.05.009
Wu, F., Qian, J., Chen, R., et al.: An effective approach to protect lithium anode and improve cycle performance for Li-S batteries. ACS Appl. Mater. Interfaces 6, 15542–15549 (2014). https://doi.org/10.1021/am504345s
Matsuda, Y., Sekiya, M.: Effect of organic additives in electrolyte solutions on lithium electrode behavior. J. Power Sources 81(82), 759–761 (1999). https://doi.org/10.1016/S0378-7753(99)00239-6
Choi, J.W., Cheruvally, G., Kim, D.S., et al.: Rechargeable lithium/sulfur battery with liquid electrolytes containing toluene as additive. J. Power Sources 183, 441–445 (2008). https://doi.org/10.1016/j.jpowsour.2008.05.038
Yang, W., Yang, W., Song, A., et al.: Pyrrole as a promising electrolyte additive to trap polysulfides for lithium-sulfur batteries. J. Power Sources 348, 175–182 (2017). https://doi.org/10.1016/j.jpowsour.2017.03.008
Baginska, M., Blaiszik, B.J., Merriman, R.J., et al.: Autonomic shutdown of lithium-ion batteries using thermoresponsive microspheres. Adv. Energy Mater. 2, 583–590 (2012). https://doi.org/10.1002/aenm.201100683
Yuge, R., Tamura, N., Manako, T., et al.: High-rate charge/discharge properties of Li-ion battery using carbon-coated composites of graphites, vapor grown carbon fibers, and carbon nanohorns. J. Power Sources 266, 471–474 (2014). https://doi.org/10.1016/j.jpowsour.2014.05.068
Kim, K.J., Lee, T.S., Kim, H.G., et al.: A hard carbon/microcrystalline graphite/carbon composite with a core-shell structure as novel anode materials for lithium-ion batteries. Electrochim. Acta 135, 27–34 (2014). https://doi.org/10.1016/j.electacta.2014.04.171
Jung, Y.S., Cavanagh, A.S., Riley, L.A., et al.: Ultrathin direct atomic layer deposition on composite electrodes for highly durable and safe Li-ion batteries. Adv. Mater. 22, 2172–2176 (2010). https://doi.org/10.1002/adma.200903951
Fang, D., Li, L.C., Xu, W.L., et al.: High capacity lithium ion battery anodes using Sn nanowires encapsulated Al2O3 tubes in carbon matrix. Adv. Mater. Interfaces 3, 1500491 (2016). https://doi.org/10.1002/admi.201500491
Feng, T., Xu, Y., Zhang, Z., et al.: Low-cost Al2O3 coating layer as a preformed SEI on natural graphite powder to improve Coulombic efficiency and high-rate cycling stability of lithium-ion batteries. ACS Appl. Mater. Interfaces 8, 6512–6519 (2016). https://doi.org/10.1021/acsami.6b00231
Xu, T., Zhou, C., Zhou, H., et al.: Synthesis of alumina-coated natural graphite for highly cycling stability and safety of Li-ion batteries. Chin. J. Chem. 37, 342–346 (2019). https://doi.org/10.1002/cjoc.201800559
Bublil, S., Sharabani, T., Turgeman, M., et al.: Improving amorphous carbon anodes for Na ion batteries by surface treatment of a presodiated electrode with Al2O3. Langmuir 35, 11670–11678 (2019). https://doi.org/10.1021/acs.langmuir.9b02141
Hu, T., Xie, M., Zhong, J., et al.: Porous Fe2O3 nanorods anchored on nitrogen-doped graphenes and ultrathin Al2O3 coating by atomic layer deposition for long-lived lithium ion battery anode. Carbon 76, 141–147 (2014). https://doi.org/10.1016/j.carbon.2014.04.060
Cheng, Y.W., Pandey, R.K., Li, Y.C., et al.: Conducting nitrogen-incorporated ultrananocrystalline diamond coating for highly structural stable anode materials in lithium ion battery. Nano Energy 74, 104811 (2020). https://doi.org/10.1016/j.nanoen.2020.104811
Ding, F., Xu, W., Choi, D., et al.: Enhanced performance of graphite anode materials by AlF3 coating for lithium-ion batteries. J. Mater. Chem. (2012). https://doi.org/10.1039/c2jm31015e
Wu, Q.H., Qu, B., Tang, J., et al.: An alumina-coated Fe3O4-reduced graphene oxide composite electrode as a stable anode for lithium-ion battery. Electrochim. Acta 156, 147–153 (2015). https://doi.org/10.1016/j.electacta.2014.12.149
Moradi, B., Wang, D., Botte, G.G.: Carbon-coated Fe3O4 nanospindles as solid electrolyte interface for improving graphite anodes in lithium ion batteries. J. Appl. Electrochem. 50, 321–331 (2020). https://doi.org/10.1007/s10800-019-01393-0
Lotfabad, E.M., Kalisvaart, P., Cui, K., et al.: ALD TiO2 coated silicon nanowires for lithium ion battery anodes with enhanced cycling stability and Coulombic efficiency. Phys. Chem. Chem. Phys. 15, 13646–13657 (2013). https://doi.org/10.1039/c3cp52485j
