Abstract
In the present work, porous ceramics with open pores were fabricated using the gel casting method, which utilizes carrageenan as a cross-linking agent. Beyond the foaming agents or templates techniques, this approach employed large-sized Al2O3 particles (~ 45 μm) as the underlying framework material, enabling the direct formation of minute open pores within the green bodies and sintered samples. In order to finely adjust the pore structure and bolster flexural strength, micropowders of α-Al2O3 and Kaolin were incorporated as filling materials, alongside the introduction of rare-earth oxides as sintering additives. The study diligently examined the impact of varying the content of sintering additives as well as the sintering temperatures on the characteristics of the porous ceramics. As a result, remarkable achievements were obtained, culminating in the production of Al2O3-based porous ceramics measuring 8 mm in thickness, exhibiting flexural strength exceeding 160 MPa, porosity surpassing 30%, and exceptional gas permeability of over 30 L/min/cm2 under a pressure drop of 100 kPa.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Porous ceramics are widely used in various fields because of their excellent properties. The porous ceramics with pore sizes ranging from 100 to 1000 μm can be used in sound absorption [1], physical filtration [2], and thermal insulation [3], while those with pore size of 1 ~ 100 μm are widely used in beneficial bacteria culture [4], catalyst support [5], porous ceramic vacuum chuck [6], and so on. Recently, gel casting has been successfully used to prepare porous ceramics. The gel casting method was initially put forward by Janney Mark A et al. [7, 8] in 1990s. In this process, acrylamide or methyl acrylamide was used as monocase in the gel casting route to prepare the dense ceramics. Later, to avoid the toxicity, non-toxic natural gel systems (such as alginate, chitosan, and so on) have been used in the gel casting route [9,10,11,12,13]. To prepare porous ceramics, gel casting combined with the foaming or sacrificial template method was always employed [14, 15]. When gel casting was combined with foaming method, the foaming agents such as ammonium lauryl sulfate [16], twelve alkyl sulfate triethanolamine [17], and sodium lauryl sulfate [18] were added to the gel system. Using this process, the porosity of as-obtained porous ceramic can be as high as 80 ~ 90%. However, the pore diameter was always higher than 100 μm, and the strength is only 10 ~ 20 MPa. On the other hand, gel casting route combined with sacrificial template method can produce pores with size of several or several tens of micrometers. For example, Zhou et al. prepared porous zirconia ceramics using acrylamide gel system and PMMA microspheres as pore forming agent. The pore size can be as low as ~ 15 μm, and compressive strength of the porous ceramics reached more than 100 MPa [19]. Using gelatin as natural gel system and polythene microspheres as pore forming agent, Tulliani et al. successfully prepared zirconia porous ceramics by gel casting process, with porosity of ~ 40% [20]. However, this method is not environmentally friendly, and sometimes leads to the closed pores inside the bulk of ceramics.
To prepare porous ceramics, the “direct stacking and sintering method” is also reported [21, 22]. Different to the sacrificial template method and foaming method, this method does not need the pore-forming agent. Using large-sized ceramic powders (tens to hundreds of micrometers) as raw materials, the open pores are directly formed during the sintering process. The pore structure formed by this method is dispersive, and the ceramics with open pores often have strong mechanical properties. With large-sized Al2O3 powders as skeleton materials, Kim [6] et al. prepared porous ceramics that can be used for ceramic chucks. The porous ceramics they obtained by dry pressing method have open pores with an average pore size of 10 ~ 30 μm.
We believe that if large ceramic powders are used as skeleton materials, porous ceramics with open pores will also be achieved through gel casting. However, this has not been reported up to date. Based on this idea, in this paper, we developed a new gel casting route to prepare porous ceramics. Using non-toxic carrageenan as cross-linking agent, we synthesized the porous ceramics using large-sized Al2O3 powders as skeleton materials. We noticed that the advantages of the gel casting also made the raw materials loosely arranged in the green body. Thus, after sintering, the porous ceramics with open pores were directly formed.
