Abstract
The synthesis and performance of La0.7Sr0.3VO3/YSZ composites are investigated as alternative anodes for the direct utilization of methane (i.e., biogas) in solid oxide fuel cells. In this study, the structural stability between Sr-doped lanthanum vanadate and yttria-stabilized zirconia was investigated by using the powder mixture annealed at various periods of time and temperatures. The chemical reaction between these two materials in the temperature ranging from 1100 to 1400 °C was examined by X-ray diffraction (XRD) and electron probe microanalysis (EPMA) analyses. According to the examination of La0.7Sr0.3VO3/YSZ powder mixture after heat treatment at 1100–1400 °C, no second phase was detected when the La0.7Sr0.3VO3/YSZ powder mixture was heated at 1100 °C. The reaction products of perovskite SrZrO3 were formed when the specimens were heated treatment at over 1200 °C. Owing to the further diffusion of Sr cations from La0.7Sr0.3VO3 toward the reaction layer/YSZ interface via the reaction layer, the reaction layer was extended into the YSZ. The interfacial reaction behavior between electrode and electrolyte pellets of the reaction couple was examined by EPMA. No reaction products were observed as La0.7Sr0.3VO3/YSZ composite co-fired at 1100 °C. The reaction products of perovskite SrZrO3 were formed when the specimens were heat treated at over 1200 °C. The bonding energy between La-O (188 kcal/mol) is stronger than Sr-O (83.6 kcal/mol). Thus, Sr ions tend to migrate and react with YSZ much faster than La ions. Furthermore, when the Sr concentration increases to 70%, excess of SrZrO3 formation leads to the phase decomposition of perovskite La0.3Sr0.7VO3.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The solid oxide fuel cell (SOFC) using yttria-stabilized zirconia (YSZ) as an electrolyte is being considered as a nearly perfect clean energy technology [1]. In order to provide sufficient mobility of oxygen ions, a high operating temperature of around 1000 °C is necessary. Recently, due to the development of a thin film electrolyte, the intermediate temperature SOFCs (IT-SOFC) operating at a lower temperature of 600–800 °C have been demonstrated [2, 3]. Owing to the reduction of operating temperature, use of anode materials with higher electrocatalysis becomes one of the major approaches to enhance the IT-SOFC performance.
However, it has not been very successful to use carbon-containing fuel (methane) for IT-SOFC. The main reason is the deposition of carbon when Ni is used as the anode. After carbon is deposited on Ni, the effective area of Ni is significantly reduced. In addition, the deposited carbon tends to block the open channel for gas transport as well.
Recently, perovskite oxides have been studied as novel anode materials because they are less prone to the problem of coking. Generally, Sr-doped lanthanum-based perovskite oxide, La1−xSrxMO3 (M = Ti, V, Nb, Mo, W etc.) is the common formula. After Sr2+ doping, trivalent La3+ lattice site is replaced by divalent ion in the perovskite structure. Due to charge-compensation effect, positive-charge defects such as electron holes (h•) and oxygen vacancies (VO••) may be created [4,5,6]. In the case of La1−xSrxMO3, the induced electron holes may associate with vanadium ions changing V+3 to V+4.
When La1−xSrxVO3 (LSV) is used as an SOFC anode operated at 800 °C [7,8,9,10,11,12], the local high-temperature areas on the YSZ/LSV interface may be higher than 800 °C, which causes material problems including thermal stress at the electrode/electrolyte interface, interdiffusion between cell components, and chemical reaction between electrode and electrolyte materials.
In the SOFC-related literature, it was found that the reactions between perovskite cathode and YSZ electrolyte have been reported. Most of the results were obtained based the XRD analyses. For instance, the diffusion of transition metal ions from perovskite oxide to YSZ plays an important role in the reaction between electrode and electrolyte materials. The diffusion of Mn causes the phase decomposition of Sr-doped lanthanum manganese oxide (LSM) and the formation of La2O3. The formation of La2O3 will further react against YSZ [13, 14]. The reaction between anode and YSZ, on the other hand, has not been extensively studied. The reason is because the state-of-the-art anode Ni has very limited reaction with YSZ electrolyte. However, the LSV anode used in this study showing perovskite structure may exhibit reaction and diffusion against YSZ electrolyte. In addition to X-ray diffraction (XRD) analyses, detailed interdiffusion based on X-ray electron probe microanalyzer (EPMA) analysis from diffusion couples was conducted. Finally, a reaction mechanism is proposed.