Li, Y., Sun, Y.J., Xu, G.J., et al.: Tuning electrochemical performance of Si-based anodes for lithium-ion batteries by employing atomic layer deposition alumina coating. J. Mater. Chem. A 2, 11417–11425 (2014). https://doi.org/10.1039/C4TA01562B
Zhu, B., Liu, N., McDowell, M., et al.: Interfacial stabilizing effect of ZnO on Si anodes for lithium ion battery. Nano Energy 13, 620–625 (2015). https://doi.org/10.1016/j.nanoen.2015.03.019
Amine, K., Belharouak, I., Chen, Z.H., et al.: Nanostructured anode material for high-power battery system in electric vehicles. Adv. Mater. 22, 3052–3057 (2010). https://doi.org/10.1002/adma.201000441
Wang, B., Hu, S.S., Gu, L., et al.: A porous mooncake-shaped Li4Ti5O12 anode material modified by SmF3 and its electrochemical performance in lithium ion batteries. Chem. Eur. J. 26, 17097–17102 (2020). https://doi.org/10.1002/chem.202002095
Jo, C., Kim, Y., Hwang, J., et al.: Block copolymer directed ordered mesostructured TiNb2O7 multimetallic oxide constructed of nanocrystals as high power Li-ion battery anodes. Chem. Mater. 26, 3508–3514 (2014). https://doi.org/10.1021/cm501011d
Tian, S., Xing, A., Tang, H., et al.: Enhanced cycling stability of TiO2-coated V2O5 nanorods through a surface sol–gel process for lithium ion battery applications. J. Mater. Chem. A 2, 2896 (2014). https://doi.org/10.1039/c3ta14364c
Wang, X.L., Zhang, J.M., Kong, X., et al.: Increasing rigidness of carbon coating for improvement of electrochemical performances of Co3O4 in Li-ion batteries. Carbon 104, 1–9 (2016). https://doi.org/10.1016/j.carbon.2016.03.027
Yesibolati, N., Shahid, M., Chen, W., et al.: SnO2 anode surface passivation by atomic layer deposited HfO2 improves Li-ion battery performance. Small 10, 2849–2858 (2014). https://doi.org/10.1002/smll.201303898
Jung, M.H.: Carbon-coated ZnO mat passivation by atomic-layer-deposited HfO2 as an anode material for lithium-ion batteries. J. Colloid Interface Sci. 505, 631–641 (2017). https://doi.org/10.1016/j.jcis.2017.06.069
Cheng, Q.H., He, W., Zhang, X.D., et al.: Modification of Li2MnSiO4 cathode materials for lithium-ion batteries: a review. J. Mater. Chem. A 5, 10772–10797 (2017). https://doi.org/10.1039/c7ta00034k
Nayak, P.K., Erickson, E.M., Schipper, F., et al.: Review on challenges and recent advances in the electrochemical performance of high capacity Li- and Mn-rich cathode materials for Li-ion batteries. Adv. Energy Mater. 8, 1702397 (2018). https://doi.org/10.1002/aenm.201702397
Liu, J., Banis, M.N., Sun, Q., et al.: Rational design of atomic-layer-deposited LiFePO4 as a high-performance cathode for lithium-ion batteries. Adv. Mater. 26, 6472–6477 (2014). https://doi.org/10.1002/adma.201401805
Dai, X.Y., Wang, L.P., Xu, J., et al.: Improved electrochemical performance of LiCoO2 electrodes with ZnO coating by radio frequency magnetron sputtering. ACS Appl. Mater. Interfaces 6, 15853–15859 (2014). https://doi.org/10.1021/am503260s
Han, B.H., Paulauskas, T., Key, B., et al.: Understanding the role of temperature and cathode composition on interface and bulk: optimizing aluminum oxide coatings for Li-ion cathodes. ACS Appl. Mater. Interfaces 9, 14769–14778 (2017). https://doi.org/10.1021/acsami.7b00595
Zhou, A.J., Lu, Y.T., Wang, Q.J., et al.: Sputtering TiO2 on LiCoO2 composite electrodes as a simple and effective coating to enhance high-voltage cathode performance. J. Power Sources 346, 24–30 (2017). https://doi.org/10.1016/j.jpowsour.2017.02.035
Teranishi, T., Inohara, M., Kano, J., et al.: Synthesis of nano-crystalline LiNbO3-decorated LiCoO2 and resulting high-rate capabilities. Solid State Ion. 314, 57–60 (2018). https://doi.org/10.1016/j.ssi.2017.11.020
Zhou, A.J., Xu, J., Dai, X.Y., et al.: Improved high-voltage and high-temperature electrochemical performances of LiCoO2 cathode by electrode sputter-coating with Li3PO4. J. Power Sources 322, 10–16 (2016). https://doi.org/10.1016/j.jpowsour.2016.04.092
Liu, H.M., Saikia, D., Wu, H.C., et al.: Towards an understanding of the role of hyper-branched oligomers coated on cathodes, in the safety mechanism of lithium-ion batteries. RSC Adv. 4, 56147–56155 (2014). https://doi.org/10.1039/c4ra09220a
