Abstract
In this study, a novel nanocomposite based on pine leaf waste and modified kaolinite was synthesized for the first time, and its efficiency for cadmium and lead removal from aqueous solutions was evaluated. First, iron oxide magnetic nanoparticles were synthesized in the medium of pine leaf waste, and then, the magnetic pine leaf waste was cross-linked by bis-acrylamide and hybridized with activated amino silane kaolinite. Parameters of pH, initial concentration of cadmium and lead, adsorbent dose, and contact time were optimized by using the design of experiments. Maximum adsorption capacity for lead was achieved at pH = 5.5, adsorbent dose = 0.04 g, initial concentration of 80 ppm, and time of 30 min, and for cadmium at pH = 5, adsorbent dose of 0.1 g, initial concentration of 120 ppm, and time of 50 min. The maximum removal percentage was 99.42 and 99.32 for cadmium and lead, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal pollution has posed a serious problem due to its non-biodegradability, bioaccumulation, and high toxicity [1]. Industrial activities such as extraction, metalworking, printing, dye industry, oil purification, petrochemical industry, and fertilizer and pesticide manufacturing are the prominent sources of heavy metal pollution [2, 3]. Cadmium (Cd) is a heavy metal with high toxicity, even at low concentrations [4, 5]. Exposure to this metal may cause renal disorders, lung cancer, and bone lesions. According to the World Health Organization (WHO), the allowable cadmium level for drinking water is < 5 µg/L [6]. Lead (Pb) is another hazardous heavy metal that may seriously harm the central nervous system, the kidneys, liver, and brain stem cells in addition to disrupting brain functions. This element can lead to renal tumors and different cancers. Regarding its high bonding tendency to red blood cells (RBCs), Pb can result in anomia. Therefore, it is essential to propose reliable, efficient, and cost-effective methods for the removal of cadmium and lead [7].
Several methods have been developed for extracting heavy metals from aqueous solutions (e.g., solvent extraction, coagulation, reverse osmosis, chemical sedimentation, and ion exchange) although some of them are costly, inefficient, or pollutant [8,9,10,11]. Absorption is a reliable and practical method for heavy metal elimination due to its high capacity, low cost, and reusability. This approach has found extensive applications using diverse adsorbents such as agricultural waste, natural polymers like chitosan and chitin, active carbon, carbon nanotubes, aluminosilicate, MCM41, and clay [12,13,14,15]. Nanotechnology has revolutionized water treatment approaches. Thanks to this technology, the costs of water treatment have been considerably reduced [16]. Magnetite is one type of iron oxide with super-paramagnetic properties in nanodimensions in addition to high surface-to-volume ratio, and biocompatibility. Owing to their unique properties, magnetite nanoparticles (NPs) have been widely used in the design of catalysts for organic reactions, adsorbents for water and wastewater treatment, imaging tumor tissues, and targeted drug delivery [17, 18]. Among nanocomposites, the highest attention has been paid to polymer-based magnetic nanocomposites due to their high mechanical strength, low weight, high thermal stability, great electric conductivity, and excellent chemical strength. Organic and mineral materials are commonly used to reinforce polymers [19, 20]. Therefore, in this project, nano-Fe3O4 and clay were used to reinforce nanocomposites. Regarding the low cost, biocompatibility, and abundance of clay, extensive effort has been made to synthesize polymeric nanocomposites based on a clay matrix. A small amount of clay can significantly improve the mechanical and thermal properties of the composite, further encouraging the design and synthesis of functionalized clay that can be used in nanocomposite synthesis [21,22,23,24,25]. Kaolinite is a hydrophilic mineral with a layered structure whose layers are bound through hydrogen bonding between its numerous OH groups. Thanks to these features, kaolinite has high porosity and water absorption capacity, making it a versatile adsorbent in different industries as well as a soil modifier for agriculture and gardening [26,27,28]. Due to the shortage of raw materials in the industry and the severe reduction in renewable sources, the use of agricultural waste seems inevitable. Agricultural products, especially those containing cellulose, have exhibited great adsorption capacity for various pollutants [29]. Hemicellulose, lignin, lipids, proteins, simple sugars, water, hydrocarbons, and starch are the main components of agricultural wastes. Agricultural wastes are benefited from several salient features such as cost-effectiveness, biocompatibility, unique chemical compositions, high accessibility, and renewability, making them a promising candidate for water and wastewater treatment. Plant leaves, stems, pits, skin, and fruit have been extensively used for this purpose. The application of these wastes resolves the problem of waste disposal and prevents environmental pollution, in addition to being economical and producing value-added products [30,31,32]. Recently, the increasing demand for the production and application of biodegradable materials for industrial purposes based on environmentally friendly methods and their replacement with chemicals and their derivatives has gained the attraction of researchers and industrialists. In this context, finding a proper solution for optimal and economical use of these materials seems necessary [33]. Agricultural wastes such as wheat straw, corn, rice bran, sugarcane bagasse, branches, and leaves from the pruning of various trees are a rich source of cellulose fibers. Biodegradable cellulosic polysaccharide is non-toxic with desirable properties such as proper reinforcement, cost-effectiveness, low density, high specific surface area, the possibility of functionalization due to its active chemical groups, desirable chemical stability, and selective pollutant adsorption [34, 35]. Some recently developed adsorbents will be reviewed in the following:
Zayed et al. modified the carboxymethyl-β-cyclodextrin with Fe3O4 nanoparticles for removing Ni2+, Cd2+, and Pb2+ [36]. Nazarzadeh et al. synthesized magnetic nanocomposite of poly(aniline-co-m-phenylenediamine)@Fe3O4 and for removing Pb(II), Co(II), and Cd(II) ions from aqueous solutions [37]. Fe3O4/talc nanocomposite was employed by Kalantari et al. for extracting Pb(II), Ni(II), and Cu(II) ions from aqueous solutions [38]. Wang et al. successfully synthesized hybrid nanocomposites of poly (γ-glutamic acid)-Fe3O4-GO-(o-MWCNTs). After optimizing the conditions, they achieved maximum absorption capacities of 625.00, 574.71, and 384.62 mg/g for the Cd(II), Cu(II), and Ni(II), respectively [39]. Simona et al. separated lead cation by using a new polymeric membrane, polyethersulfone montmorillonite, from an aqueous medium [40]. Mittal et al. modified montmorillonite with L-cysteine by mixing it with alginate. Their adsorbent efficiently removed Cu2+, Ni2+, and Pb2+ [41]. A biological hydrogel nanocomposite was synthesized by Moharrami et al. through bonding starch to co-polymer 2-acrylamido-2-methyl propane sulfonate and acrylic acid followed by hybridization with cellulose nanocrystal obtained from sugar beet waste. The resulting nanocomposite was evaluated for removing two cationic dyes: crystal violet and methylene blue [42]. Zhang et al. employed eggshell for preparing CuFe2O4 magnetic nanocomposite. This nanocomposite had unique morphology with layered multi-channel coral-like structures, quite different from pure CuFe2O4. This nanocomposite was then used for the removal of Congo red dye [43]. Ahmed et al. synthesized the hybrid nanocomposite gum g-polyacrylonitrile (GPCM) L-methionine MMT by using radical co-polymerization from acrylonitrile monomer with guar gum biopolymer and montmorillonite modified with l-methionine and used it for removing Cu(II) and Pb(II) [44]. Struijk et al. activated the surface of kaolinite by dithia-1,8-octanediol-3,6; after stabilizing the structure, it was used for removing zinc from aqueous solutions [45]. He et al. reported the successful synthesis of carboxymethyl chitosan-kaolinite composite hydrogel with excellent mechanical properties, low cost, and absorption capacity of 206 mg/g for Cu2+ [46]. Due to the necessity of synthesizing efficient adsorbents and continue research, for the first time in this study, pine leaf waste was used to prepare the adsorbent. To increase the absorption ability, pine leaf waste was reinforced with Fe3O4 magnetic nanoparticles and cross-linked with bis-acrylamide. Then, it was hybridized with amino silane–modified kaolinite. To identify the structure of the nanocomposite, SEM, FTIR, TGA, VSM, BET, and XRD were performed. The efficiency of the nanocomposite for removing cadmium and lead cations was evaluated in different conditions in terms of pH, initial cation concentration, contact time, and adsorbent dose.
Experimental
Materials
N,N′-methylene bis-acrylamide, ferrous chloride (FeCl2·4H2O), ferric chloride (FeCl3·6H2O), lead(II) nitrate (Pb(NO3)2), cadmium nitrate (Cd(NO3)2·4H2O), NaOH, HCl (37% purity), potassium persulfate, n-hexane, (3-aminopropyl) triethoxysilane, and NH3 solution were purchased from Merck. Kaolinite was bought from Sigma-Aldrich. Deionized water was utilized for preparing solutions.
Instrumental analysis
FTIR spectra of samples in the form of KBr pellets were recorded by a Bruker tensor 27 spectrophotometer. X-ray powder diffraction (XRD) patterns were obtained by a Rigaku diffractometer, model X Pert PHILIPS using Cu-Kα radiation. Scanning electron microscopy (SEM) images were taken on VEGA// TESCAN. The dynamic weight loss tests were conducted on a NETZSCH STA 409 PC/PG thermogravimetric analyzer (TG). All tests were conducted using sample weights of 5–20 mg over a temperature range of 25–600 °C at a scan rate of 10 °C/min. The specific surface area and pore size were measured by Brunauer–Emmett–Teller (BET) method (BELSORP mini II, Japan).
Methods
Preparation of pine leaf waste
First, the leave waste of pine tree was collected and washed with water. Then, the washed leaves were dried completely and powdered with pestle.
Preparation of modified kaolinite
To activate the kaolinite, 14 g of kaolinite was added to 50 mL of n-hexane and 10.5 mL of amino silane and stirred at the ambient temperature for 120 min. Then, the resulting product was washed with a 1:1 mixture of alcohol 96% and n-hexane. Finally, once the product was dried, the activated kaolinite was prepared.
Preparation of magnetic pine leaf waste
To obtain magnetic leaf waste, first, 7.5 g of the powdered pine leaf waste was mixed with 12 mL of the FeCl2 0.5 M solution and 48 mL of the FeCl3 0.25 M solution. After a few minutes, 1 M ammonia was added to the mixture drop by drop in order for darkening the product and formation of the magnetic Fe3O4 nanoparticles. The resulting product was separated by a powerful magnet and, then, washed with water and, finally, dried.
Preparation of magnetic pine leaf waste-cl-MBA nanocomposite
Among the particles of the product obtained in the previous stage, transverse bridges were created using bis-acrylamide. For this purpose, 3 g of the magnetic pine leaf obtained in the previous stage was mixed with 1.2 g of the proxy sulfate and 1.8 g of bis-acrylamide and, then, refluxed at 90 °C for 60 min. The resulting mixture was washed with water and dried.