Further, considering the application of the ceramic chucks, high strength and moderate permeability must be considered. The permeability is closely related to the porosity of open pores. However, the enhanced porosity must result in low strength. Thus, maintaining a high strength together with a moderate permeability for the porous ceramics becomes a challenge. To realize this, we employed the active α-Al2O3 with size of 1 ~ 3 μm as raw materials and La2O3, MgO, and Y2O3 as sintering additives. After processing optimization, the obtained Al2O3 porous ceramic shows a high strength over 160 MPa, together with a moderate porosity of 32%, and a permeability of 36.14 L/min/cm2 at 100 kPa, which is of potential for application of commercial ceramic chucks.
Materials and methods
Experimental materials and reagents
In this paper, large-sized Al2O3 particles were used as skeleton materials. Kaolin powders and active α-Al2O3 micropowders were used as filling materials. The Kaolin powders (4000 mesh) and skeleton Al2O3 particles (320 mesh) were bought from Hebei Zhanteng Mineral Products Co., Ltd. Active α-alumina micropowders with purity of 99.5% were obtained from Henan Tenai Engineering Materials Co., Ltd. The SEM images in Fig. 1 show that the active α-Al2O3 micropowders are in the size range of 1 ~ 3 μm, and the size of Al2O3 skeleton particles is about 45 μm. The sintering additives of Y2O3, La2O3, and MgO were obtained from Shanghai Aladdin Biochemical Technology Co., Ltd. The carrageenan and ammonium polyacrylate were bought from Shanghai Xianding Biotechnology Co., Ltd. All the chemicals were used without further treatment.
Sample preparation
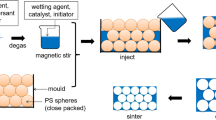
The experimental process is shown in Fig. 2. Firstly, the large-sized alumina powders, active α-alumina powders, and the sintering additives of Y2O3, La2O3, and MgO were mixed with deionized water and then ball milled for 24 h. After that, the ball-milled mixture was dried and ground, obtaining the mixture ceramic powders. Secondly, ammonium polyacrylate (0.3 g), carrageenan (0.24 g), and KCl (0.24 g) were added into deionized water (30 g). Certain amount of ammonia was added to the mixture solution to make the pH = 8. The mixture solution was then heated to 80 °C and stirred for 1 h so as to make the carrageenan completely dissolved. Thirdly, the mixture ceramic powders (totally 70 g) were slowly added to mixture solution at 80 °C, obtaining the ceramic slurry. The ceramic slurry was then stirred for 1 h at 80 °C and poured into the mold. After the slurry was cooled to room temperature, self-solidified green bodies were obtained, which were then dried at 50 °C for 12 h, and sintered at 1400–1600℃ for 4 h. The porous ceramics were finally obtained, which were machined into pieces with diameter of 50 mm and thickness of 8 mm.
Test method and instruments
The phase composition of the porous ceramics was examined by X-ray diffraction (XRD-7000, Shimadzu, Japan). Microstructure of samples was investigated by scanning electron microscopy (JSM-6700, Japan Electronics Corporation, Japan). The porosity and bulk density were measured by Archimedes method. The pore size distribution was measured by an automatic mercury porosimeter (Auto Pore V 9600, Micromeritics, USA). The flexural strength of the porous ceramics was tested by a universal testing machine (HT-2402, Taiwan Hongda Group Co., Ltd., China). The gas permeability was tested by a self-made device, as shown in Fig. 3. In different literatures, the definition of the gas permeability varies [6, 23, 24]. Considering the application of the porous ceramics in the vacuum chucks, the flux of gas passing through the porous ceramics at a fixed pressure drop is more suitable to be used to express the gas permeability [6]. Thus, in this paper, the gas permeability was used to describe the volume of the gas passing through a unit area per minute (gas flux) at a fixed pressure drop (ΔP). During the measurement of the gas permeability, the nitrogen gas passed through the porous ceramic, the pressure drop (ΔP) between the inlet gas and outlet gas was tuned by the gas flowmeter, and the gas flow rate was tested. The gas permeability Kg (L/min/cm2) is determined by Kg = Q/A, where Q is the gas flow rate (L/min) and A is the surface area of the porous ceramics (cm2). Considering the effect of the ceramic thickness on the permeability, the thickness of all the samples was controlled at 8 mm.