Experimental
Sample preparation
The La0.7Sr0.3VO3 powder material was synthesized by a conventional solid-state reaction. After an appropriate amount of La2O3 (Alfa, USA, 99.99%), SrCO3 (Aldrich, Italy, 99.9%), and V2O5 (Alfa, USA, 98%) was mixed and ball-milled in an ethanol solution for 24 h, the powder mixture was then dried and synthesized at 1100 °C for 24 h in reducing atmosphere (80%Ar:20%H2). The YSZ powder with 8 mol% Y2O3 was supplied by Tosoh (Tokyo, Japan).
La0.7Sr0.3VO3/YSZ powder mixture with 1:1 ratio was homogeneously mixed by wet ball-milling for 12 h. The powder mixture was compressed into pellets with 15 mm in diameter by cold isostatically pressing at 100 MPa to increase contact between La0.7Sr0.3VO3 and YSZ powders. The powder mixtures were heat treated at 1100–1400 °C for 24 h in reducing atmosphere (80%Ar: 20%H2).
La0.7Sr0.3VO3/YSZ diffusion couple was prepared by La0.7Sr0.3VO3 pellet (1 mm in thickness and 15 mm in diameter) and YSZ slurry. The YSZ slurry was coated on La0.7Sr0.3VO3 pellets with the polished surfaced facing each other to form a diffusion couple. A small strip of platinum was coated on the surface of the La0.7Sr0.3VO3 pellet as a marker for the diffusion reaction. The diffusion couple was heat treated at 1400 °C for 24 h in reducing atmosphere (80%Ar:20%H2) with pressure.
XRD analyses
The crystal structures of La0.7Sr0.3VO3 and YSZ powders as well as the compacted La0.7Sr0.3VO3/YSZ powder mixtures after heat treatment were analyzed by XRD (Shimadzu X-ray diffraction System: model XD-D1, Japan) at room temperature with CuKα1 radiation. The XRD traces were obtained at a scanning rate of 2°/min, covering a 2θ range from 20 to 80°.
EPMA analyses
For the interface investigation, the heat-treated La0.7Sr0.3VO3/YSZ diffusion couple was cut, ground, and polished using the standard metallographic technique. Quantitative chemical analyses of the interfacial reaction zone were performed using wavelength-dispersive X-ray (WDX) analysis by an EPMA (High Resolution Hyper Probe JXA-8500F).
Results and discussion
Reaction of La0.7Sr0.3VO3 /YSZ powder mixture
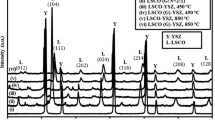
The structural stability of La0.7Sr0.3VO3 against YSZ at operating temperature plays an important role in the performance of SOFCs. To identify this, the La0.7Sr0.3VO3/YSZ powder mixture was co-fired at 1100 to 1400 °C for 24 h in reducing atmosphere (20%H2). The XRD patterns of the specimens after heat treatment are shown in Fig. 1. Based on the XRD results, no reaction products were observed when the specimen was heat treated at 1100 °C. However, the impurity phase was formed at temperatures higher than 1200 °C.
Typically, perovskite structure oxides containing La+3 tend to form a pyrochlore structure La2Zr2O7 (LZ) at interface between YSZ electrolyte in systems such as La1-xSrxMnO3/YSZ and La1-xSrxCr1-yMnyO3/YSZ. However, in this study, the LZ was not detected at the temperature range between 1100 and1400 °C.
Based on these results, the formation of this reaction product SZ in La0.7Sr0.3VO3/YSZ composite by heat treatment may be represented by the following simplified reaction (1) (yttrium ions in the zirconia has been neglected for simplicity):
Under certain conditions, highly electrically resistive phases SrZrO3 are formed at the La0.7Sr0.3VO3−YSZ interface, leading to degradation of the cell performance.