Preparation of magnetic pine leaf waste-cl-MBA/modified kaolinite nanocomposite
In this stage, 6 g of magnetic pine leaf waste-cl-MBA and 6 g of the modified kaolinite were added to 100 mL of deionized water and, then, refluxed at 50 °C for 4 h. The product was washed with deionized water twice and, then, dried.
Cd(II) and Pb(II) adsorption experiments
Using central composite through Design Expert 10.0 software, 60 sets of 60 experiments were designed for removing each of the Cd2+ and Pb2+ ions with different initial concentrations, pH values, adsorbent doses, and times. Considering the experimental conditions, the pH of the solution was adjusted using HCl and NaOH 0.1 M. A certain amount of the nanocomposite was added to 25 mL of the cadmium or lead solution with a certain concentration. After a certain time, the mixture was filtered and the concentration of the remaining cadmium and lead ions was obtained through atomic adsorption. The adsorption capacity of nanocomposite and removal efficiency was determined from the following equations:
where C0 and Ce are the initial and equilibrium Cd(II) concentrations (mg/L), respectively, m is the mass of nanocomposite (g), and V is the volume of the solution (L).
Determining the zero point of nanocomposite
The point of zero charge (pHpzc) of magnetic pine leaf waste-cl-MBA/modified kaolinite nanocomposite was determined by the batch equilibration technique according to literature [47]. For this purpose, 0.5 g of adsorbent was mixed with 100 mL electrolyte (NaCl, 0.01 M) and pH was adjusted using NaOH or HCl in the range between 2.0 and 12.0. After shaking for 24 h at room temperature, the solution was filtered and the final pH of the solution was determined. The pHpzc of the sample was determined by plotting the initial pH (pHinitial) vs ΔpH (pHfinal_pHinitial) as the point where ΔpH = 0.
Results and discussion
Synthesis and characterization of the nanocomposite
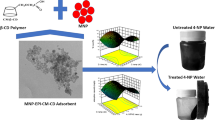
Optimal use of agricultural wastes and turning them into products with desirable properties is one of the achievements of this research. Regarding the availability and abundance of pine leaf debris, a nanocomposite comprising magnetic pine leaf waste and modified kaolinite was prepared for the elimination of cadmium and lead cations from aqueous solutions. Biosorbents from agricultural waste can be employed to improve effluent; these biosorbents offer several advantages including facile use, high compounding tendency toward heavy metals, cost-effectiveness, availability, and recovery. Chemical modification of these adsorbents can enhance their efficiency. To make the nanocomposite, magnetic nanoparticles were first synthesized in a matrix of pine leaf waste. Then, using bis-acrylamide and during radical reaction, the pine leaf waste was cross-linked, so that the functional groups suitable for heavy metal adsorption would increase and sufficient space would be provided for adsorption. The kaolinite surface was modified with amino silane and a new nanocomposite was prepared by a hybrid of magnetic pine leaf waste-cl-MBA and modified kaolinite (Fig. 1). To confirm the nanocomposite structure, various techniques were used and the spectra were interpreted.
FTIR spectroscopy
Figure 2 presents the Fourier transform–infrared (FTIR) spectra of (a) modified kaolinite, (b) magnetic pine leaf waste-cl-MBA, and (c) magnetic pine leaf waste-cl-MBA/modified kaolinite nanocomposite. Based on Fig. 2a, the characteristic peaks at 3696.44 and 3620.98/cm can be assigned to O–H stretching vibrations, while the one emerging at 3364.12/cm is due to NH stretching vibrations. C–H and Si–O stretching vibrations are manifested as peaks at 2931.76 and 1032.65/cm, respectively. All the mentioned peaks confirmed the formation of the modified kaolinite. In Fig. 2b, the wide vibration at 3439.86/cm is related to the OH groups. The OH stretching vibrations overlapped with NH vibration peaks for the as-prepared materials. Moreover, the characteristic peaks at 2935.86 and 1665.08/cm can be attributed to C–H and C = O stretching vibration, respectively. The peak at 615.50 is due to the Fe–O bond vibration in Fe3O4 nanoparticles. Concerning Fig. 2c, the characteristic peaks at 3696.48 (OH kaolinite), 3620.86 (OH kaolinite), 3431.49 (NH and OH), 2929.1 (aliphatic C–H), 1660.33 (C = O carboxylic acid), 1033.24 (Si–O), and 538.75/cm (Fe–O) confirm the successful synthesis of magnetic pine leaf waste-cl-MBA/modified kaolinite nanocomposite.
X-ray diffraction
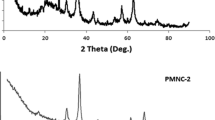
XRD patterns of the magnetic pine leaf waste-cl-MBA/modified kaolinite nanocomposite demonstrates several sharp peaks at 2θ = 18.44, 20.96, 26.88, 30.16, and 35.24° which related to Fe3O4 nanoparticles and 2θ = 12.48, 20.52, 25.08, 37.88, 55.04, and 62.52° which indicate the structure of kaolinite (Fig. 3). These results show the successful integration of Fe3O4 particles on the surface of pine leaf waste.
SEM
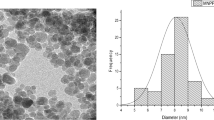
The morphology of nanocomposite was studied by using a scanning electron microscope. As shown in Fig. 4, the Fe3O4 nanoparticles are in spherical shape. The average particle size of the Fe3O4 magnetite particles is approximately 30–50 nm. The SEM micrograph illustrates the presence of Fe3O4 nanoparticles adhered to the pine leaf waste matrix.