Results
Effect of sintering temperature on the properties of porous ceramics
The sintering temperature greatly influences the crystallization and flexural strength of the final sintered samples. Also, the content of sintering additive has a strong relationship with the sintering temperature. Therefore, optimizing the sintering temperature of the porous ceramics is necessary. We prepared a series of samples sintered at different temperatures (1400 ~ 1600 °C). All the samples were prepared using the gel casting route with 41.65 g of Al2O3 skeleton particles, 17.85 g of α-Al2O3 micropowders, 7.00 g of Kaoling powders, and 3.50 g of sintering additives (including 1.75 g of Y2O3, 1.05 g of La2O3, and 0.7 g of MgO).
The XRD patterns of the porous ceramics sintered at different temperatures are shown in Fig. 4. Typical Al2O3 characteristic peaks appear at 2θ of 25.578°, 35.154°, 43.357°, and 57.507°, respectively, corresponding to (012), (104), (113), and (116) planes. Although the Y2O3, La2O3, MgO, and SiO2 in the Kaoling powders are added to the precursor powders, no diffraction peaks related to them are detected. Since the mass percentage of these powders is low, the amount the phases related to these oxides would be less, which is beyond the resolution limit of the XRD.
Figure 5 shows the SEM images of porous ceramics sintered at different temperatures. Basically, the morphologies of all the samples are almost the same. Careful investigation discloses that at low sintering temperatures (1400℃ and 1450℃), some small particles cover the surface of the skeleton Al2O3 particles. This means that the small-sized powders (including the α-Al2O3 micropowders, Kaoling powders, and sintering additives) may not be tightly connected with the large-sized Al2O3 skeleton particles. The increase of sintering temperature to 1500℃ makes the open pores more clear, although the pore size is slightly decreased. When temperature is higher than 1500℃, the Al2O3 skeleton particles are tightly interconnected, and the ceramic samples exhibit a denser structure. To make a comparison, a sample with same compositions was also prepared by dry pressing method and sintered at 1500℃. As compared with the sample prepared by the gel casting method, the morphology of the dry pressed sample shows a denser structure.
The porosity of open pores and volume density of the samples sintered at different temperatures are shown in Fig. 6. As can be seen, with the increase of sintering temperature from 1400 to 1600℃, the porosity of open pores decreases from 36.20 to 27.72%, the volume density increases from 2.44 to 2.77 g/cm3, and their linear shrinkage of ceramics increases from 7.7 to 10.8%. The sample prepared by the dry pressing method has a porosity of only ~ 27%. It has been noticed that both density and porosity are very close to that of the 1600℃-sintered sample. All the samples sintered at different temperatures show strengths higher than 100 MPa. For samples sintered over 1500℃, their flexural strength is over 160 MPa.
The permeabilities of the samples sintered at different temperatures are shown in Fig. 7. The permeability of samples sintered at 1500℃ reaches a high value of 36.14 L/min/cm2, higher than that of samples sintered at 1400℃ (23.13 L/min/cm2) and 1600℃ (22.65 L/min/cm2). The 1400℃-sintered sample is not well crystallized, and the filling materials are not fused with the Al2O3 skeleton particles, thus blocking the gas flow. For 1600 °C-sintered samples, both the pore size and porosity are decreased. Therefore, its permeability is still lower than that of the 1500℃-sintered sample. It is very interesting that the dry pressed sample shows a close permeability to the 1600℃-sintered sample, since both have almost similar porosity, pore size, flexural strength, and density.
