Abstract
Bongo and Sekyere South districts, both in the northern and southern parts of Ghana, respectively, have high populations living in rural areas and most of them use groundwater for drinking purposes. The groundwater in these areas is prone to contamination from natural and/or artificial sources. Therefore this study aims; (1) to present a comparative analysis of the fluoride concentration in groundwater samples from Bongo and Sekyere South districts and the associated groundwater–rock interaction that may be the cause for the varied fluoride concentrations, (2) to determine the leaching potential of fluoride from the host rocks as the possible mechanism for groundwater contamination. Sixty (60) groundwater samples from active pumping wells and twelve (12) rock samples from outcrops were collected from various communities in the two districts for fluoride concentration and mineralogical analysis. Based on the variations in fluoride concentration, fluoride spatial distribution maps were prepared using empirical Bayesian kriging interpolation method and analysed by means of hierarchical cluster analysis. The fluoride concentration in Bongo district varies between 1.71 and 4.0 mg/L, whereas that in Sekyere South district changes from 0.3 to 0.8 mg/L. From the mineralogical studies, biotite has the highest percentage in the Bongo district and has positive correlation with fluoride concentration in the analysed water samples than in the Sekyere South district. The elevated fluoride concentration in the Bongo district relative to the Sekyere South district is due to the dissolution of biotite in the groundwater and the sufficient groundwater–rock interaction since the water samples are mainly sourced from deeper boreholes. This high fluoride concentration has resulted in a plethora of reported cases of dental fluorosis and other health-related issues in Bongo.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Groundwater is the most appropriate portable and widely used source of drinking water for many rural communities in the world and its quality has special health significance and needs great attention of all concerned (Wright et al. 2004; Furi et al. 2011; Kanyerere et al. 2012; Sunkari and Danladi 2016; Raj and Shaji 2017; Rashed and Niyazi 2017). However, natural processes and increased human activities that release harmful chemicals pose a great threat to the groundwater quality. One of these released chemical constituents is fluoride which exists in ionic form (F−) in aqueous solution (Sreedevi et al. 2006; Jabal et al. 2014). Fluoride concentration in groundwater is of enormous importance and must be known due to its health-related issues. For example, in lower concentrations below 1.5 mg/L (World Health Organisation—WHO guideline), fluoride has a significant mitigating effect against dental caries and promotes bone development (Apambire et al. 1997; Alfredo et al. 2014) and more than 50% of developed countries fluoridate their water below this level (Alfredo et al. 2014). However, at levels greater than 1.5 mg/L, fluoride can impact significant negative health effects on the teeth through incorporation into growing enamel crystals (Aoba and Fejerskov 2002) coupled with substitution for hydroxyl ions in the apatite structure (Elliott et al. 1985). It is well established that prolonged ingestion of highly fluoridated water during the developmental stages of life can lead to mild fluorosis (mottled or brittle teeth) or in extreme cases skeletal fluorosis (porous bone structures) (Marshall 1990). Therefore, the WHO has concluded that the myriad of attendant effects of ingesting water containing fluoride above 1.5 mg/L is a major health concern and has since used this limit as the guideline. Many countries stick to the WHO guideline in developing their own standards. For example, in Ghana the acceptable standard set by the Ghana Standards Board for fluoride content in drinking water is the same 1.5 mg/L.
The concomitant health problems emanating from the intake of drinking water with elevated fluoride content are especially critical in developing countries largely because of lack of suitable infrastructure for treatment. Additionally, the fluoride content in the groundwater of these countries are reported to be from natural sources (Gaciri and Davies 1993; Ayenew 2008; Rango et al. 2010).