Xie, M., Li, B., Zhou, Y.: Free-standing high-voltage LiCoO2/multi-wall carbon nanotube paper electrodes with extremely high areal mass loading for lithium ion batteries. J. Mater. Chem. A 3, 23180–23184 (2015). https://doi.org/10.1039/c5ta06823a
Tebbe, J.L., Holder, A.M., Musgrave, C.B.: Mechanisms of LiCoO2 cathode degradation by reaction with HF and protection by thin oxide coatings. ACS Appl. Mater. Interfaces 7, 24265–24278 (2015). https://doi.org/10.1021/acsami.5b07887
Xiao, B., Liu, H., Chen, N., et al.: Size-mediated recurring spinel sub-nanodomains in Li- and Mn-rich layered cathode materials. Angew. Chem. Int. Ed. 59, 14313–14320 (2020). https://doi.org/10.1002/anie.202005337
Sarkar, S., Patel, R.L., Liang, X., et al.: Unveiling the role of CeO2 atomic layer deposition coatings on LiMn2O4 cathode materials: an experimental and theoretical study. ACS Appl. Mater. Interfaces 9, 30599–30607 (2017). https://doi.org/10.1021/acsami.7b06988
Xiao, B., Liu, J., Sun, Q., et al.: Unravelling the role of electrochemically active FePO4 coating by atomic layer deposition for increased high-voltage stability of LiNi0.5Mn1.5O4 cathode material. Adv. Sci. 2, 1500022 (2015). https://doi.org/10.1002/advs.201500022
Shapira, A., Tiurin, O., Solomatin, N., et al.: Robust AlF3 atomic layer deposition protective coating on LiMn1.5Ni0.5O4 particles: an advanced Li-ion battery cathode material powder. ACS Appl. Energy Mater. 1, 6809–6823 (2018). https://doi.org/10.1021/acsaem.8b01048
Cheng, F., Xin, Y., Huang, Y., et al.: Enhanced electrochemical performances of 5 V spinel LiMn1.58Ni0.42O4 cathode materials by coating with LiAlO2. J. Power Sources 239, 181–188 (2013). https://doi.org/10.1016/j.jpowsour.2013.03.143
Kim, C.A., Choi, H.J., Lee, J.H., et al.: Influence of surface modification on electrochemical performance of high voltage spinel ordered-LiNi0.5Mn1.5O4 exposed to 53 V for 100 h before and after surface modification with ALD method. Electrochim. Acta 184, 134–142 (2015). https://doi.org/10.1016/j.electacta.2015.10.041
Zou, Y., Yang, X., Lv, C., et al.: Multishelled Ni-rich Li(NixCoyMnz)O2 hollow fibers with low cation mixing as high-performance cathode materials for Li-ion batteries. Adv. Sci. 4, 1600262 (2017). https://doi.org/10.1002/advs.201600262
Li, X., Liu, J., Banis, M.N., et al.: Atomic layer deposition of solid-state electrolyte coated cathode materials with superior high-voltage cycling behavior for lithium ion battery application. Energy Environ. Sci. 7, 768–778 (2014). https://doi.org/10.1039/c3ee42704h
Sun, B., El Kazzi, M., Müller, E., et al.: Toward high-performance Li(NixCoyMnz)O2 cathodes: facile fabrication of an artificial polymeric interphase using functional polyacrylates. J. Mater. Chem. A 6, 17778–17786 (2018). https://doi.org/10.1039/c8ta03954b
Srur-Lavi, O., Miikkulainen, V., Markovsky, B., et al.: Studies of the electrochemical behavior of LiNi0.80Co0.15Al0.05O2 electrodes coated with LiAlO2. J. Electrochem. Soc. 164, A3266–A3275 (2017). https://doi.org/10.1149/2.1631713jes
Hu, E., Yu, X., Lin, R., et al.: Evolution of redox couples in Li- and Mn-rich cathode materials and mitigation of voltage fade by reducing oxygen release. Nat. Energy 3, 690–698 (2018). https://doi.org/10.1038/s41560-018-0207-z
Hu, E., Bak, S.M., Liu, Y., et al.: Utilizing environmental friendly iron as a substitution element in spinel structured cathode materials for safer high energy lithium-ion batteries. Adv. Energy Mater. 6, 1501662 (2016). https://doi.org/10.1002/aenm.201501662
He, W., Liu, P., Qu, B., et al.: Uniform Na+ doping-induced defects in Li– and Mn-Rich cathodes for high-performance lithium-ion batteries. Adv. Sci. 6, 1802114 (2019). https://doi.org/10.1002/advs.201802114
Zhou, F., Zhao, X., Dahn, J.R.: Impact of Al or Mg substitution on the thermal stability of Li1.05Mn1.95−zMzO4 (M = Al or Mg). J. Electrochem. Soc. 157, A798–A801 (2010). https://doi.org/10.1149/1.3425606
Ji, W., Wang, F., Liu, D., et al.: Building thermally stable Li-ion batteries using a temperature-responsive cathode. J. Mater. Chem. A 4, 11239–11246 (2016). https://doi.org/10.1039/c6ta03407a
