Abstract
Tin-doped zinc sulfide was coated onto glass plates and subjected to a post-air annealing treatment at 300 °C for 2 h. The air-annealed thin films were characterized for observing the tailoring in structural, surface, chemical, optical, photoluminescence, and magnetic properties due to annealing. The properties of thin films after air-annealing were compared with as-synthesized Kumar et al. (J. Supercond. Novel Magn. 32(6), 1725-1734, 2019), and vacuum-annealed Kunapalli et al. (Opt. Mater.114, 110961, 2021) thin films. Annealing in presence of air leads to finer crystallite structures of ZnS thin films. The air annealing also enhanced the transmittance property of the thin films. Two prominent peaks at 420 nm and 440 nm were observed in photoluminescence spectra. From magnetic studies, it was found that the films were paramagnetic in nature, and magnetization increased with an increase in the applied field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It has been known that the process of inclusion of magnetic materials into semiconductors in very small amounts is called dilute magnetic semiconductors (DMS). These DMS materials find application in future electronics or spintronic devices [1, 2]. A theoretical prediction about the DMS was given by Dietl et al. [3]. Currently, high importance has been given to II-VI semiconductor thin films which have the ability to exhibit ferromagnetism at room temperature [4,5,6]. Among them, zinc sulfide (ZnS) has been one of the most important wide bandgap semiconductors. The interesting feature of ZnS is when a small quantity of impurity ions is added to the host semiconductor, a change in optical, electrical, and magnetic properties has been observed.
The transition metal ion-doped ZnS thin film has been studied by many research groups as its peculiar properties find in many spintronic and storage applications [7,8,9,10,11]. Generally, in dilute magnetic semiconductors, the host ions, i.e., cations, are partially substituted by impurity ions and these impurity ions would have been randomly confined over the host lattice. Moreover, the Curie temperature for II-VI semiconductors has been reported to be very near to room temperature. So, room temperature ferromagnetism in these semiconductors has been attained by adding suitable impurities or suitable doping concentration or specific synthesis method [12]. ZnS observed half metallic behavior by reporting magnetic behaviors like ferromagnetic and antiferromagnetic in presence of chromium, iron and nickel in the lattice of ZnS [13,14,15].
Annealing of thin films led to strengthening and enhancing the properties of the films. The thin films were formed by the process of nucleation and growth of the molecules on the substrate. Annealing has been used on thin films as post-synthesis treatments. They have been used to tailor the physical properties of thin films. It has been found that annealing at different atmosphere had shown different influences on various thin films. Annealing at different atmosphere might generate more vacant sites of the halides. The increase in the number of vacant sites could lead to enhancement in the optical and magnetic properties of dilute magnetic semiconductors. As annealing in presence of different atmosphere might lead to change in the growth of the crystallites and might also affect the number of vacant sites, the influence of them on the properties had been studied in detail for various dilute magnetic semiconductors. One more significance of studying the influence of annealing at various atmospheric condition on the thin films, could help to predict the nature of the film at various seasons in earth. A spintronic device should be able to work efficiently at all seasons, so the influence of weather condition on the thin film could be checked by annealing them in different atmospheres.
ZnS has been well known for its spintronic applications and so the influence of dopant after annealing in air and vacuum environment needs to be compared. A spintronic device has its backbone on the optical and magnetic properties of the thin films or nanoparticles. And annealing at optimized temperature could strengthen the bonds in the thin films leading to increased adhesivity, finer crystallite size, and strong bonding. It would also lead to increase in the number of vacant sites leading to increase in charge carriers for optical conductivity and magnetic properties. The magnetic properties in ZnS had been reported as ferromagnetic only when there had been presence of sulfide vacant sites.
Previously, the role of dopants and vacuum annealed on the physical properties of transition metal doped ZnS thin films were reported in detail [16,17,18,19]. As very few reports have been available on the role of air annealing on magnetic properties of doped ZnS thin films, here we have aimed to study the effect of air annealing on the structural, optical, photoluminescence, and magnetic properties of Sn-doped ZnS thin films prepared by electron beam evaporation technique.