Figure 2 shows the XRD pattern with different concentration of Sr ions in La1-xSrxVO3 (x = 0, 0.5, and 0.7). It is very clearly to see no pyrochlore structure LZ product was detected even LaVO3 (X = 0) /YSZ composite which was heat treated at 1400 °C for 24 h in reducing atmosphere (20%H2). Due to vanadium ion exhibiting an average valence close to V3+ in La0.7Sr0.3VO3, the bond-dissociation energy between La-O (798 kJ/mol) is stronger than Sr-O (426±6.3 kJ/mol). SrZrO3 were formed when La0.7Sr0.3VO3 react with ZrO2. Although the XRD analysis presented in Fig. 1 is only semi-quantitative, it reflects a significant increase in the relative amount of SZ, and a decrease in the perovskite phase when the specimen was heated treatment from 1100 to 1200 °C. With increasing amount of strontium doping up to 70% (x = 0.7), the intensity of SrZrO3 becomes stronger and VO2 phase was obtained. The formation of the segregated of VO2 can be explained by the phase decomposition of perovskite La0.3Sr0.7VO3 because of the formation of SZ accompanying the consumption of Sr in La0.3Sr0.7VO3. It had been investigated that perovskite La0.3Sr0.7VO3 was synthesized by an appropriate amount of strontium addition. Therefore, when an excess amount of strontium reacted against YSZ and formed SZ (Eq. (2)), the structural stability of perovskite La0.3Sr0.7VO3 was degraded due to the loss of strontium. Consequently, the perovskite La0.3Sr0.7VO3 was decomposed to LaVO3 and VO2. It is represented by the following simplified reaction (yttria in the zirconia has been neglected for simplicity).
Figure 3 shows the EDS micrographics and the La, Sr, V, Y, and Zr element distributions of the compressed La0.7Sr0.3VO3/YSZ powder mixture after heat treatment at 1400 °C for 24 h. As shown in the EDS image, it is very clear to see Sr ions were diffused to YSZ zone at 1400 °C.
EPMA analyses of La0.7Sr0.3VO3/YSZ diffusion couple
Owing to the requirement of defect-free specimen for the EMPA analyses, the La0.7Sr0.3VO3/YSZ diffusion couple was heat treated under pressure. The elemental distribution on the cross section of the specimen was revealed by X-ray mapping. After being heated at 1400 °C for 24 h, the elemental distribution and the back-scattering image of the cross section of the La0.7Sr0.3VO3/YSZ diffusion couple are shown in Fig. 4. The chemical compositions of these regions are shown in Table 1. Figure 5 shows the back-scattering image of the La0.7Sr0.3VO3/YSZ interface with the Pt markers. Based on the EDS analysis, the bright spots marked by arrows were found to be Pt markers. There is only one reaction layer (zone B) between the original La0.7Sr0.3VO3 and YSZ interface.
Figure 5 illustrates the schematic diagram showing reaction zones labeled in Fig. 4 microstructure image and the diffusion pass way of the Sr2+ and Zr4+ ions.
An SZ layer was formed, which was indicated as the B layer, due to the interdiffusion of Sr2+ and Zr4+ ions. This result is consistent with the XRD analyses of the La0.7Sr0.3VO3/YSZ mixture.