Natural sources of fluoride are connected to various types of rocks and volcanic activity. Several factors such as variations in regional geology, rock weathering, mineralogy of watersheds and aquifers, water–rock and water–soil interactions are a contributory factor to the elevated levels of fluoride in groundwater (Islam et al. 2017). Moreover, chemical reactions including dissolution, precipitation, ion exchange and various biological processes commonly take place below the surface. The commonest fluoride-bearing minerals involved in these mechanisms in the environment include fluorite (CaF2), fluorapatite (Ca5(PO4)3F), micas (muscovite and biotite), amphiboles (Ca,Na,K)0 1(Ca,Fe,Li,Mg,Mn,Na)2(Al,Cr,Fe,Mg,Mn,Ti)5(Al,Si,Ti)8O22(OH,F,Cl)2, cryolite (Na3AlF6), villiaumite (NaF), and topaz (Al2(SiO4)F2) (Ozsvath 2009). The dissolution of these minerals, for example, fluorapatite (Ca5(PO4)3F) in most magmatic and sedimentary terranes can be accomplished by calcite precipitation that removes Ca2+ from the solution by the following reaction:
hence, reducing the aqueous Ca2+ activity (Pickering 1985; Nordstrom et al. 1989; Wenzel and Blum 1992; Shah and Danishwar 2003). The removal of Ca2+ can also be attained by ion exchange especially with Na+ from clay minerals (Sarma and Rao 1997), which changes the saturation state of the solution coupled with increase in fluorite/fluorapatite dissolution. Fluorapatite is chosen as the representative fluoride-bearing mineral in this kind of environment, since its dissolution will release calcium and fluoride, and calcite as the representative carbonate mineral precipitation has the proclivity to sequestrate the released calcium and enhance fluorapatite dissolution. The mechanisms that derive the release of fluoride by fluorapatite dissolution are explained in two ways from the following chemical reactions:
Dissolution of fluorapatite in this reaction is derived from calcite precipitation. Accordingly, the reaction for calcite precipitation is as follows:
where fluorapatite dissolution (2) and calcite precipitation (3) are chemically bonded by Ca2+. This suggests that calcite precipitation is the main catalyst for fluorapatite dissolution, which is achieved by simply reducing the activity of calcium in the solution. The combined mass balance equation (Banerjee 2015) representing these two chemical phenomena where groundwater is in contact with both calcite and fluorapatite is given as:
Nevertheless, groundwater that has an alkaline geochemical signature is usually associated with HCO3− and Na+ contents (Möller et al. 2016). This implies that such groundwater is prone to having hydroxyl ions (OH−) which are capable of exchanging replaceable fluoride ions (F−) present in fluoride-bearing minerals. As a result, the dissolution of micas (muscovite and biotite) and the resultant increase in groundwater fluoride take place spontaneously via the following reactions:
It has recently been established that there is a positive correlation of fluoride with Na/Na + Ca, which is associated with the replacement of Ca2+ with Na+ by cation exchange capacity (Fantong et al. 2010), increasing the dissolution rate of fluoride from the mineral phases into water. Previously, Jeong et al. (1996) described such type of process as preferential adsorption of Ca2+ and Mg2+ within clay minerals such as montmorillonite, illite, and kaolinite. Recently, Naseem et al. (2010) suggested the presence of Na/Na + Cl ratio in groundwater as 0.45 which is indicative of the normative mineral composition of albite, a mineral very common in granitic rocks. Ozsvath (2006) also proposed a good relationship between albite and high-fluoride-contaminated groundwater. It is thus obvious that the positive correlation of fluoride with Na+ and (K+ + Na+)/(K+ + Na+ + Ca2+ + Mg2+) ratio reveals the influence of fluoride mobility during the water softening process.
Owing to these phenomena, hydrogeochemical and mineralogical analysis of groundwater and the underlying rocks will provide a conceptual framework for understanding the processes that are responsible for groundwater contamination.