Effect of sintering additive content on the performance of porous ceramics
The above-mentioned results indicate that we successfully obtain the porous ceramic with high strength, moderate porosity, and permeability using the gel casting route. Significantly, the porosity of ~ 32% and permeability of ~ 36 L/min/cm2 at 100 kPa are not easily realized by dry pressing method. Moreover, the sample also shows a flexural strength over 160 MPa, which is enough for porous ceramic chucks.
However, some issues need to be addressed: (1) although the sintering temperature exerts great influence on the crystallization of the ceramics, the compositions and their dozes affect the sintering temperature; (2) the composition design of the ceramics also shows great influence on the pore size, porosity, and flexural strength of the porous ceramics. Therefore, the effect of the composition on the properties of the porous ceramics needs to be explored.
To design the composition of the ceramics, six samples (samples A, B, C, D, E, and F) with mass percentage of sintering additives of 0 wt.%, 1 wt.%, 3 wt.%, 5 wt.%, 7 wt.%, and 9 wt.%, respectively, were prepared. Their compositions are shown in Table 1. As can be seen, the samples’ mass percentage of sintering additives of the samples are changed by tuning the mass of skeleton alumina powders (45 μm), active α-alumina micropowders (1 ~ 3 μm), and the sintering additives. But the mass of the Kaoling powders was fixed at 7 g, the mass ratio of the skeleton alumina powders to active α-alumina micropowders was fixed at 7:3, and the mass ratio of Y2O3, La2O3, and MgO was controlled at 5:3:2. The total mass of ceramic powders of every sample was fixed at 70 g. All the samples were sintered at 1500 °C for 4 h.
The X-ray diffraction experiments were performed on the samples A–F. The samples A–D (with additive of 0 ~ 5 wt.%) have almost the same XRD patterns. Only Al2O3 diffraction peaks can be observed, as shown by Fig. 8. The XRD patterns of samples with sintering additive percentage of 5 wt.%, 7 wt.%, and 9 wt.% are shown in Fig. 5. When the amount of additives reaches 7 wt. %, some tiny peaks corresponding to other phases that appear at 2θ of 29.65°, 30.70°, 32.07°, and 36.16° were observed. Further, when the percentage of the additives reaches 9 wt.%, these peak intensities are enhanced. Careful investigation of these tiny peaks discloses that these second phases are mainly Al2Y4O9 and MgAl11LaO19. Considering the resolution limit of the XRD, we believe that in the samples with additive percentage less than 7 wt. %, the second phases of Al2Y4O9 and MgAl11LaO19, and even other phases such as MgAl2O4 phase, may also be existed.
The microstructures of sintered ceramic samples A ~ F are shown in Fig. 9a ~ f. Without or with 1 wt.% percentage of sintering additives, some powders are attached to the skeleton particles. This indicates that the samples A and B are not well crystallized. The unreacted powders inside the ceramics would lead to a low strength value of the porous ceramic. When the percentage of sintering additives is increased to 3 wt.%, these unreacted powders disappear, and open pores in the ceramics are clearly observed. With further increase of the sintering additives to 5 ~ 9 wt.%, the microstructure keeps almost the same.
The apparent porosity and bulk density of samples A–F is shown in Fig. 10a. With the increase of the percentage of the sintering additives from 0 to 9 wt.%, the porosity of open pores decreases continuously from 44.9 (sample A) to 26.7% (sample F). Correspondingly, the volume density increases from 2.12 to 2.89 g/cm3. However, the strength of the samples does not show a continuous increase with the percentage of sintering additives. As can be seen from Fig. 10d, the samples A and B shows a strength value lower than 40 MPa. This is due to their not-well-crystallized nature, whereas the flexural strength values of samples C, D, E, and F are higher than 120 MPa. Especially, sample D shows a flexural strength as high as 160 MPa. However, samples E and F show a lower strength than that of sample D. The reasons may be as follows: on the one hand, the increase of liquid phase during sintering leads to abnormal grain growth, resulting in the decrease of grain size uniformity and the decrease of ceramic strength. On the other hand, the formation of new-phase MgAl11LaO19 during sintering may also lead to the decrease of strength.