Kise, M., Yoshioka, S., Kuriki, H.: Relation between composition of the positive electrode and cell performance and safety of lithium-ion PTC batteries. J. Power Sources 174, 861–866 (2007). https://doi.org/10.1016/j.jpowsour.2007.06.224
Zhong, H., Kong, C., Zhan, H., et al.: Safe positive temperature coefficient composite cathode for lithium ion battery. J. Power Sources 216, 273–280 (2012). https://doi.org/10.1016/j.jpowsour.2012.05.015
Klein, M.J., Dolocan, A., Zu, C., et al.: An effective lithium sulfide encapsulation strategy for stable lithium-sulfur batteries. Adv. Energy Mater. 7, 1701122 (2017). https://doi.org/10.1002/aenm.201701122
Zu, C., Klein, M., Manthiram, A.: Activated Li2S as a high-performance cathode for rechargeable lithium-sulfur batteries. J. Phys. Chem. Lett. 5, 3986–3991 (2014). https://doi.org/10.1021/jz5021108
Ren, Y.X., Zhao, T.S., Liu, M., et al.: A self-cleaning Li-S battery enabled by a bifunctional redox mediator. J. Power Sources 361, 203–210 (2017). https://doi.org/10.1016/j.jpowsour.2017.06.083
Kim, K.R., Lee, K.S., Ahn, C.Y., et al.: Discharging a Li-S battery with ultra-high sulphur content cathode using a redox mediator. Sci Rep 6, 32433 (2016). https://doi.org/10.1038/srep32433
Liu, M., Ren, Y.X., Jiang, H.R., et al.: An efficient Li2S-based lithium-ion sulfur battery realized by a bifunctional electrolyte additive. Nano Energy 40, 240–247 (2017). https://doi.org/10.1016/j.nanoen.2017.08.017
Yu, M., Yuan, W., Li, C., et al.: Performance enhancement of a graphene-sulfur composite as a lithium-sulfur battery electrode by coating with an ultrathin Al2O3 film via atomic layer deposition. J. Mater. Chem. A 2, 7360–7366 (2014). https://doi.org/10.1039/c4ta00234b
Johnson, L., Li, C., Liu, Z., et al.: The role of LiO2 solubility in O2 reduction in aprotic solvents and its consequences for Li-O2 batteries. Nat. Chem. 6, 1091–1099 (2014). https://doi.org/10.1038/nchem.2101
Gao, X., Chen, Y., Johnson, L., et al.: Erratum: promoting solution phase discharge in Li-O2 batteries containing weakly solvating electrolyte solutions. Nat. Mater. 15, 918–918 (2016). https://doi.org/10.1038/nmat4691
Feng, W., Long, P., Feng, Y., et al.: Two-dimensional fluorinated graphene: synthesis, structures, properties and applications. Adv. Sci. 3, 1500413 (2016). https://doi.org/10.1002/advs.201500413
Sato, Y., Kume, T., Hagiwara, R., et al.: Reversible intercalation of HF in fluorine-GICs. Carbon 41, 351–357 (2003). https://doi.org/10.1016/S0008-6223(02)00311-1
Dubecky, M., Otyepkova, E., Lazar, P., et al.: Reactivity of fluorographene: a facile way toward graphene derivatives. J. Phys. Chem. Lett. 6, 1430–1434 (2015). https://doi.org/10.1021/acs.jpclett.5b00565
Matsuo, Y., Nakajima, T.: Carbon-fluorine bondings of fluorinated fullerene and graphite. Z. Anorg. Allg. Chem. 621, 1943–1950 (1995). https://doi.org/10.1002/zaac.19956211119
Zhang, W., Dubois, M., Guerin, K., et al.: Effect of curvature on C-F bonding in fluorinated carbons: from fullerene and derivatives to graphite. Phys. Chem. Chem. Phys. 12, 13881398 (2010). https://doi.org/10.1039/b914853a
Lam, P., Yazami, R.: Physical characteristics and rate performance of (CFx)n (0.33<x<0.66). https://doi.org/10.1016/j.jpowsour.2005.05.022
Panich, A.M., Nakajima, T.: Physical properties and C-F bonding in fluorine-graphite intercalation compounds as seen by NMR. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A Mol. Cryst. Liq. Cryst. 340, 77–82 (2006). https://doi.org/10.1080/10587250008025446
Li, Y., Chen, Y., Feng, W., et al.: The improved discharge performance of Li/CFx batteries by using multi-walled carbon nanotubes as conductive additive. J. Power Sources 196, 2246–2250 (2011). https://doi.org/10.1016/j.jpowsour.2010.10.005
Yang, W., Dai, Y., Cai, S., et al.: Graphene/Au composite paper as flexible current collector to improve electrochemical performances of CFx cathode. J. Power Sources 255, 37–42 (2014). https://doi.org/10.1016/j.jpowsour.2013.12.122