Worryingly, Bongo district in the northern part of Ghana mainly composed of unique granitic rocks known as ‘the Bongo Granite’ is reported in the literature to have high fluoride content in the underlying groundwater (Apambire et al. 1997, Smedley et al. 2002; Rossiter et al. 2010; Alfredo et al. 2014). The previous studies in the district were comprehensive on fluoride concentration in groundwater within some communities without a coverage in most of the communities under this study. Although these studies identified elevated levels of fluoride in the study area, limited interpretation is being provided for the influence of the underlying rocks on the groundwater which could be a possible source for the high fluoride content. The fluoride content in Sekyere South district in southern Ghana is still speculative and there exist no literature regarding it. Thus, this research is the first report on fluoride concentrations in the Sekyere South district.
In the present study, we compared the fluoride content in groundwater samples from Bongo in Northern Ghana to fluoride content in groundwater samples sourced from Sekyere South district in Southern Ghana to understand the geological controls causing the variations in both districts. Contextually, we present the relationship between fluoride concentration in groundwater and the underlying rocks and to possibly identify the major mineral(s) that cause the variation in the concentration of fluoride in groundwaters in the Northern and Southern parts of Ghana. The study seeks to determine the leaching potential of fluoride from the host rocks as the mechanism for groundwater contamination. Health implications emanating from elevated fluoride concentrations in groundwater within the two districts would be highlighted as a secondary objective only from field observation and from scientific literature. Additionally, for the first time, we present the spatial distribution of fluoride contents in groundwater in the two districts using geographic information systems (GIS) software version 10.2 and analysed by means of hierarchical cluster analysis (HCA).
2 Study Setting
2.1 Location and Demographic Information
2.1.1 Bongo District
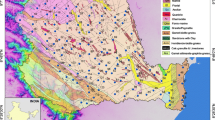
Bongo District lies between longitudes 1°W and 0.62° W and latitudes 10.83°N and 11.05°N (Fig. 1). It covers an area of 460 km2 with a total population of 77,885 (Apambire et al., 1997). The district is bordered by the Kassena-Nankana District in the west, by the Bolgatanga Municipal Assembly in the south and by the Talensi-Nabdam District in the southeast. Topographically, the district is flat, low-lying and is underlain by Paleoproterozoic Basin-type Granitoids and Birimian volcanics (Fig. 1). In some locations, the area is covered with phyllites, quartz, sericite schist, greywacke and associated late granitic intrusions. The granites cover majority of the area with low gently rolling relief between 90 and 300 m above the surrounding lands (Apambire et al. 1997).
Location and geological map of the study areas (modified from Sunkari and Danladi 2016)
2.1.2 Sekyere South District
The Sekyere South district is in the southern part of Ghana and was previously known as the Afigya-Sekyere district. It lies between Latitudes 6º50′N. and 7º10′N and Longitudes 1º40′W and 1º25′W (Fig. 1). The district covers an area of 416.8 square kilometres. Topographically, the area falls within a plateau with a height ranging from 800 to 1200 m above sea level. The plateau forms part of the Mampong-Gambaga scarp. The district is bordered to Ejura Sekyedumasi (north), Mampong Municipal and Sekyere East (east), Kwabre East (south) and Offinso Municipal (west). According to the Ghana Statistical Service, the population of the district is about 94,009. Sekyere South district lies within upper Birimian, intruded by Paleoproterozoic Belt-type Granitoids (Fig. 1).
2.2 Climatic and Vegetation Information
2.2.1 Bongo District
Like any other area in northern Ghana, the Bongo district is characterized by distinct climatic conditions made up of one rainy season which usually occurs between May and October having an average annual rainfall of 900–1200 mm, followed by a prolonged dry season between November and April (Apambire et al. 1997). Savannah grassland is the major vegetation type in the area with clusters of shrubs, short trees and drought-resistant trees such as the baobab, ebony, shea, dawadawa, thorn, acacia and many other related species. The trees in the area are mainly destroyed by anthropogenic activities such as bush burning, construction and farming. The district is noted for high day temperatures with dry harmattan winds blowing from November to March creating dust, thus reducing visibility.