TGA
N2 was employed as a neutral atmosphere; the test temperature ranged from 25 to 600 °C. Figure 5a indicates magnetic pine leaf waste. Two stages of weight loss can be observed in the figure. The first stage is related to moisture loss in the compound, occurring up to 100 °C, while the second stage can be attributed to the elimination of pine leaf waste. At 600 °C, 35% of the nanocomposite weight was retained. Figure 5b illustrates no weight loss other than what occurred in the previous stage which is related to the breaking of transverse bridges caused by bis-acrylamide. At 600 °C, 43% of the compound weight was retained. Figure 5c indicates the thermogram of the final nanocomposite; as seen, no weight loss occurred up to 220 °C. This compound exhibited about 73% residual weight at 600 °C, suggesting high thermal stability of the nanocomposite due to the presence of modified kaolinite (see Fig. 5).
VSM analysis
The saturation magnetization of (a) magnetic pine leaf waste, (b) magnetic pine leaf waste-cl-MBA, and (c) magnetic pine leaf waste-cl-MBA/modified kaolinite nanocomposite is found to be 6, 1.3, and 0.6 emu/g. These lower Ms value than the values for bulk magnetite particles (92–100 emu/g) can be related to the formation of the Fe3O4 nanoparticles in the pine leaf waste, their cross-link with MBA, and hybridization with modified kaolinite (Fig. 6).
BET analysis
Using the Brunauer–Emmett–Teller (BET) method, the specific surface area (SBET) was calculated. Vtot was then evaluated by converting the volume of nitrogen adsorbed at p/ps ≈ 0.99 to the volume of liquid adsorbate. Using the Barrett–Joyner–Halenda (BJH) method, the mesopore surface area SBJH and the mesopore volume VBJH were calculated. The micropore volume was calculated by the t-plot method (Table 1).
Adsorption of heavy metal ions
A statistically optimized procedure in the form of response surface methodology (RSM) was adopted to overcome the difficulties of the conventional optimization methods. In this study to determine the effect of the operating parameters on the removal efficiency of Pb(II) and Cd(II) ions (response variable), 4 influential parameters were selected: initial Pb(II) and Cd(II) ions concentration, pH, adsorbent content, and contact time. Experimental data were analyzed using the statistical software Design Expert 11.0.0 (Stat-Ease, Inc. Minneapolis, USA). The levels of variables are shown in Tables 2 and 3.
Statistical ANOVA for Pb 2+
The final empirical reduced quadratic model equation in terms of coded factors for adsorption capacity of Pb2+ using nanocomposite
The final empirical reduced quadratic model equation in terms of coded factors for the removal efficiency of Pb2+
In the above equation, A, B, C, and D represent pH, adsorbent dose, initial concentration of heavy metal, and contact time, respectively. The minus sign of terms shows an antagonistic effect on adsorption capacity while the positive sign depicts the synergistic impacts on heavy metal sequestration.
The F-value of 10.03 implies the significance of the model. P-values less than 0.0500 also indicate that the model terms are significant. In this case, A, B, C, AB, and AC are significant model terms. P-values greater than 0.1000 indicate the model terms are not significant. The lack of fit F-value of 0.77 implies the lack of fit is not significant relative to the pure error. Non-significant lack of fit is desirable. For better fitting of the model, the closer the value of R2 (here, 0.8186) to one, the better is the model. Adeq precision measures the signal-to-noise ratio. A ratio greater than 4 is desirable. The ratio of 14.234 indicates an adequate signal. This model can be used to navigate the design space (Table 4).
The model F-value of 7.05 implies the model is significant. The lack of fit F-value of 0.40 reflects the insignificance of the lack of fit relative to the pure error. R2 = 0.8327 and Adeq precision = 9.1135 confirm that this model can be used to navigate the design space (Table 5).
Statistical ANOVA for Cd 2+
The final empirical reduced cubic model equation in terms of coded factors for adsorption capacity of Cd2+ removal using nanocomposite
The model F-value of 95.57 implies the model is significant. P-values less than 0.0500 indicate model terms are significant. In this case, A, B, C, D, AD, BC, CD, A2, B2, D2, ABC, ABD, ACD, BCD, A2B, A2D, and AB2 are significant model terms. The lack of fit F-value of 2.48 reflects the insignificance of the lack of fit relative to the pure error. The R2 = 0.9936 and Adeq precision = 37.967 indicate that this model can be used to navigate the design space (Table 6).
The final empirical reduced quadratic model equation in terms of coded factors for Cd2+ removal efficiency.
The model F-value of 35.34 implies the significance of the model. In this case, A, B, D, AD, BC, CD, A2, B2, D2, ABC, ABD, ACD, BCD, A2D, AB2, and A2B2 are significant terms of the model. The lack of fit F-value of 0.43 implies that the lack of fit is not significant relative to the pure error. Non-significant lack of fit is desirable (Table 7).
The next test indicates the quality of the model (Fig. 7). In this test, the points predicted by the model are plotted versus the experimental data. In a proper model, the points resulting from this diagram would be positioned around the 45° line, indicating the proper fit of the model to the laboratory data. The diagram of the predicted values versus laboratory data indicates that the model properly predicts the data as they are placed around the 45° line.
Effects of model variables and their interactions
Effect on pH
pH is a major factor affecting the degree of adsorption. Since metal ions settle as hydroxide at pH > 9, the pH ranges of 1–7 and 1–9 were selected for Pb2+ and Cd2+, respectively. At very low pH levels, the OH and NH groups are protonated on the nanocomposite surface and lose their ability for adsorbing metal ions. Therefore, with increasing pH, the adsorption capacity and removal percentage increased.