Experimental details
Before annealing, the nanoparticles and thin films of tin doped ZnS composition were prepared and reported in earlier publications [16, 17]. Then, the prepared thin films were annealed in presence of air at 300 °C to study the role of air annealing on the physical properties of tin-doped ZnS thin films. The air annealing was performed by keeping small pieces of 3 cm × 3 cm dimension of thin films in horizontal furnace. The thin films were positioned to be at the middle of the horizontal furnace to heat it in air at 300 °C for 2 h. Then, the air annealed films were characterized by X-ray diffractometer using BRUKER- D8 ADVANCE model, JASCO V-670 model of UV–Vis-NIR spectrophotometer and Hitachi F700 model of Fluoroscence spectrophotometer. The air annealed films were characterized by Lakeshore 7410 model of vibrating sample magnetometer.
Results and discussions
Structural properties
The structural properties of air annealed thin films were analyzed from the diffraction pattern as shown in Fig. 1a obtained from X-ray diffractometer. The diffraction pattern reported three peaks at positions 28.6°, 47.6°, and 56.6°. The respective miller indices for those peaks are found to be (1 1 1), (2 2 0), and (3 1 0). The miller indices were obtained by comparing the obtained pattern with the zinc blende structured JCPDS pattern of #08–0020 [20]. Fityk software has been used to fit the patterns. A small shift in diffraction peak was observed with increase in Sn concentration as shown in Fig. 1b. The average crystallite size for the annealed thin films was calculated using the Scherrer’s formula. The air annealed thin films attained a size of 8 to 12 nm for increasing dopant concentration. But the average crystallite size of air annealed thin films was found to be the least compared to as-deposited and vacuum annealed thin films reported earlier [17, 19]. The finer average crystallite size was observed for air annealed thin films due to the slow heating and cooling mechanism exhibited during annealing in presence of air. The micro strain in air annealed thin films was calculated to increase from 47 × 10−4 to 50 × 10−4. Thus, the annealing in presence of air leads to increase in the strain of the nanoparticles deposited on glass compared to the thin films annealed in vacuum [19] and unannealed thin films [17]. In air annealing, the thin films were heat treated in presence of air molecules. Thus, in presence of air molecules, the heating of the thin films would have taken some more time. As the deposition was already done on the glass plate, this meant that the nucleation of Sn-doped ZnS molecules was already completed. On heat treating them, the growth of the nanoparticles would have been changed due to change in temperature gradient. While annealing in presence of vacuum, the thin films were heated in the absence of any kind of molecules, so the heat would have remained for longer duration on the thin films, then cooling. This would lead to a slow change in temperature gradient. It has been known that growth of nanoparticles has been always inversely proportional to the rate of change in temperature gradient. Also, the annealing in vacuum might lead to instability of structure. But in case of annealing in presence of air, the cooling had occurred faster. So, in air annealing, the temperature gradient had changed very quickly leading to hinder the growth of the nanoparticles. So, the films annealed in air attained the finest crystallites compared to vacuum annealed and as-deposited thin films as shown in Table 1 with other physical parameters’ comparison.
Optical properties
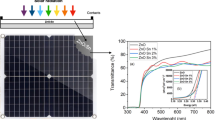
The optical absorbance, reflectance, and transmittance spectra of the Zn1−xSnxS thin films were reported in Fig. 2a–d. The optical properties of the corresponding thin films annealed in vacuum and as-deposited films were discussed earlier in previous reports [17, 19]. The graph plotted between wavelength (nm) verses transmittance (%) for different Sn-doped ZnS thin films has been shown in Fig. 2c. From the figure, one can see that the annealed thin films exhibited high optical transmittance (~ 90%). The increase in optical transmittance with annealing temperature was reported by Sara A Mohamed et al. [20] in thin films. The thin films that exhibit high optical transmittance find suitable applications in solar cells. Tauc’s relation [21] was used for determining the optical band gap as shown in Fig. 2d. The optical band gap of the air annealed thin films was found to increase from 3.4 eV to 3.45 eV. On comparing these with the optical band gap of as-deposited Sn doped ZnS [17] and vacuum annealed Sn doped ZnS thin films [19], it was found to be slightly decreased. But no significant change in the optical band gap was observed due to the annealing in air. The decreasing optical band with doping concentration was previously reported in manganese-doped zinc sulfide thin films [22]. The band gap in the range of 3.4 to 3.45 eV with high visible region transmittance increased the probability of using air annealed films to act as buffer layer in solar cell as observed in ZnSe thin films [23].