Figure 11 shows the permeability curves of samples B, D, and E. It can be found that when the percentage of sintering additives is 5 wt.%, the porous ceramic has the highest permeability, up to 36 L/min/cm2 at a pressure drop of 100 kPa. Although sample A has a high porosity, its permeability is low. This is due to the unreacted powders inside the ceramic bulk, which block the transport of the gas. The permeability of sample E is lower than sample D because its porosity is low. However, its permeability is still higher than that of sample B.
Figure 12 shows the pore size percentage of samples prepared using different content of sintering additives. It can be seen that when the sintering additive content is 1 wt.%, the small pore size is 0–10 μm. The volume percentage for pores with size less than 10 μm is as high as 90%. As the content of sintering additives increases to 5 ~ 7 wt.%, the pores with size of 10 ~ 20 μm takes a large proportion. This phenomenon is closely related to the formation of quasi liquid phase induced by the sintering additives, which will be discussed later.
Porous ceramics have wide applications in different fields. The open-pore ceramics prepared in this work aims to be applied for ceramic chucks. Thus, the gas permeability and the strength of the open-pore ceramics are of great importance. The properties of the open-pore ceramics prepared in this work are compared with the data in other literatures, as shown in Table 2. It is difficult to compare our data with all the references since different papers use different units for permeability. However, the unit of permeability in references [6, 25], and [26] are same to ours. We found that not the permeability of our open-pore ceramics is slightly higher, but the flexural strength is much higher than the data as compared with references [6, 25], and [26]. This indicates that the open-pore ceramics prepared by our novel gel casting process is of potential in application of ceramic chucks.
Discussion
According to the results mentioned above, we can conclude that (1) at low temperature, although the sample’s porosity is high, filling materials are not well crystallized. Thus, the gas cannot flow smoothly, resulting in low gas permeability, as illustrated by the left two graphs in Fig. 13. Only after being sintered at high temperatures, the gas permeability becomes better. But there are still several issues that need to be discussed here. Firstly, in our porous ceramics, the Al2O3 skeleton particles have a size of ~ 45 μm. And it is noted that the sintering temperature is only 1500–1600℃. At this temperature, these large-sized skeleton particles are almost inert in the solid sintering process. Even with the help of the sintering additives, the atoms inside these large particles are not easy to diffuse. Therefore, the solid reaction between these large-sized Al2O3 skeleton particles at 1500–1600℃ is almost impossible. Otherwise, the open pores would be filled, and the porosity of the porous ceramic must be low.
It should be noted that, in the total 70 g of precursor powders, there are 40 ~ 45 g of large-sized Al2O3 skeleton particles. That means, the inert Al2O3 skeleton particles take up ~ 60 wt.% in the precursor powders. Therefore, the Al2O3 skeleton particles take up almost half of the volume of the samples. However, the porous ceramics still show a high strength over 160 MPa. Thus, an issue arises: what happens during the sintering process? and how the flexural strength is enhanced?
Since the Al2O3 skeleton particles are inert during the sintered process, the high strength of the final porous ceramics would be related to the reactions of the filling materials, including the active α-Al2O3 micropowders, the Kaoling powders, and the sintering additives. It is known that the rare-earth oxides Y2O3 and La2O3 are good surface-active substances, which can improve the surface wettability of Al2O3 and reduce the sintering temperature of alumina ceramic materials. The addition of rare-earth materials promotes the solid reaction of Al2O3 micropowders and can form a low melting point liquid phase [30,31,32]. The SiO2 in the Kaoling powders also promotes the formation of the liquid phase [33]. Therefore, during the sintering process, there are two main phases: one is the inert Al2O3 particles (skeleton particles), and the other is the quasi liquid phase.