According to the XRD and EPMA analyses, the reactants presented in zone B are marked. Combining the EPMA and XRD results, the following reactions and diffusion behaviors are proposed. It can be reasoned that the Y3+ ions diffused to the La0.7Sr0.3VO3 and La0.7Sr0.3VO3/YSZ interface (Fig. 4(Y)); however, the low concentration of Y3+ ions in zone C cannot be detected in Table 1. La3+ ions were not diffused into the YSZ lattice and La0.7Sr0.3VO3/YSZ interface (Fig. 4(La)), but the diffusion of Sr2+ ions into the YSZ and La0.7Sr0.3VO3/YSZ interface was significant. Based on these results, the diffusion pass ways of these cations are also illustrated in Fig. 5. The bonding energy between La-O (188 kcal/mol) is stronger than Sr-O (83.6 kcal/mol). So the La3+ ions diffused to YSZ lattice are limited. As a result, the reaction behavior between La0.7Sr0.3VO3 and YSZ is much different from that of cathode material LSM and YSZ. The mechanism of reaction between LSM and YSZ had been widely investigated [15,16,17,18,19]. When LSM/YSZ powder mixture or a diffusion couple was heated at 1200 °C for more than 120 h, the reaction products of LZ or SZ were formed. It is believed that the reaction between LSM and YSZ is caused by the diffusion of Mn ions into the YSZ lattice [15]. Because of the Mn diffusion, the La2O3 (SrO) was separated from LSM and then reacted with YSZ to form La2Zr2O7 and/or SrZrO3.
In the case of the reaction between La0.7Sr0.3VO3 and YSZ, the concentration profiles in the reaction zone are shown in Fig.6. The concentrations of La and V in the YSZ phase were almost zero. Also, the concentrations of Zr and Y in the La0.7Sr0.3VO3 phase are almost zero. However, the concentration changes of these cations in the reaction layers were obvious. This phenomenon elucidates that the cations diffused and reacted at the La0.7Sr0.3VO3/YSZ interface instead of diffusing deeply into the opposite material. Furthermore, Sr ion diffused from the La0.7Sr0.3VO3 phase into the YSZ via reaction layers. The difference in reaction behaviors between LSM/YSZ and La0.7Sr0.3VO3/YSZ may be due to the material’s nature. It is known that the LSM is a poor ionic conductor while La0.7Sr0.3VO3 is an electrical conductor. According to Taimatsu et al.’s [15] study, in order to maintain local charge neutrality in the material, oxygen ions or electrons must migrate together with the cations. However, YSZ is a predominant oxygen-ion conductor, and the electrical conductivity of YSZ is quite poor. The migration of Zr/Y ions, together with oxygen vacancies can be expected. The presence of oxygen vacancies enhances the migration of the oxygen vacancies in La0.7Sr0.3VO3; therefore, a higher reactivity of La0.7Sr0.3VO3 toward YSZ can be expected.
Conclusion
According to the examination of La0.7Sr0.3VO3/YSZ powder mixture after heat treatment at 1100–1400 °C, no second phase was detected when the La0.7Sr0.3VO3/YSZ powder mixture was heated at 1100 °C. When the La0.7Sr0.3VO3/YSZ powder mixture was heat treated at 1200 °C, the reaction product of perovskite structure SrZrO3 was formed at the La0.7Sr0.3VO3/YSZ interface initially. No pyrochlore structure La2Zr2O7 product was detected even LaVO3 (X = 0) /YSZ which were heated treatment at 1400 °C for 24 h. Owing to the further diffusion of Sr cations from La0.7Sr0.3VO3 toward the reaction layer/YSZ interface via the reaction layer, the reaction layer was extended into the YSZ. Due to the decrease in strontium concentration of the perovskite structure that leads to La0.7Sr0.3VO3 transforms LaVO3 and the conductivity is decreased.