Li, Y., Feng, W.: The tunable electrochemical performances of carbon fluorides/manganese dioxide hybrid cathodes by their arrangements. J. Power Sources 274, 1292–1299 (2015). https://doi.org/10.1016/j.jpowsour.2014.10.150
Wang, J., Sun, M., Liu, Y., et al.: Unraveling nanoscale electrochemical dynamics of graphite fluoride by in situ electron microscopy: key difference between lithiation and sodiation. J. Mater. Chem. A 8, 6105–6111 (2020). https://doi.org/10.1039/d0ta00093k
Han, J.G., Kim, K., Lee, Y., et al.: Scavenging materials to stabilize LiPF6-containing carbonate-based electrolytes for Li-ion batteries. Adv. Mater. 31, e1804822 (2019). https://doi.org/10.1002/adma.201804822
Yang, Y., Yu, D., Wang, H., et al.: Smart electrochemical energy storage devices with self-protection and self-adaptation abilities. Adv. Mater. 29, 1703040 (2017). https://doi.org/10.1002/adma.201703040
Liu, K., Liu, Y., Lin, D., et al.: Materials for lithium-ion battery safety. Sci. Adv. 4, eaas9820 (2018). https://doi.org/10.1126/sciadv.aas9820
Lin, X., Zhou, G., Liu, J., et al.: Rechargeable battery electrolytes capable of operating over wide temperature windows and delivering high safety. Adv. Energy Mater. 10, 2001235 (2020). https://doi.org/10.1002/aenm.202001235
Zheng, J., Engelhard, M.H., Mei, D., et al.: Electrolyte additive enabled fast charging and stable cycling lithium metal batteries. Nat. Energy 2, 17012 (2017). https://doi.org/10.1038/nenergy.2017.12
Sun, H., Zhu, G., Zhu, Y., et al.: High-safety and high-energy-density lithium metal batteries in a novel ionic-liquid electrolyte. Adv. Mater. 32, e2001741 (2020). https://doi.org/10.1002/adma.202001741
Ma, Y., Zhou, Z., Li, C., et al.: Enabling reliable lithium metal batteries by a bifunctional anionic electrolyte additive. Energy Storage Mater. 11, 197–204 (2018). https://doi.org/10.1016/j.ensm.2017.10.015
Wang, G., Xiong, X., Xie, D., et al.: Suppressing dendrite growth by a functional electrolyte additive for robust Li metal anodes. Energy Storage Mater. 23, 701–706 (2019). https://doi.org/10.1016/j.ensm.2019.02.026
Han, J.G., Hwang, E., Kim, Y., et al.: Dual-functional electrolyte additives toward long-cycling lithium-ion batteries: ecofriendly designed carbonate derivatives. ACS Appl. Mater. Interfaces 12, 24479–24487 (2020). https://doi.org/10.1021/acsami.0c04372
Han, J.G., Hwang, C., Kim, S.H., et al.: An antiaging electrolyte additive for high-energy-density lithium-ion batteries. Adv. Energy Mater. (2020). https://doi.org/10.1002/aenm.202000563
Pham, H.Q., Mirolo, M., Tarik, M., et al.: Multifunctional electrolyte additive for improved interfacial stability in Ni-rich layered oxide full-cells. Energy Storage Mater. 33, 216–229 (2020). https://doi.org/10.1016/j.ensm.2020.08.026
Han, J.G., Jeong, M.Y., Kim, K., et al.: An electrolyte additive capable of scavenging HF and PF5 enables fast charging of lithium-ion batteries in LiPF6-based electrolytes. J. Power Sources 446, 227366 (2020). https://doi.org/10.1016/j.jpowsour.2019.227366
Dagger, T., Grützke, M., Reichert, M., et al.: Investigation of lithium ion battery electrolytes containing flame retardants in combination with the film forming electrolyte additives vinylene carbonate, vinyl ethylene carbonate and fluoroethylene carbonate. J. Power Sources 372, 276–285 (2017). https://doi.org/10.1016/j.jpowsour.2017.10.058
Zeng, Z., Wu, B., Xiao, L., et al.: Safer lithium ion batteries based on nonflammable electrolyte. J. Power Sources 279, 6–12 (2015). https://doi.org/10.1016/j.jpowsour.2014.12.150
Rong, H., Xu, M., Xie, B., et al.: A novel imidazole-based electrolyte additive for improved electrochemical performance at elevated temperature of high-voltage LiNi0.5Mn1.5O4 cathodes. J. Power Sources 329, 586–593 (2016). https://doi.org/10.1016/j.jpowsour.2016.07.120
Wang, R., Li, X., Wang, Z., et al.: Electrochemical analysis graphite/electrolyte interface in lithium-ion batteries: p-toluenesulfonyl isocyanate as electrolyte additive. Nano Energy 34, 131–140 (2017). https://doi.org/10.1016/j.nanoen.2017.02.037
Ignatova, A.A., Yarmolenko, O.V., Tulibaeva, G.Z., et al.: Influence of 15-crown-5 additive to a liquid electrolyte on the performance of Li/CFx-systems at temperatures up to −50 °C. J. Power Sources 309, 116–121 (2016). https://doi.org/10.1016/j.jpowsour.2016.01.075