2.2.2 Sekyere South District
The climate of the district is equatorial. It has a double rainfall with the maximum rainy season occurring between March and July. The minor rainfall season occurs between September and November. The mean annual rainfall ranges between 855 mm and 1500 mm. December–March are usually dry and characterized by relatively high temperatures and early morning moist/fog and cold weather conditions. The mean monthly temperatures are about 27 °C with high humidity during the rainy season. The months of December–February, however, record very low humidity. Lying within the rain forest belt, the vegetation can best be described as moist semi-deciduous. The rain forest consists of three layers namely the Upper, Middle and Lower layers. The district is drained by many rivers and streams. And notable among them are the Offin, Oyon and Abankro rivers.
3 Materials and Methods
3.1 Sample Collection, Preparation and Storage
Selection of the communities for sampling was based on the relative concentration of fluoride from secondary hydrogeochemical data from the two districts. Prior to groundwater sample collections in January, 2015, plastic bottles and sample bags were pre-cleaned and sterilized. A total of sixty (60) groundwater samples were collected from operational boreholes and wells in the two districts. All the water samples from the wells were collected using a Klyen downhole stainless steel borehole sampler. After carefully collecting the samples, they were put in the sterilized plastic sample bottles and completely filled and tightened with double-sealed plastic caps to prevent leakage. Each sample bottle was correctly labelled with the collection point identification number, date, time, and sample designation and later stored in cardboard boxes. Since water samples for fluoride concentration analysis do not require any preservation except proper storage for a holding time of 28 days (Simon et al. 2008), the collected samples were transported to the Water Research Institute in Tamale, Ghana for laboratory analysis a day after the sample collection.
Twelve rock samples were also collected from outcrops within the two districts. In-situ samples were taken as close as possible to the sampled water sources. Out of these 12 samples, 6 were granites and the remaining 6 were metasediments.
3.2 Analytical Methods
The fluoride concentrations were measured by an Ion Chromatograph (Basic IC Plus, Metrohm). The Basic IC Plus, Metrohm has high detection sensitivity, and therefore, allows analyses to be performed in the μg/L, mg/L and g/L ranges (Rozalen and Huertas 2013). This remarkable sensitivity is based both on the minimal baseline noise of the chromatogram and on the performance of the intelligent conductivity detector.
In analysing the samples, they were first filtered using a luer-lock syringe and a 25 mm, 0.45 µm pore size syringe filter. The samples were filtered as many times as needed to obtain clear, solute-free samples. This was followed by dissolving 0.2210 g sodium fluoride (NaF, CASRN 7681-49-4) in reagent water and diluted to 100 mL in a volumetric flask. A small aliquot of the sample was then introduced into the Ion Chromatograph and the fluoride ionic composition was later separated and measured, by means of a system comprised of a guard column, analytical column, suppressor device, and conductivity detector (details in Simon et al. 2008). Careful quality controls were achieved by checking sample containers, reagents for background readings, preparation of blank and standard samples. Duplicate samples and standard materials were used to ascertain the precision and accuracy of the analysis. Analytical error was generally below 2%, well within the accuracy of the Ion Chromatograph. The analysis was repeated for each sample and the average estimated.
The mineralogical analysis of the rock samples was carried out at the Department of Geological Engineering of the University of Mines and Technology, Tarkwa-Ghana. This was done by employing the comparison chart for visual mineralogical percentage estimation of Folk (1951). In principle, the chart contains twelve squares and each square has a given percentage of black area with the percentage printed below the square. Each quarter of the square contains the same amount of black, but the arrangement is varied (Folk 1951). A paper mask was constructed with a hole cut in the center, the same size as the squares; this was moved over the chart until a square was found in which the percentage of black area most nearly matched the percentage of the desired mineral in the hand specimen of the rock.