Thus, we can explain the phenomena mentioned above. The sintering temperature’s effect on the porous ceramic’s properties can be explained as follows: (1) at a relatively low temperature, the quasi liquid phase may not be fully formed, or its fluidity is low. Thus, some of the filling materials would be left inside the big pores formed by the skeleton particles. In this case, the gas does not flow freely, decreasing the permeability of the ceramic. However, there porosity is still high, because at the low sintering temperature, the ceramic does not experience a large shrinkage; (2) with the increase of the sintering temperature, more filling materials are consumed and transferred to liquid phases. The liquid phase preferentially fills the sharp corners of the neighbor skeleton particles to decrease the system’s surface energy, as shown in Fig. 14a. And the increased content of the liquid phase caused by elevated temperature will tightly wrap and connect the skeleton Al2O3 powders, thus decreasing the diameter of pores caused by neighbor skeleton Al2O3 particles, as shown in Fig. 14b. As a result, the strength of the ceramic is enhanced, and their porosity is decreased. However, the filling materials that block the gas flow transport are consumed by the liquid phase, so the gas permeability is still enhanced. However, as indicated by Fig. 14c, further increase of the temperature would make the pore diameter decrease and also decrease the gas permeability.
Schematic illustration of the liquid phase formation inside the ceramics during the sintering process: a at low sintering temperature or with low content of sintering additives; b at moderate sintering temperature or with suitable content of sintering additives; c at high sintering temperature or with high content of the sintering additives
The liquid phase is also related to the content of the sintering additives. As illustrated in Fig. 14, when the content of the sintering additives is low, the formed liquid phase will be of low content. And increase of the content of the sintering additives will bring about more amount of the liquid phase. Therefore, the effect of the content of the sintering additives on the properties of final porous ceramic is similar to the effect of the sintering temperature. However, too much content of the additives would lead to more content of second phases inside the ceramic. As indicated by XRD, the second phases of Al2Y4O9 and MgAl11LaO19, and even other phases such as MgAl2O4 phase, may form inside the ceramics. Thus, the second phase (such as MgAl11LaO19) will decrease the strength of the porous ceramic [34].
Conclusions
Using non-toxic carrageenan as a cross-linking agent, a novel gel casting method was developed to fabricate porous ceramics with open pores. We especially synthesized the porous ceramics using large-sized Al2O3 powders as skeleton materials, by which the micropores were directly formed after sintering. In addition, to improve its strength, we also employed the active α-Al2O3 with size of 1~3 μm as raw materials and La2O3, MgO, and Y2O3 as sintering additives. The obtained Al2O3 porous ceramic shows a high strength over 160 MPa, together with a high porosity of 32%, and a permeability of 36.14 L/min/cm2 at 100 kPa, which is of potential for commercial ceramic chucks.
References
Du, Z., Yao, D., Xia, Y., Zuo. K., Yin, J., liang, H., Zheng, Y.P.: The sound absorption properties of highly porous silicon nitride ceramic foams. J. Alloys Compd. 820 (2020)