References
Setoguchi, T., Sawano, M., Eguchi, K., Arai, H.: Application of the stabilized zirconia thin film prepared by spray pyrolysis method to SOFC. Solid State Ionics. 40–41, 502–505 (1990)
Charpentier, P., Fragnaud, P., Schleich, D.M., Gehain, E.: Preparation of thin film SOFCs working at reduced temperature. Solid State Ionics. 135, 373–380 (2000)
Fukui, T., Ohara, S., Murata, K., Yoshida, H., Miura, K., Inagaki, T.: Performance of intermediate temperature solid oxide fuel cells with La(Sr)Ga(Mg)O3 electrolyte film. J Power Sources. 106, 142–145 (2002)
Bringley, J.F., Scott, B.A., Placa, S.J., MnGuire, T.R., Mehran, F., McElfresh, M.W., Cox, D.E.: Structure and properties of the LaCuO3-δ perovskites. Phys Rev B. 47(15), 269–275 (1993)
Petrov, A.N., Kononchuk, O.F., Andreev, A.V., Cherepanov, V.A., Kofstad, P.: Crystal structure, electrical and magnetic properties of La1 − xSrxCoO3 − y. Solid State Ionics. 80, 189–199 (1995)
Karppinen, M., Yamauchi, H., Suematsu, H., Fukunaga, O.: Synthesis of various LaCuO3−y phases by a high-pressure technique and subsequent post-annealing treatments. Physica C. 264, 268–274 (1996)
Dougier, P., Casalot, A.: Sur Quelques Nouvelles Series de Composes Oxygenes du Vanadium et des Lanthanides de Structure Perovskite. J Solid State Chem. 2, 396–403 (1970)
Dougier, P., Fan, J.C.C., Goodenough, J.B.: Etude des proprietes magnetiques, electriques et optiques des phases de structure perovskite SrVO2.90 et SrVO3. J Solid State Chem. 14, 247–259 (1975)
Mahajan, A.V., Johnston, D.C., Torgeson, D.R., Borsa, F.: Structural, electronic, and magnetic properties of LaxSr1-xVO3 (0.1 <= x <= 1.0). Phys Rev B. 46, 10973–10985 (1992)
Giannakopoulou, V., Odier, P., Bassat, J.M., Loup, J.P.: SrVO3 and Sr2VO4, electrical properties below and above room T. Solid State Commun. 93, 579–583 (1995)
Hui, S.Q., Petric, A.: Conductivity and stability of SrVO3 and mixed perovskites at low oxygen partial pressures. Solid State Ionics. 143, 275–283 (2001)
Ge, X.M., Chan, S.H.: Lanthanum strontium vanadate as potential anodes for solid oxide fuel cells. J Electrochem Soc. 156(3), B386–B391 (2009)
Brugnoni, C., Ducati, U., Scagliotti, M.: SOFC cathode/electrolyte interface. Part I: reactivity between La0.85Sr0.15MnO3 and ZrO2-Y2O3. Solid State Ionics. 76, 177–182 (1995)
Kuge’er, D., Holc, J., Hrovat, M., Bernik, S., Samardmija, Z., Kolar, D.: Interactions between a thick film LaMnO3 cathode and YSZ SOFC electrolyte during high temperature ageing. Solid State Ionics. 78, 79–85 (1995)
Taimatsu, H., Wada, K., Yamamura, H.: Mechanism of reaction between lanthanum manganite and yttria-stabilized zirconia. J Am Ceram Soc. 75, 401–405 (1992)
Yokokawa, H., Sakai, N., Kawada, T., Dokiya, M.: Thermodynamic analysis on interface between perovskite electrode and YSZ electrolyte. Solid State Ionics. 40/41, 398–401 (1990)
van Roosmalen, J.A.M., Cordfunke, E.H.P.: Chemical reactivity and interdiffusion of (La, Sr)MnO3 and (Zr, Y)O2 solid oxide fuel cell cathode and electrolyte materials. Solid State Ionics. 52, 303–312 (1992)
Yokokawa, H., Sakai, N., Kawada, T., Dokiya, M.:Chemical thermodynamic stability of the interface. In: Badwal, S.P.S., Bannister, M.J., Hannink, R.H.J. (eds.) Science and Technology of Zirconia V, pp. 752–763. Technomic Publishing Co., Lancaster (1993)
Stochniol, G., Syskakis, E., Naoumidis, A.: Chemical compatibility between strontium-doped lanthanum manganite and yttria-stabilized zirconia. J Am Ceram Soc. 78, 929–932 (1995)
Funding
The authors acknowledge the financial support provided by the Ministry of Science and Technology Taiwan under grant MOST-105-2923-E-006-004-MY3. This work was also financially supported by the Hierarchical Green-Energy Materials (Hi-GEM) Research Center from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, CY., Tsai, SY., Ni, CT. et al. Interfacial reaction between YSZ electrolyte and La0.7Sr0.3VO3 perovskite anode for application. J Aust Ceram Soc 55, 97–102 (2019). https://doi.org/10.1007/s41779-018-0215-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41779-018-0215-2