ArcGIS software 10.2 was later used by means of Empirical Bayesian Kriging interpolation method to produce the fluoride spatial distribution maps for the two districts. Empirical Bayesian Kriging is a modern form of interpolation method which operates through sub-setting and simulations of multiple variograms rather than single variograms (Chilès and Delfiner 1999; Pilz and Spöck 2008). The method is user friendly and can be easily applied with the help of EBK tool incorporated in ArcGIS 10.2 software. Also, hierarchical cluster analysis (HCA) in the form of dendrograms was used to outline the groundwater spatial relationships in the two districts. Centroid linkage was employed to link the clusters in the dendrograms which produced the best classification and revealed the spatial association of the groundwater from the Northern and Southern parts of Ghana.
4 Results
4.1 Fluoride Concentrations
Fluoride ion distributions in groundwater in Bongo and Sekyere South districts are presented in Table 1. The fluoride content in Bongo district ranges from 1.71 to 4.0 mg/L, whereas in the Sekyere South district, it varies between 0.3 and 0.8 mg/L.
4.2 Spatial Distribution of Fluoride in Groundwaters of the Northern and Southern Zones of Ghana
4.2.1 Spatial Map of the Bongo District in Northern Ghana
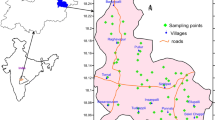
The concentrations of fluoride in groundwaters in the Bongo district (Table 1) were input into ArcGIS 10.2 software to produce the fluoride spatial distribution map shown in Fig. 2. The smaller communities in Table 1 were grouped under the major towns within which they are located in Fig. 2. Also, in areas where more than one sample was collected, the average was calculated and used in plotting the spatial map. The World Health Organisation (WHO) upper limit for fluoride concentration (1.5 mg/L) in groundwater abetted in the delineation of the anomalous areas.
Spatial distribution of fluoride concentrations in groundwater in the Bongo district. The WHO guideline value of 1.5 mg/L aided in delineating areas with high fluoride content. Note that the major communities with very high fluoride content (3.5–4.0 mg/L) are indicated with the violet to white colour on the spatial map whilst the lowest fluoride content are indicated with light green colour
The highest fluoride concentrations ranging from 3.5 to 4.0 mg/L were observed in water samples taken from Tarongo, Gambrugo and Vea (Fig. 2). The samples analysed in Gowrie and Bongo Tingre recorded high fluoride concentrations which varied from 3.0 to 3.5 mg/L (Fig. 2). But the moderate to high fluoride concentrations (1.8–2.5 mg/L) were logged in some samples from Balungu, Namoi Central, Kadare and Nayire (Fig. 2). The spatial distribution map given in Fig. 2 will provide a conceptual basis for assessing the significance of the data collected from boreholes in all the communities.
4.2.2 Spatial Map of the Sekyere South District in Southern Ghana
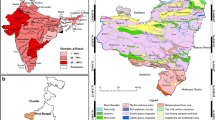
As in the Bongo district, the fluoride concentrations in groundwaters in the Sekyere South district shown in Table 1 were transposed onto the ArcGIS 10.2 software and later projected with the coordinate system of the communities to produce the spatial map of fluoride in groundwaters within the district (Fig. 3). Again, the WHO upper limit of fluoride concentration in drinking water set the basis for anomalous areal delineation. On the spatial map, the individual communities were put under the major ones in the district based on the available map features with average fluoride concentrations for areas where many samples were taken.