From the pH initial vs ΔpH plot (Fig. 8), the pHpzc of was found to be ~ 5.8. At this pH value, the total positive charges on the surface of are equal to the total negative charges. In other words, the magnetic pine leaf waste-cl-MBA/modified kaolinite nanocomposite surface is positively charged when pH < pHpzc and is negatively charged when pH > pHpzc.
In addition to the electrostatic forces that cause the adsorption of cations on the adsorbent, chemical bonding can be an important factor in the adsorption of cations. In the synthetic nanocomposite structure, there are functional groups such as NH2 and OH. Some of them at a pH of about 5.5 are not protonated and can be bound to cations with their free electron pairs.
Effect on adsorbent dose
The dosage of adsorbent is another important parameter determining the adsorption capacity of nanocomposite for a specified initial concentration. An increase in the content of the adsorbent, due to the high surface area of the specimen, raised the number of active sites for metal ion complexes, thus enhancing the adsorption process. The adsorption capacity was reduced by increasing the adsorbent dose while the removal efficiency showed an increment. A reduction in the adsorption capacity of metal ions with a rise in adsorbent dose is due to the increased interference between linking sites at higher doses of adsorbent.
Effect of contact time
In adsorption processes, the adsorption capacity increases over time, followed by a decrement to achieve equilibrium as all the active sites on the adsorbent are occupied. The results show that the adsorption of cadmium ions increased over time, but lead adsorption reached maximum equilibrium in 30 min. This trend can be attributed to a large number of accessible active groups for complexing with the metal ions; adsorption centers are gradually filled over time.
Effect on the initial concentration
The final examined parameter is the initial concentration of metal cations. At low initial concentrations of metal ions, they were adsorbed by specific sites. By enhancing the initial concentration of metal, these specific sites became saturated and could not adsorb more ions. The ratio of the initial number of metal ions to the number of available adsorption sites was increased, suggesting a decline in the adsorption yield. However, the adsorption capacity rose by increasing the concentration of metals.
Figure 9 displays the changes affecting the system at one point of the test space. The effects of pH and initial cation concentration on lead adsorption capacity exhibited a rising trend, while increasing the adsorbent dose reduced the adsorption capacity and time had no prominent effect. Increasing pH and adsorbent dose and reducing initial concentration incremented the lead removal percentage. pH, initial concentration, and time had a rising trend on the cadmium adsorption capacity, but increasing the adsorbent dose acted quite the opposite and reduced the adsorption capacity. However, incrementing pH, adsorbent dose, and time as well as reducing the initial concentration of cadmium raised the removal percentage.
The contour lines express the important interaction between the variables and the heavy metals adsorption efficiency of the nanocomposite. The adsorption capacity was increased by a simultaneous increase in the initial concentration of lead and reduction in the adsorbent dose (Fig. 10a), an increase in pH and a reduction in adsorbent dose (Fig. 10b), and an increase in pH and initial concentration (Fig. 10c). Based on Fig. 11a, with increasing the adsorbent dose, the lead adsorption percentage first increased followed by a decrease. pH increment also increased the adsorption. Figure 11b shows no significant effect of time on removal percentage. According to Fig. 11c, by increasing pH and reducing the initial concentration, the lead removal percentage grew. As depicted in Fig. 12a, a rise in the initial concentration of cadmium and pH to 5 enhanced the adsorption capacity followed by a gradual decrease. Figure 12 b shows that prolonging the time to 70 min enhanced the adsorption capacity. An increase in adsorption capacity can be observed in Fig. 12c by incrementing the initial Cd2+ concentration and reducing the adsorbent dose. Figure 12d illustrates the simultaneous effect of initial concentration and contact time while Fig. 12e shows the simultaneous effect of pH and contact time. Figure 13a-f display the simultaneous influences of pH and adsorbent dose, pH and Cd2+ initial concentration, pH and contact time, Cd2+ initial concentration and adsorbent dose, adsorbent dose and time, and Cd2+ initial concentration and time on Cd2+ removal. Overall, by increasing pH and time, the cadmium removal percentage showed an increase. A rise in adsorbent dose first increased the adsorption followed by a decline. Increasing the initial concentration of the metal has no prominent effect on the removal percentage.
The comparison of the synthesized adsorbent in this study is compared with other adsorbents to evaluate the feasibility of the adsorbent. It is evident that magnetic pine leaf waste-cl-MBA/modified kaolinite nanocomposite has the highest adsorption capacity among all the adsorbents listed in Table 8.
Conclusions
Due to the importance of water treatment, especially heavy metal removal, and the application of nanocomposites for this purpose, a novel nanocomposite based on pine leaf waste and clay modified with amino silane was prepared for the first time. To facilitate composite exit from the aqueous medium, iron oxide magnetic nanoparticles were synthesized on a matrix of pine leaf waste. To promote efficiency, the magnified pine leaf waste was cross-linked to bis-acrylamide. After confirming the structure using TGA, XRD, FTIR, VSM, and SEM techniques, its application for removing cadmium and lead ions was evaluated. Heavy metal adsorption on the adsorbent occurs due to the inverse charges between the adsorbent and the heavy metal ions. The hydroxyl and amide groups on the composite surface can adsorb heavy metal ions. This is managed by the solution basicity. In the media with a high degree of acidity, removal efficiency declines. The lower the acidity of the medium, the higher the negative charges on the surface would be. The results revealed that the adsorption of heavy metals on the active sites of the nanocomposite is increased by increasing solution pH. Maximum adsorption capacity for lead was achieved at pH = 5.5, adsorbent dose = 0.04 g, initial concentration = 80 ppm, and time of 30 min and for cadmium at pH = 5, adsorbent dose = 0.1 g, initial concentration = 120 ppm, and time of 50 min. The reduction in adsorption by increasing adsorbent dose was due to the overlap between the active sites of the nanocomposite, which decreased the surface accessible for adsorption. The adsorption capacity increased by increasing concentration, while the passage of time had a slight effect on it. The cadmium removal percentage increased by reducing initial concentration, increasing pH, adsorbent dose, and time, but lead removal percentage did not greatly change by increasing time.