Wen, L., Liang, J., Chen, J., et al.: Smart materials and design toward safe and durable lithium ion batteries. Small Methods 3, 1900323 (2019). https://doi.org/10.1002/smtd.201900323
Janssen, P., Streipert, B., Krafft, R., et al.: Shutdown potential adjustment of modified carbene adducts as additives for lithium ion battery electrolytes. J. Power Sources 367, 72–79 (2017). https://doi.org/10.1016/j.jpowsour.2017.09.023
Noelle, D.J., Shi, Y., Wang, M., et al.: Aggressive electrolyte poisons and multifunctional fluids comprised of diols and diamines for emergency shutdown of lithium-ion batteries. J. Power Sources 384, 93–97 (2018). https://doi.org/10.1016/j.jpowsour.2018.02.068
Xia, L., Wang, D., Yang, H., et al.: An electrolyte additive for thermal shutdown protection of Li-ion batteries. Electrochem. Commun. 25, 98–100 (2012). https://doi.org/10.1016/j.elecom.2012.09.038
Dagger, T., Lürenbaum, C., Schappacher, F.M., et al.: Electrochemical performance evaluations and safety investigations of pentafluoro(phenoxy)cyclotriphosphazene as a flame retardant electrolyte additive for application in lithium ion battery systems using a newly designed apparatus for improved self-extinguishing time measurements. J. Power Sources 342, 266–272 (2017). https://doi.org/10.1016/j.jpowsour.2016.12.007
Fan, X., Ji, X., Chen, L., et al.: All-temperature batteries enabled by fluorinated electrolytes with non-polar solvents. Nat. Energy 4, 882–890 (2019). https://doi.org/10.1038/s41560-019-0474-3
Zhao, C.Z., Zhao, B.C., Yan, C., et al.: Liquid phase therapy to solid electrolyte-electrode interface in solid-state Li metal batteries: a review. Energy Storage Mater. 24, 75–84 (2020). https://doi.org/10.1016/j.ensm.2019.07.026
Cheng, Q., Li, A., Li, N., et al.: Stabilizing solid electrolyte-anode interface in Li-metal batteries by boron nitride-based nanocomposite coating. Joule 3, 1510–1522 (2019). https://doi.org/10.1016/j.joule.2019.03.022
Jiang, T., He, P., Wang, G., et al.: Solvent-free synthesis of thin, flexible, nonflammable garnet-based composite solid electrolyte for all-solid-state lithium batteries. Adv. Energy Mater. 10, 1903376 (2020). https://doi.org/10.1002/aenm.201903376
Zhao, N., Khokhar, W., Bi, Z., et al.: Solid garnet batteries. Joule 3, 1190–1199 (2019). https://doi.org/10.1016/j.joule.2019.03.019
Huo, H., Chen, Y., Luo, J., et al.: Rational design of hierarchical “ceramic-in-polymer” and “polymer-in-ceramic” electrolytes for dendrite-free solid-state batteries. Adv. Energy Mater. 9, 1804004 (2019). https://doi.org/10.1002/aenm.201804004
Sun, X., Stavola, A.M., Cao, D., et al.: Operando EDXRD study of all-solid-state lithium batteries coupling thioantimonate superionic conductors with metal sulfide. Adv. Energy Mater. 11, 2002861 (2020). https://doi.org/10.1002/aenm.202002861
Xu, H., Zhang, H., Ma, J., et al.: Overcoming the challenges of 5 V spinel LiNi0.5Mn1.5O4 cathodes with solid polymer electrolytes. ACS Energy Lett. 4, 2871–2886 (2019). https://doi.org/10.1021/acsenergylett.9b01871
Zhao, Q., Liu, X., Stalin, S., et al.: Solid-state polymer electrolytes with in-built fast interfacial transport for secondary lithium batteries. Nat. Energy 4, 365–373 (2019). https://doi.org/10.1038/s41560-019-0349-7
Liang, J., Chen, D., Adair, K., et al.: Insight into prolonged cycling life of 4 V all-solid-state polymer batteries by a high-voltage stable binder. Adv. Energy Mater. 11, 2002455 (2020). https://doi.org/10.1002/aenm.202002455
Zhang, M., Yu, S., Mai, Y., et al.: A single-ion conducting hyperbranched polymer as a high performance solid-state electrolyte for lithium ion batteries. Chem. Commun. 55, 6715–6718 (2019). https://doi.org/10.1039/c9cc02351h
Bouchet, R., Maria, S., Meziane, R., et al.: Single-ion BAB triblock copolymers as highly efficient electrolytes for lithium-metal batteries. Nat. Mater. 12, 452–457 (2013). https://doi.org/10.1038/nmat3602
Cao, C., Li, Y., Feng, Y., et al.: A solid-state single-ion polymer electrolyte with ultrahigh ionic conductivity for dendrite-free lithium metal batteries. Energy Storage Mater. 19, 401–407 (2019). https://doi.org/10.1016/j.ensm.2019.03.004