Spatial distribution of fluoride concentrations in groundwater in the Sekyere South district. Note that major areas with high fluoride content (0.8 mg/L) are indicated with the light grey colour on the spatial map, whereas the lowest fluoride content (0.3 mg/L) shown with the light green to cream colours
The highest fluoride content (0.8 mg/L) was observed in Embik Farms near Abrakaso and changed to about (0.6 mg/L) in Agona (the district capital) (Fig. 3). Towards the northeastern part of the district in Akrofonso community, the fluoride content decreased to 0.5 mg/L and in the central part around Abroma similar concentration (0.5 mg/L) was recorded in the water samples taken for fluoride content analysis (Fig. 3). In the southern part of the district just close to Kwamang, the fluoride content in the groundwater is 0.4 mg/L (Fig. 3). But in the southwestern segment around Nkwantakese the lowest fluoride content (0.3 mg/L) was observed (Fig. 3). The general distribution of fluoride in groundwaters of the Sekyere South district as shown in Fig. 3 is the first of its kind in the literature and gives a better understanding of the fluoride mapping that was carried out in the district.
4.3 Mineralogy
4.3.1 Samples from Bongo (Bongo Granite)
Most of the rock samples here display hypidiomorphic granular texture and are primarily composed of quartz, microcline, plagioclase, biotite, muscovite with variable amounts of hornblende and amphibole. The accessory phases include zirconium and opaque minerals (apatite). Sericite and epidote are locally present as secondary minerals. The minerals identified in the samples are the most important fluoride-bearing minerals. Among the fluoride-bearing minerals in the samples taken from the Bongo district, biotite is the most abundant mineral and the fluoride in it can be leached to some extent. The mineralogical composition of rock samples analysed from the Bongo district are summarized in Table 2.
4.3.2 Samples from Sekyere South (Muscovite-Gneiss)
The analysed samples have a granular texture. The main constituents include quartz, plagioclase and muscovite. Subhedral biotite is partly replaced by Fe-chlorite in some of the samples as a result of low-grade metamorphism. Mafic phases in the samples include amphiboles with little hornblende (in one sample) and other undifferentiated accessory minerals like apatite. In the Sekyere South district, muscovite, however, presents higher values in the analysed samples and represents the most abundant fluoride-bearing mineral as shown in Table 3.
5 Discussions
The acceptable limit of fluoride concentration proposed by the World Health Organization (WHO 1984; Edition F 2011) in drinking water is pegged at 1.5 mg/L. This clearly posits that the fluoride concentration in the Bongo district of Northern Ghana is above the desired limit, whereas the fluoride content in the Sekyere South district in Southern Ghana is below the permissible limit. Previous studies in the Bongo district have also revealed similar concentrations of fluoride in groundwater (Apambire et al. 1997; Smedley et al. 2002; Anku et al. 2009; Atipoka 2009; Yidana et al. 2012; Firempong et al. 2013; Craig et al. 2015). The upper limits of fluoride concentrations recorded for the Bongo groundwater in the previous studies generally peaked between 2.36 and 6.97 mg/L which is comparable to the 4.0 mg/L detected in the current study (Fig. 4).
Histogram showing a comparison of previous studies with the current studies. The fluoride concentration of groundwater in Bongo district has consistently increased throughout all the studies. The upper limit obtained in the current study is comparable to the previous studies. Sekyere South district, however, has very low fluoride concentration in the analysed samples when correlated with the samples collected from Bongo district and the WHO guideline of 1.5 mg/L
The preponderance of studies on the ‘Bongo groundwater fluoride menace’ suggests that the situation is getting out of hand on yearly basis in view of the increasing fluoride concentration in drinking water within the Bongo district. The concomitant problems this menace puts people into across the globe are enormous. In the literature, health implications of high fluoride contents in drinking water have been widely reported (Craig et al. 2015). The intake of water with fluoride concentration above 1.5 mg/L can cause dental fluorosis (mottling of the teeth) whereas concentrations below 1.5 mg/L may be adequately sufficient to prevent mottling of the teeth, thereby enhancing good teeth development especially in children (Edition F 2011; WHO). Dental fluorosis is a medical condition of the enamel-forming cells which become highly calcified (Ghosh et al. 2013). When the enamel becomes fluorosed due to excessive intake of fluorine, it is weakened and becomes porous creating undesirable brown pits. In severe cases, the fluorosed enamel is brittle and is liable to breakage.