Photoluminescence studies
The emission spectra for air annealed Sn-doped ZnS thin films were analyzed from photoluminescence spectra as shown in Fig. 3. A source with an excitation wavelength 330 nm was used and emission peaks were observed at 420 nm and 440 nm for all air annealed thin films. Thus, no significant shift in emission spectra was observed after annealing in air compared to annealing in vacuum [19]. Thus, the air annealing did not significantly affect the defects in the thin films. It was considered that the peak at 420 nm and 440 nm might be due to vacancies of sulfur ions and zinc ions, respectively [16].
Magnetic properties
The magnetic property of tin-doped zinc sulfide annealed in air showed the paramagnetic nature as shown in Fig. 4. Similar to the magnetic properties of vacuum annealed Sn-doped ZnS thin films [19], the paramagnetic nature has been reported due to the decrease in sulfur defect. The maximum magnetization decreased from 4 memu/g to 2 memu/g on increasing the dopant concentration. Decrease in the coercivity and retentivity values were observed for the air annealed films from 60.98 Oe to 14.5 Oe and 22.2 µemu/g to 1.5 µemu/g, respectively. The narrowing of band gap and decrease in average crystallite size on annealing in presence of air did not induce the ferromagnetic property in the thin films. This has occurred, as the sulfur vacancy was not created even after air annealing to undergo superexchange interaction in the thin films. So, the annealing treatment did not significantly change the behavior of spins leading to Sn-doped ZnS showing paramagnetic property even in presence of air.
Conclusions
The influence of air during annealing of Sn-doped ZnS thin films in their physical properties was studied in detail. The structural properties showed an enhancement in the average crystallite size behavior of air annealed thin films compared to vacuum annealed and as-deposited thin films. With the finer crystallite size of about 8 to 12 nm, the air annealing led to decrease in the crystallite sizes. A decrease in optical band gap was found for the Zn1−xSnxS when compared with that of optical band gaps of the as prepared thin films. The Zn1−xSnxS exhibited paramagnetic behavior at room temperature which might be due to absence of any secondary phases or vacancies in the air annealed thin films.
References
Ohno, H.: Making nonmagnetic semiconductors ferromagnetic. Science 281(5379), 951 (1998). https://doi.org/10.1126/science.281.5379.951
Furdyna, J.K.: Diluted magnetic semiconductors. J. Appl. Phys. 64(4), R29–R64 (1988)
Dietl, T., Ohno, H., Matsukura, F., Cibert, J., Ferrand, D.: Zener model description of ferromagnetism in zinc-blende magnetic semiconductors. Science 287(5455), 1019 (2000). https://doi.org/10.1126/science.287.5455.1019
Alver, Ü., Bacaksız, E., Yanmaz, E.: Structural, magnetic and optical properties of Co-diffused CdTe thin films. J. Alloy. Compd. 456(1–2), 6–9 (2008)
Yuan, H.J., Yan, X.Q., Zhang, Z.X., Liu, D.F., Zhou, Z.P., Cao, L., Wang, J.X., Gao, Y., Song, L., Liu, L.F.: Synthesis, optical, and magnetic properties of Zn1− xMnxS nanowires grown by thermal evaporation. J. Cryst. Growth 271(3–4), 403–408 (2004)
Fukumura, T., Jin, Z., Kawasaki, M., Shono, T., Hasegawa, T., Koshihara, S., Koinuma, H.: Magnetic properties of Mn-doped ZnO. Appl. Phys. Lett. 78(7), 958–960 (2001)
Bartholomew, D.U., Furdyna, J.K., Ramdas, A.K.: Interband Faraday rotation in diluted magnetic semiconductors: Zn 1–x Mn x Te and Cd 1–x Mn x Te. Phys. Rev. B 34(10), 6943 (1986)