References
Nasiri, R., Arsalani, N., Panahian, Y.: One-pot synthesis of novel magnetic three-dimensional graphene/chitosan/nickel ferrite nanocomposite for lead ions removal from aqueous solution: RSM modelling design. J. Clean. Prod. 201, 507–515 (2018)
Chen, M., Qin, X., Zeng, G., Li, J.: Impacts of human activity modes and climate on heavy metal “spread” in groundwater are biased. Chemosphere 152, 439–445 (2016)
Ma, J., Qin, G., Zhang, Y., Sun, J., Wang, S., Jiang, L.: Heavy metal removal from aqueous solutions by calcium silicate powder from waste coal fly-ash. J. Clean. Prod. 182, 776–782 (2018)
Waalkes, M.P.: Cadmium carcinogenesis in review. J. Inorg. Biochem. 79, 241–244 (2000)
Jiang, H.Y., Zhao, Q.X., Zeng, Y.: Removal of Cd(II) and Pb(II) from aqueous solutions by modified polyvinyl alcohol. Desalin. Water Treat. 57, 6452–6462 (2016)
Jiang, L., Ye, Q., Chen, J., Chen, Z., Gu, Y.: Preparation of magnetically recoverable bentonite–Fe3O4–MnO2 composite particles for Cd(II) removal from aqueous solutions. J. Colloid. Interf. Sci. 513, 748–759 (2018)
Zhang, X., Du, T., Jia, H.: Efficient activation of coal fly ash for silica and alumina leaches and the dependence of Pb(II) removal capacity on the crystallization conditions of Al-MCM-41. Int. J. Mol. Sci. 22, 6540 (2021)
Filice, S., D’Angelo, D., Libertino, S., Nicotera, I., Kosma, V., Privitera, V., Scalese, S.: Graphene oxide and titania hybrid Nafion membranes for efficient removal of methyl orange dye from water. Carbon 82, 489–499 (2015)
Lam, B., D_eon, S., Morin-Crini, N., Crini, G., Fievet, P.: Polymer-enhanced ultrafiltration for heavy metal removal: influence of chitosan and carboxymethyl cellulose on filtration performances. J. Clean. Prod. 171, 927–933 (2018)
Li, J., Wang, X., Zhao, G., Chen, C., Chai, Z., Alsaedi, A., Hayat, T., Wang, X.: Metaleorganic framework-based materials: superior adsorbents for the capture of toxic and radioactive metal ions. Chem. Soc. Rev. 47(7), 2322–2356 (2018)
Panahian, Y., Arsalani, N., Nasiri, R.: Enhanced photo and sono-photo degradation of crystal violet dye in aqueous solution by 3D flower like F-TiO2 (B)/fullerene under visible light. J. Photochem. Photobiol. Chem. 365, 45–51 (2018)
Nasseh, N., Barikbin, B., Taghavi, L., Nasseri, M.A.: Adsorption of metronidazole antibiotic using a new magnetic nanocomposite from simulated wastewater (isotherm, kinetic and thermodynamic studies). Compos. Part B Eng. 159, 146–156 (2019)
Siyal, A.A., Shamsuddin, M.R., Khan, M.I., Rabat, N.E., Zulfiqar, M., Man, Z., Siame, J., Azizli, K.A.: A review on geopolymers as emerging materials for the adsorption of heavy metals and dyes. J. Environ. Manage. 224, 327–339 (2018)
Gupta, V.K., Nayak, A., Agarwal, S., Tyagi, I.: Potential of activated carbon from waste rubber tire for the adsorption of phenolics: effect of pre-treatment conditions. J. Colloid. Interf. Sci. 417, 420–430 (2014)
Ghorbanzade Zaferani, S.P., Sarmasti Emami, M.R., Kiannejad Amiri, M., Binaeian, E.: Optimization of the removal Pb (II) and its Gibbs free energy by thiosemicarbazide modified chitosan using RSM and ANN modeling. Int. J. Biol. Macromol. 139, 307–319 (2019)
Mahmoud, M.E., Nabil, G.M., Zaki, M.M., Saleh, M.M.: Starch functionalization of iron oxide by-product fromsteel industry as a sustainable low cost nanocomposite for removal of divalent toxic metal ions from water. Int. J. Biol. Macromol. 137, 455–468 (2019)
Oroujeni, M., Kaboudin, B., Xia, W., Jönsson, P., Ossipov, D.A.: Conjugation of cyclodextrin to magnetic Fe3O4 nanoparticles via polydopamine coating for drug delivery. Prog. Org. Coat. 114, 154–161 (2018)
Cao, H., He, J., Deng, L., Gao, X.: Fabrication of cyclodextrin-functionalized superparamagnetic Fe3O4/amino-silane core–shell nanoparticles via layer-by-layer method. Appl. Surf. Sci. 255, 7974–7980 (2009)
Jazzar, A., Alamri, H., Malajati, Y., Mahfouz, R., Bouhrara, M., Fihri, A.: Recent advances in the synthesis and applications of magnetic polymer nanocomposites. J. Ind. Eng. Chem. 99, 1–18 (2021)
Khodakarami, M., Bagheri, M.: Recent advances in synthesis and application of polymer nanocomposites for water and wastewater treatment. J. Clean. Prod. 296, 126404 (2021)