In Bongo district, most of the inhabitants in the areas where anomalous concentrations of fluoride (Fig. 2) were recorded in their groundwater suffer from dental mottling. Interestingly, the nearby communities where moderate fluoride concentrations were observed tend to also suffer from this health-related quagmire as they were slightly higher than the recommended value by the WHO. Apart from this being a major health issue, the attendant problems could be high dental costs and psychological implications which may make the victims feel inferior in the society.
Nevertheless, in the Sekyere South district, the inhabitants are less prone to dental fluorosis since the groundwater in the communities where samples were sourced for this study are generally below the WHO (2011) guideline value of 1.5 mg/L. But the 0.8 mg/L recorded in the groundwater at Embik Farms close to Abrakaso suggests that the people might be prone to mild dental fluorosis since the observed value in the groundwater is closer to the 0.9 mg/L threshold value for mild dental fluorosis (Dean 1942). The moderate fluoride concentration in the Embik farms (Fig. 3) than the other localities in the Sekyere South district might be due to the farming activities in this area. The use of phosphate fertilizers in the area could be a contributory factor to the moderate concentration recorded. Besides, low concentrations of fluoride have been reported to be a precursor of dental caries, especially in children and thus, very low concentrations in drinking water could also be a recipe for diverse health implications (Edmunds and Smedley 2013).
Increasing intake of fluorine supersaturated-water may cause the bones to become weakened and in some cases fragile within the human body. This is termed skeletal fluorosis and it has been widely reported as one of the problems related to elevated fluoride concentration in groundwater (Dissanayake 1991; Jacks et al. 2005; Tekle-Haimanot et al. 2006). The WHO (1984), postulated that for skeletal fluorosis to occur in humans the fluoride concentrations in drinking water should be between 4 and 8 mg/L. However, when the concentration is above 10 mg/L, it may lead to chronic skeletal fluorosis (WHO 1970). The effects of these health-related issues (dental fluorosis and skeletal fluorosis) of elevated fluoride content in drinking water have become a major problem in sub-Saharan Africa where the study areas are located especially in Bongo.
The anomalous fluoride concentrations in the Bongo district relative to the low concentrations observed in the Sekyere South district are peradventure attributable to the groundwater–rock interaction incipient in these districts. This is evident from the minerals observed in Tables 3 and 4. From these Tables, higher concentrations of fluoride were observed in areas with greater percentage of the fluoride-bearing minerals particularly biotite, indicating a positive correlation (statistically significant R2 = 0.9407) between fluoride concentration and biotite percentage (Fig. 5). Given this correlation, a lot can be said about the groundwater–rock interaction in view of the mineralogical composition of the underlying rocks. First, elemental fluorine occurs primarily in granitic, volcanic and meta-volcanic rocks, which are rich in black mica (biotite) where it substitutes for hydroxyl positions in the biotite (Banerjee 2015; Edmunds and Smedley 2013). During magmatic activities and weathering, fluorine tends to be released preferentially from the biotite \(\left( {{\text{K}}\left( {{\text{Mg}},{\text{Fe}}^{ 2+ }_{ 3} } \right)\left( {{\text{Al}},{\text{Fe}}^{ 3+ } } \right){\text{Si}}_{ 3} {\text{O}}_{ 10} \left( {{\text{OH}},{\text{F}}} \right)_{ 2} } \right)\) within the Bongo Granites and this again mixes with the underlying groundwater causing progressive contamination (Fig. 6). Also, carbonate bearing rocks within the basement may have released fluorine as fluorine phosphate during igneous and metamorphic activities in Bongo, thereby enriching the natural fluorine content of the groundwater (Fig. 6). The relationship amongst F−, Ca2+ and CO32− (released from the carbonate rocks) can be explained by the following reaction:
The equilibrium constant for this reaction is expressed as:
suggesting that any change in the fluoride concentration of groundwater is directly proportional to change in carbonate concentration and inversely proportional to the phosphate concentration. Recently, inverse correlation of phosphate and fluoride is documented in Vedaraniyam, South India and Langtang area, Nigeria (Krishnakumar et al. 2014; Dibal et al. 2017). This corroborates well with the present understanding that fluoride concentration is directly related to the degree of water–rock interaction that enhances the dissolution of fluoride-bearing minerals in the rock (Saxena and Ahmed 2001; Chae et al. 2006).