Li, Z., Liu, F., Chen, S., et al.: Single Li ion conducting solid-state polymer electrolytes based on carbon quantum dots for Li-metal batteries. Nano Energy 82, 105698 (2021). https://doi.org/10.1016/j.nanoen.2020.105698
Zhou, J., Qian, T., Liu, J., et al.: High-safety all-solid-state lithium-metal battery with high-ionic-conductivity thermoresponsive solid polymer electrolyte. Nano Lett. 19, 3066–3073 (2019). https://doi.org/10.1021/acs.nanolett.9b00450
Yang, H., Leow, W.R., Chen, X.: Thermal-responsive polymers for enhancing safety of electrochemical storage devices. Adv. Mater. 30, e1704347 (2018). https://doi.org/10.1002/adma.201704347
Yuan, M., Liu, K.: Rational design on separators and liquid electrolytes for safer lithium-ion batteries. J. Energy Chem. 43, 58–70 (2020). https://doi.org/10.1016/j.jechem.2019.08.008
Lee, J., Lee, C.-L., Park, K., et al.: Synthesis of an Al2O3-coated polyimide nanofiber mat and its electrochemical characteristics as a separator for lithium ion batteries. J. Power Sources 248, 1211–1217 (2014). https://doi.org/10.1016/j.jpowsour.2013.10.056
Zhong, G., Wang, Y., Wang, C., et al.: An AlOOH-coated polyimide electrospun fibrous membrane as a high-safety lithium-ion battery separator. Ionics 25, 2677–2684 (2018). https://doi.org/10.1007/s11581-018-2716-y
Liu, J., Qin, J., Mo, Y., et al.: Polyphenylene sulfide separator for high safety lithium-ion batteries. J. Electrochem. Soc. 166, A1644–A1652 (2019). https://doi.org/10.1149/2.1041908jes
Li, W., Li, X., Yuan, A., et al.: Al2O3/poly(ethylene terephthalate) composite separator for high-safety lithium-ion batteries. Ionics 22, 2143–2149 (2016). https://doi.org/10.1007/s11581-016-1752-8
Tian, Y., Lin, C., Wang, Z., et al.: Polymer of intrinsic microporosity-based macroporous membrane with high thermal stability as a Li-ion battery separator. RSC Adv. 9, 21539–21543 (2019). https://doi.org/10.1039/c9ra02308a
Lee, Y., Ryou, M.H., Seo, M., et al.: Effect of polydopamine surface coating on polyethylene separators as a function of their porosity for high-power Li-ion batteries. Electrochim. Acta 113, 433–438 (2013). https://doi.org/10.1016/j.electacta.2013.09.104
Li, Y., Pu, H., Wei, Y.: Polypropylene/polyethylene multilayer separators with enhanced thermal stability for lithium-ion battery via multilayer coextrusion. Electrochim. Acta 264, 140–149 (2018). https://doi.org/10.1016/j.electacta.2018.01.114
Ma, S., Lin, H., Yang, L., et al.: High thermal stability and low impedance polypropylene separator coated with aluminum phosphate. Electrochim. Acta (2019). https://doi.org/10.1016/j.electacta.2019.07.039
Xiao, W., Gong, Y., Wang, H., et al.: Preparation and electrochemical performance of ZrO2 nanoparticle-embedded nonwoven composite separator for lithium-ion batteries. Ceram. Int. 41, 14223–14229 (2015). https://doi.org/10.1016/j.ceramint.2015.07.048
Li, Y., Pu, H.: Facile fabrication of multilayer separators for lithium-ion battery via multilayer coextrusion and thermal induced phase separation. J. Power Sources 384, 408–416 (2018). https://doi.org/10.1016/j.jpowsour.2018.02.086
Xiong, M., Tang, H., Wang, Y., et al.: Expanded polytetrafluoroethylene reinforced polyvinylidenefluoride-hexafluoropropylene separator with high thermal stability for lithium-ion batteries. J. Power Sources 241, 203–211 (2013). https://doi.org/10.1016/j.jpowsour.2013.04.064
Woo, J.J., Zhang, Z., Dietz Rago, N.L., et al.: A high performance separator with improved thermal stability for Li-ion batteries. J. Mater. Chem. A 1, 8538–8540 (2013). https://doi.org/10.1039/c3ta12154b
Lee, T., Lee, Y., Ryou, M.H., et al.: A facile approach to prepare biomimetic composite separators toward safety-enhanced lithium secondary batteries. RSC Adv. 5, 39392–39398 (2015). https://doi.org/10.1039/c5ra01061f
Liu, K., Liu, W., Qiu, Y., et al.: Electrospun core-shell microfiber separator with thermal-triggered flame-retardant properties for lithium-ion batteries. Sci. Adv. 3, e1601978 (2017). https://doi.org/10.1126/sciadv.1601978