Voigt, C., Jäckel, E., Taina, F., Zienert, T., Salomon, A., Wolf, G., Aneziris, C.G.: Le Brun P Filtration efficiency of functionalized ceramic foam filters for aluminum melt filtration. Metall. Mater. Trans. B. 48, 497–505 (2016)
Zhou, W., Yan, W., Li, N., Li, Y., Dai, Y., Zhang, Z., Ma, S.: Fabrication of mullite-corundum foamed ceramics for thermal insulation and effect of micro-pore-foaming agent on their properties. J. Alloys Compd. 785, 1030–1037 (2019)
Hong, Y., Wang, Y., Li, B., Pan, G.: Immobilizing nitrifying bacteria with Fe2O3-CaO-SiO2 porous glass-ceramics. Int. J. Appl. Glass Sci. 10, 228–234 (2019)
Liao, M., Guo, C., Guo, W., Hu, T., Qin, H., Gao, P., Xiao, H.: Hydrogen production in microreactor using porous SiC ceramic with a pore-in-pore hierarchical structure as catalyst support. Int. J. Hydrogen Energy. 45, 20922–20932 (2020)
Kim, J., Ha, J.H., Lee, J., Song, I.-H.: Optimization for permeability and electrical resistance of porous alumina-based ceramics. J. Korean Ceram. Soc. 53, 548–556 (2016)
Omatete, O.O., Janney, M.A., Nunn, S.D.: Gelcasting: from laboratory development toward industrial production. J. Eur. Ceram. Soc. 17, 407–413 (1997)
Omatete, O.O., Janney, M.A., Strehlow, R.A.: Gelcasting: a new ceramic forming process. Am. Ceram. Soc. Bull. 70, 1641–1649 (1991)
Milla’n, A.J., Nieto, M.I., Moreno, R.: Near-net shaping of aqueous alumina slurries using carrageenan. J. Eur. Ceram. Soc. 22, 297–303 (2002)
Xu, J., Liu, S., Wang, Y., Guo, Y., Zhao, J., Yan, H., Gao, F.: Enhanced dielectric properties of highly dense Ba0. 5Sr0. 5TiO3 ceramics via non-toxic gelcasting. J. Mater. Sci-Mater. El. 31, 17819–17827 (2020)
Adolfsson, E.: Gelcasting of zirconia using agarose. J. Am. Ceram. Soc. 89, 1897–1902 (2006)
Li, Y., Sun, S., Gao, P., Zhang, M., Fan, C., Lu, Q., Li, C., Chen, C., Lin, B.: A tough chitosan-alginate porous hydrogel prepared by simple foaming method. J. Solid State Chem. 294 (2021)
Peter, M., Binulal, N.S., Nair, S.V., Selvamurugan, N., Tamura, H., Jayakumar, R.: Novel biodegradable chitosan–gelatin/nano-bioactive glass ceramic composite scaffolds for alveolar bone tissue engineering. Chem. Eng. J. 158, 353–361 (2010)
Han, L., Li, F., Deng, X., Wang, J., Zhang, H., Zhang, S.: Foam-gelcasting preparation, microstructure and thermal insulation performance of porous diatomite ceramics with hierarchical pore structures. J. Eur. Ceram. Soc. 37, 2717–2725 (2017)
Chen, Y., Guo, W., Luo, Y., Ma, Z., Zhang, L., Yue, Z.: Microwave and terahertz properties of porous Ba4 (Sm, Nd, Bi) 28/3Ti18O54 ceramics obtained by sacrificial template method. J. Am. Ceram. Soc. 104, 5679–5688 (2021)
Zhang, F.Z., Kato, T., Fuji, M., Takahashi, M.: Gelcasting fabrication of porous ceramics using a continuous process. J. Eur. Ceram. Soc. 26, 667–671 (2006)
Han, L., Wu, W., Huang, Z., Lei, W., Li, S., Zhang, H., Jia, Q., Zhang, S.: Preparation and characterization of a novel fluorine-free and pH-sensitive hydrophobic porous diatomite ceramic as highly efficient sorbent for oil–water separation. Sep. Purif. Technol. 254 (2021)
Ivanova, Y.A., Monteiro, J.F., Teixeira, L.B., Vitorino, N., Kovalevsky, A.V., Frade, J.R.: Designed porous microstructures for electrochemical reduction of bulk hematite ceramics. Mater. Des. 122, 307–314 (2017)
Zhou, J., Wang, C.A.: Porous yttria-stabilized zirconia ceramics fabricated by nonaqueous-based gelcasting process with PMMA microsphere as pore-forming agent. J. Am. Ceram. Soc. 96, 266–271 (2013)