Ghorbel-Abid, I., Trabelsi-Ayadi, M.: Competitive adsorption of heavy metals on local landfill clay. Arab. J. Chem. 8, 25–31 (2015)
Khalil, N., Charef, A., Khiari, N., Gomez Pérez, C.P., Andolsi, M., Hjiri, B.: Influence of thermal and marine water and time of interaction processes on the Cu, Zn, Mn, Pb, Cd and Ni adsorption and mobility of silty-clay peloid. Appl. Clay. Sci. 162, 403–408 (2018)
Kumar, K.Y., Raj, T.N.V., Archana, S., Prasad, S.B.B., Olivera, S.: Muralidhara HB SnO2 nanoparticles as effective adsorbents for the removal of cadmium and lead from aqueous solution: adsorption mechanism and kinetic studies. J. Water. Process. Eng. 13, 44–52 (2016)
Pawar, R.R., Lalhmunsiama, Bajaj, H.C., Lee, S.M., Activated bentonite as a low-cost adsorbent for the removal of Cu(II) and Pb(II) from aqueous solutions: batch and column studies. J. Ind. Eng. Chem. 34, 213–223 (2016)
Wang, J., Sun, T., Saleem, A., Chen, Y.: Enhanced adsorptive removal of Cr(VI) in aqueous solution by polyethyleneimine modified palygorskite. Chin. J. Chem. Eng. 28, 2650–2657 (2020)
Fida, H., Guo, Sh., Zhang, G.: Preparation and characterization of bifunctional Ti–Fe kaolinite composite for Cr(VI) removal. J. Colloid. Interf. Sci. 442, 30–38 (2015)
Carretero, M.I.: Clay minerals and their beneficial effects upon human health. A review. Appl. Clay. Sci. 21, 155–163 (2002)
Wang, Z., Zheng, W., Zhang, Z., Chen, L., Zhang, Z., Li, Y., Ma, N., Du, P.: Formation of a kaolinite-serine intercalation compound via exchange of the pre-intercalated transition molecules in kaolinite with serine. Appl. Clay. Sci. 135, 378–385 (2017)
Dayanidhi, K., Vadivel, P., Jothi, Sh., Sheik, Eusuff, N.: Facile synthesis of silver@eggshell nanocomposite: a heterogeneous catalyst for the removal of heavy metal ions, toxic dyes and microbial contaminants from water. J. Environ. Manage. 271, 110962 (2020).
Farirzadeh, I., Samani, M.R., Toghraie, D.: Lead removal from aqueous medium using fruit peels and polyaniline composites in aqueous and non-aqueous solvents in the presence of polyethylene glycol. Chin. J. Chem. Eng. in press (2020).
Tokay, B., Akpınar, I.: A comparative study of heavy metals removal using agricultural waste biosorbents. Bioresour. Technol. Rep. 15, 100719 (2021)
Nguyen, T.A.H., Ngo, H.H., Guo, W.S., Zhang, J., Liang, S., Yue, Q.Y., Li, Q., Nguyen, T.V.: Applicability of agricultural waste and by-products for adsorptive removal of heavy metals from wastewater. Bioresour. Technol. 148, 574–585 (2013)
Gisi, S.D., Lofrano, G., Grassi, M., Notarnicol, M.: Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: a review. Sustain. Mater. Techno. 9, 10–40 (2016)
Singh, N.B., Nagpal, G., Agrawal, S.: Rachna,: Water purification by using adsorbents: a review. Environ. Technol. Innov. 11, 187–240 (2018)
Oladoye, P.O.: Natural, low-cost adsorbents for toxic Pb(II) ion sequestration from (waste) water: a state-of-the-art review. Chemosphere 287, 132130 (2022)
Badruddoza, A.Z.M., Shawon, Z.B.Z., Daniel, T.W.J., Hidajat, K., ShahabUddin, M.: Fe3O4/cyclodextrin polymer nanocomposites for selective heavy metals removal from industrial wastewater. Carbohydr. Polym. 91, 322–332 (2013)
Nazarzadeh Zare, E., Motahari, A., Sillanpää, M.: Nanoadsorbents based on conducting polymer nanocomposites with main focus on polyaniline and its derivatives for removal of heavy metal ions/dyes: a review. Environ. Res. 162, 173–195 (2018)
Kalantari, K., Ahmad, M.B., Fard Masoumi, H.R., Shameli, K., Basri, M., Khandanlou, R.: Rapid adsorption of heavy metals by Fe3O4/talc nanocomposite and optimization study using response surface methodology. Int. J. Mol. Sci. 15(7), 12913–12927 (2014)
Wang, L., Hu, D., Kong, X.K., Liu, J., Li, X., Zhou, K., Zhao, H., Zhou, Ch.: Anionic polypeptide poly(γ-glutamic acid)-functionalized magnetic Fe3O4-GO-(o-MWCNTs) hybrid nanocomposite for high-efficiency removal of Cd(II), Cu(II) and Ni(II) heavy metal ions. Chem. Eng. J. 346, 38–49 (2018)
Simona, C., Raluca, I., Anita-Laura, Andrei, S., Raluca, S., Bogdan, T., Elvira, A., Catalin-Ilie, S., Claudiu, F.R., Daniela, IE., Silviu, P., Leonard-Ionut, A., Dan, D.: Synthesis, characterization and efficiency of new organically modified montmorillonite polyethersulfone membranes for removal of zinc ions from waste wasters. Appl. Clay. Sci. 137, 135–142 (2017).