Hwang, Y.H., Um, Y.H., Furdyna, J.K.: Temperature dependence of the band-edge photoluminescence of Zn1− x Mn x Se films. Semicond. Sci. Technol. 19(5), 565 (2004)
Islam, M.M., Ishizuka, S., Yamada, A., Sakurai, K., Niki, S., Sakurai, T., Akimoto, K.: CIGS solar cell with MBE-grown ZnS buffer layer. Sol. Energy Mater. Sol. Cells 93(6–7), 970–972 (2009)
Tauchi, T., Yamada, Y., Ohno, T., Mullins, J.T., Masumoto, Y.: Ultraviolet laser and photodetector of CdZnS/ZnS multiple quantum wells. Physica B 191(1–2), 136–139 (1993)
Noda, D., Hagiwara, K., Yamamoto, T., Okamoto, S.: Electron emission properties of ZnS-based thin-film cold cathode for field emission display. Jpn. J. Appl. Phys. 44(6R), 4108 (2005)
Holub, M., Chakrabarti, S., Fathpour, S., Bhattacharya, P., Lei, Y., Ghosh, S.: Mn-doped InAs self-organized diluted magnetic quantum-dot layers with Curie temperatures above 300 K. Appl. Phys. Lett. 85(6), 973–975 (2004)
Stern, R.A., Schuler, T.M., MacLaren, J.M., Ederer, D.L., Perez-Dieste, V., Himpsel, F.J.: Calculated half-metallic behavior in dilute magnetically doped ZnS. J. Appl. Phys. 95(11), 7468–7470 (2004)
Tablero, C.: Electronic and magnetic properties of ZnS doped with Cr. Phys. Rev. B 74(19), 195203 (2006)
Sambasivam, S., Joseph, D.P., Reddy, D.R., Reddy, B.K., Jayasankar, C.K.: Synthesis and characterization of thiophenol passivated Fe-doped ZnS nanoparticles. Mater. Sci. Eng., B 150(2), 125–129 (2008). https://doi.org/10.1016/j.mseb.2008.03.009
Kumar, K.C., Rao, N.M., Kaleemulla, S., Rao, G.V.: Structural, optical and magnetic properties of Sn doped ZnS nano powders prepared by solid state reaction. Physica B 522, 75–80 (2017). https://doi.org/10.1016/j.physb.2017.07.071
Kumar, K.C., Kaleemulla, S., Krishnamoorthi, C., Rao, N.M., Rao, G.V.: Evidence of Room Temperature Ferromagnetism in Zn1−xSnxS Thin Films. J. Supercond. Novel Magn. 32(6), 1725–1734 (2019). https://doi.org/10.1007/s10948-018-4868-4
Kunapalli, C.K., Shaik, K.: Room-temperature ferromagnetic Zn1−xNixS nanoparticles. Appl. Phys. A 124(5), 384 (2018). https://doi.org/10.1007/s00339-018-1811-2
Kunapalli, C.K., Chakraborty, D., Shaik, K.: Effect of vacuum annealing on structural, optical and magnetic properties of Sn doped ZnS thin films. Opt. Mater. 114, 110961 (2021). https://doi.org/10.1016/j.optmat.2021.110961
Mohamed, S.A, Ahmed. M.R, Ali, H.M and Abdel Hakeem A.M.: Structural, electrical and optical properties investigation of nano-sized Sb0.1(SnO2)0.9. Physics Scripta,97, 045810 (2022). https://doi.org/10.1088/1402-4896/ac5bc4
Tauc, J.: Amorphous and Liquid Semiconductors, 2nd edn. Plenum Press, New York, NY, USA (1974)
Kannan, S., Subiramaniyam, N.P., Sathishkumar, M.: Effect of annealing temperature and Mn doping on the structural and optical properties of ZnS thin films for enhanced photocatalytic degradation under visible light irradiation. Inorg. Chem. Commun. 119, 108068 (2020)
Sharma, R., Himanshu, Patel, S.L., Chander, S., Kannan, M.D., Dhaka, M.S.: Physical properties of ZnSe thin films: Air and vacuum annealing evolution to buffer layer applications. Physics Letters A 384(4), 126097 (2020). https://doi.org/10.1016/j.physleta.2019.126097
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kunapalli, C.K., Chakraborty, D. & Shaik, K. Structural, optical, and magnetic properties of Sn-doped ZnS thin films: role of post-annealing. J Aust Ceram Soc 58, 1105–1110 (2022). https://doi.org/10.1007/s41779-022-00786-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41779-022-00786-3