Tulliani, J.M., Bartuli, C., Bemporad, E., Baglieri, V., Sebastiani, M.: Preparation and mechanical characterization of dense and porous zirconia produced by gel casting with gelatin as a gelling agent. Ceramics. Int. 35, 2481–2491 (2009)
Xia, B., Wang, Z., Gou, L., Zhang, M., Guo, M.: Porous mullite ceramics with enhanced compressive strength from fly ash-based ceramic microspheres: facile synthesis, structure, and performance. Ceramics. Int. 48, 10472–10479 (2022)
Huo, W., Zhang, X., Chen, Y., Lu, Y., Liu, J., Yan, S., Wu, J.M., Yang, J.: Novel mullite ceramic foams with high porosity and strength using only fly ash hollow spheres as raw material. J. Eur. Ceram. Soc. 38, 2035–2042 (2018)
Bautista, M.A., Cancapa, J.Q., Fernandez, J.M., Rodríguez, M.A., Singh, M.: Microstructural and mechanical evaluation of porous biomorphic silicon carbide for high temperature filtering applications. J. Eur. Ceram. Soc. 31, 1325–1332 (2011)
Kumar, A., Mohanta, K., Kumar, D., Parkash, O.: Low cost porous alumina with tailored gas permeability and mechanical properties prepared using rice husk and sucrose for filter applications. Micropor. Mesopor. Mat. 213, 48–58 (2015)
Song, I.H., Park, M.J., Kim, H.D., Kim, Y.W., Bae, J.S.: Microstructure and permeability property of Si bonded porous SiC with variations in the carbon content. J. Korean Ceram. Soc. 47, 546–552 (2010)
Kim, J., Ha, J. H., Lee, J., Song, I. H., Kim, J., Ha, J. H., ... & Song, I. H.: The effect of MnO2 content on the permeability and electrical resistance of porous alumina-based ceramics. J. Korean Ceram. Soc. 54, 331–339 (2017)
Li, Y., Yang, X., Liu, D., Chen, J., Zhang, D., Wu, Z.: Permeability of the porous Al2O3 ceramic with bimodal pore size distribution. Ceramics Int. 45, 5952–5957 (2019)
Yin, Y., Ma, B., Hu, C., Liu, G., Li, H., Su, C., Ren, X., Yu, J., Zhang, Y., Yu, J.: Preparation and properties of porous SiC–Al2O3 ceramics using coal ash. Int. J. Appl. Ceram. Tec. 16, 23–31 (2019)
Su, Z., Xi, X., Hu, Y., Fei, Q., Yu, S., Li, H., Yang, J.: A new Al2O3 porous ceramic prepared by addition of hollow spheres. J. Porous Mat. 21, 601–609 (2014)
Sato, E., Carry, C.: Yttria doping and sintering of submicrometer-grained α-alumina. J. Am. Ceram. Soc. 79, 2156–2160 (1996)
Cho, J., Harmer, M.P., Chan, H.M., Rickman, J.M., Thompson, A.M.: Effect of yttrium and lanthanum on the tensile creep behavior of aluminum oxide. J. Am. Ceram. Soc. 80, 1013–1017 (1997)
Yang, Q., Zeng, Z., Xu, J., Zhang, H., Ding, J.: Effect of La2O3 on microstructure and transmittance of transparent alumina ceramics. J. Rare Earth. 24, 72–75 (2006)
Park, C.W., Yoon, D.Y.: Effects of SiO2, CaO2, and MgO additions on the grain growth of alumina. J. Am. Ceram. Soc. 83, 2605–2609 (2000)
Chen, X., Zhao, Y., Huang, W., Ma, H., Zou, B., Wang, Y., Cao, X.: Thermal aging behavior of plasma sprayed LaMgAl11O19 thermal barrier coating. J. Eur. Ceram. Soc. 31, 2285–2294 (2011)
Funding
This work was supported by the Science and Technology Project of Shaanxi Province (No. 2023-YBGY-160).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Z., Chen, Y., Demir, M. et al. Air permeable alumina-based porous ceramics with high performance prepared by novel gel casting route. J Aust Ceram Soc 59, 1303–1313 (2023). https://doi.org/10.1007/s41779-023-00910-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41779-023-00910-x