Mittal, A., Ahmad, R., Hasan, I.: Biosorption of Pb2+, Ni2+ and Cu2+ ions from aqueous solutions by L-cystein-modified montmorillonite-immobilized alginate nanocomposite. Desalin. Water. Treat. 57, 17790–17807 (2016)
Moharrami, P., Motamedi, E.: Application of cellulose nanocrystals prepared from agricultural wastes for synthesis of starch-based hydrogel nanocomposites: efficient and selective nanoadsorbent for removal of cationic dyes from water. Bioresour. Technol. 313, 123661 (2020)
Zhang, Y., Chen, Y., Kang, Z.W., Gao, X., Zeng, X., Liu, M., Yang, D.P.: Waste eggshell membrane-assisted synthesis of magnetic CuFe2O4 nanomaterials with multifunctional properties (adsorptive, catalytic, antibacterial) for water remediation. Colloids Surf. A: Physicochem. Eng. Asp. 612, 125874 (2021)
Ahmad, R., Hasan, I.: l-methionine montmorillonite encapsulated guar gum-g-polyacrylonitrile copolymer hybrid nanocomposite for removal of heavy metals. Groundw. Sustain. Dev. 5, 75–84 (2017)
Struijk, M., Rocha, F., Detellier, Ch.: Novel thio-kaolinite nanohybrid materials and their application as heavy metal adsorbents in wastewater. Appl. Clay. Sci. 150, 192–201 (2017)
He, G., Wang, Ch., Cao, J., Fan, L., Zhao, S., Chai, Y.: Carboxymethyl chitosan-kaolinite composite hydrogel for efficient copper ions trapping. J. Environ. Chem. Eng. 7, 102953 (2019)
Lazarević, S., Janković-Častvan, I., Jovanović, D., Milonjić, S. Janaćković D, Petrović R.: Appl. Clay Sci. 37, 47–57 (2007).
Unuabonah, E.I., Adebowale, K.O., Olu-Owolabi, B.I., Yang, L.Z.: Comparison of sorption of Pb2+ and Cd2+ on kaolinite clay and polyvinyl alcohol-modified kaolinite clay. Adsorption 14, 791–803 (2008)
Kim, K.H., Keller, A.A., Yang, J.K.: Removal of heavy metals from aqueous solution using novel composite of recycled materials. Colloids Surf. A: Physicochem. Eng. Asp. 425, 6–14 (2013)
Bhattacharyya, K.G., Gupta, S.S.: Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: a review. Adv. Colloid Interface Sci. 140, 114–131 (2008)
Lan, S., Leng, Z., Guo, N., Wu, X., Gan, S.: Sesbania gum-based magnetic carbonaceous nanocomposites: facile fabrication and adsorption behavior. Colloids Surf. A: Physicochem. Eng. Asp. 446, 163–171 (2014)
Ahmad, R., Hasan, I.: L-methionine montmorillonite encapsulated guar gum-g- polyacrylonitrile copolymer hybrid nanocomposite for removal of heavy metals. Groundw. Sustain. Dev. 5, 75–84 (2017)
Yan, L., Li, S., Yu, H., Shan, R., Du, B., Liu, T.: Facile solvothermal synthesis of Fe3O4/bentonite for efficient removal of heavy metals from aqueous solution. Powder Technol. 301, 632–640 (2016)
Unuabonah, E.I. Taubert. A.: 2014 Clay–polymer nanocomposites (CPNs): adsorbents of the future for water treatment. Appl. Clay. Sci. 99, 83–92
Kumar, U., Bandyopadhyay, M.: Sorption of cadmium from aqueous solution using pretreated rice husk. Bioresour. Technol. 97, 104–109 (2006)
Akinyeye, J., Ibigbami, T.B., Odeja, O.O., Sosanolu, O.M.: Evaluation of kinetics and equilibrium studies of biosorption potentials of bamboo stem biomass for removal of lead (II) and cadmium (II) ions from aqueous solution. Afr. J. Pure Appl. Chem. 14, 24–41 (2020)
Ofomaja, A.E., Naidoo, E.B., Modise, S.J.: Biosorption of Cu(II) and Pb(II) on to potassium hydroxide treated pine cone powder. J. Environ. Manage. 91, 1674–1685 (2010)
Çelebi, H., Gök, G., Gök, O.: Adsorption capability of brewed tea waste in waters containing toxic lead (II), cadmium (II), nickel (II), and zinc (II) heavy metal ions, Sci. Rep. Ii 1–12 (2020).
Acknowledgements
The authors thank the Higher Education Complex of Zarand and Shahid Bahonar University of Kerman for supporting this investigation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qhalandari, M.A., Fozooni, S. & Darezereshki, E. Magnetic pine leaf waste-cl-MBA/modified kaolinite nanocomposite: synthesis, characterization, and optimization by response surface methodology for Pb2+ and Cd2+ ion adsorption. J Aust Ceram Soc 58, 705–724 (2022). https://doi.org/10.1007/s41779-022-00725-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41779-022-00725-2