Jiang, X., Xiao, L., Ai, X., et al.: A novel bifunctional thermo-sensitive poly(lactic acid)@poly(butylene succinate) core-shell fibrous separator prepared by a coaxial electrospinning route for safe lithium-ion batteries. J. Mater. Chem. A 5, 23238–23242 (2017). https://doi.org/10.1039/c7ta08063h
Ji, W., Jiang, B., Ai, F., et al.: Temperature-responsive microspheres-coated separator for thermal shutdown protection of lithium ion batteries. RSC Adv. 5, 172–176 (2015). https://doi.org/10.1039/c4ra11500g
Zhang, C., Li, H., Wang, S., et al.: A polyethylene microsphere-coated separator with rapid thermal shutdown function for lithium-ion batteries. J. Energy Chem. 44, 33–40 (2020). https://doi.org/10.1016/j.jechem.2019.09.017
Lei, T., Chen, W., Hu, Y., et al.: A nonflammable and thermotolerant separator suppresses polysulfide dissolution for safe and long-cycle lithium-sulfur batteries. Adv. Energy Mater. 8, 1802441 (2018). https://doi.org/10.1002/aenm.201802441
Liao, H., Zhang, H., Qin, G., et al.: Novel core-shell ps-co-pba@SiO2 nanoparticles coated on pp separator as “thermal shutdown switch” for high safety lithium-ion batteries. Macromol. Mater. Eng. 302, 1700241 (2017). https://doi.org/10.1002/mame.201700241
Ye, Y., Chou, L.Y., Liu, Y., et al.: Ultralight and fire-extinguishing current collectors for high-energy and high-safety lithium-ion batteries. Nat. Energy 5, 786–793 (2020). https://doi.org/10.1038/s41560-020-00702-8
Wang, M., Shi, Y., Noelle, D.J., et al.: Internal short circuit mitigation of high-voltage lithium-ion batteries with functional current collectors. RSC Adv. 7, 45662–45667 (2017). https://doi.org/10.1039/c7ra08277k
Sharma, A., Tyagi, V.V., Chen, C.R., et al.: Review on thermal energy storage with phase change materials and applications. Renew. Sust. Energ. Rev. 13, 318–345 (2009). https://doi.org/10.1016/j.rser.2007.10.005
Lv, Y., Yang, X., Li, X., et al.: Experimental study on a novel battery thermal management technology based on low density polyethylene-enhanced composite phase change materials coupled with low fins. Appl. Energy 178, 376–382 (2016). https://doi.org/10.1016/j.apenergy.2016.06.058
Jiang, G., Huang, J., Fu, Y., et al.: Thermal optimization of composite phase change material/expanded graphite for Li-ion battery thermal management. Appl. Therm. Eng. 108, 1119–1125 (2016). https://doi.org/10.1016/j.applthermaleng.2016.07.197
Alipanah, M., Li, X.: Numerical studies of lithium-ion battery thermal management systems using phase change materials and metal foams. Int. J. Heat Mass Transf. 102, 1159–1168 (2016). https://doi.org/10.1016/j.ijheatmasstransfer.2016.07.010
Umair, M.M., Zhang, Y., Iqbal, K., et al.: Novel strategies and supporting materials applied to shape-stabilize organic phase change materials for thermal energy storage-a review. Appl. Energy 235, 846873 (2019). https://doi.org/10.1016/j.apenergy.2018.11.017
Wang, Q., Rao, Z., Huo, Y., et al.: Thermal performance of phase change material/oscillating heat pipe-based battery thermal management system. Int. J. Therm. Sci. 102, 9–16 (2016). https://doi.org/10.1016/j.ijthermalsci.2015.11.005
Wang, Q., Jiang, B., Xue, Q.F., et al.: Experimental investigation on EV battery cooling and heating by heat pipes. Appl. Therm. Eng. 88, 54–60 (2015). https://doi.org/10.1016/j.applthermaleng.2014.09.083
Hallaj, S.A., Selman, J.R.: A novel thermal management system for electric vehicle batteries using phase-change material. J. Electrochem. Soc. 147, 3231 (2000). https://doi.org/10.1149/1.1393888
Acknowledgements
This work was financially supported by the State Key Program of the National Natural Science Foundation of China (No. 51633007) and the National Natural Science Foundation of China (Nos. 51773147 and 51973151).
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Consent for publication
Written informed consent for publication was obtained from all participants.
Rights and permissions
About this article
Cite this article
Kong, L., Li, Y. & Feng, W. Strategies to Solve Lithium Battery Thermal Runaway: From Mechanism to Modification. Electrochem. Energy Rev. 4, 633–679 (2021). https://doi.org/10.1007/s41918-021-00109-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41918-021-00109-3