Moreover, Smedley et al. (2002) reported a fluorine concentration of 0.2% in the Bongo granites with a maximum value of 4.4 mg/L far above the WHO (1984) guideline value of 1.5 mg/L for drinking water. This accounts for the elevated fluoride concentrations in the groundwater in Bongo district. Rocks which are generally aggregates of various minerals, harmful and harmless alike do weather under varying conditions. If a rock is rich in fluoride and water percolates through it, the fluoride is capable of been released with time if the rock becomes weathered or in severe cases sheared. The fluoride released in this case can drain into surrounding waterbodies and may remain within the overburden (regolith) for a long time which will naturally pollute water being tapped from such reservoirs (Fig. 6). It is suggested that biotite and muscovite are the main fluoride-bearing minerals in the study areas based on the mineralogical composition (see Tables 2 and 3). These micas may have released hydroxyl ions (OH−) and fluoride during magmatic activities and weathering through the reactions below:
Also, the association of the rocks with accessory apatite may have played a role in dissolution of fluorapatite. In this case, fluoride may have been leached from the rocks alongside calcite as the representative carbonate mineral precipitation, which may have sequestrated the released calcium and enhanced fluorapatite dissolution through the following reaction:
Therefore, the principal mechanism responsible for the release of fluoride by the dissolution of fluoride-bearing minerals such as micas and fluorapatite in the two districts is mainly hydroxyl ions (capable of exchanging replaceable F ions present in fluoride-bearing micas) and calcite precipitation, which is the main catalyst for fluorapatite dissolution and may have been achieved by simply reducing the activity of calcium in the solution.
The high fluoride concentrations observed in borehole water samples taken from the Bongo district could also be due to the fact that boreholes drilled in this area are at substantial depths intersecting the biotite super-saturated underlying Bongo Granites, further releasing fluoride in higher concentrations (Edmunds and Smedley 2013). In Sekyere South district, the main drinking water sources are generally shallow boreholes as compared to waters of deeper origin (deeper boreholes) in Bongo district. Although, the district is underlain by Belt-type granitoids of the Upper Birimian mainly composed of meta-volcanics and meta-granites which are rich in micas, the fluoride concentrations tend to be low. Edmunds and Smedley (2013) reiterated that shallow groundwaters that had no interaction with the underlying granitic rocks have no proclivity for high fluoride concentrations. This holds for the Sekyere South district scenario. Besides, fluoride concentrations generally tend to be lower in the humid tropics because of the enormity of rainfall causing dilution of the groundwater chemical composition (Smedley et al. 2002). This might be one of the overriding reasons for the low fluoride concentrations in the Sekyere South district as juxtaposed to the extremely anomalous values encountered in water samples from the Bongo district which is characterized by semi-arid climatic conditions.
Furthermore, by means of Hierarchical Cluster Analysis (HCA) in the form of a dendrogram using centroid linkage, three spatial groundwater associations were observed in the Bongo district (Fig. 7). The first cluster involves groundwaters from communities which have fluoride concentrations less than 3.5 mg/L but greater than 2.0 mg/L, the second cluster encompasses communities with fluoride concentrations in their groundwater generally less than 2.0 mg/L but greater than 1.7 mg/L. However, the third cluster comprises communities with groundwater fluoride concentrations less than 4.1 mg/L yet greater than 2.7 mg/L. These variations suggest that the second cluster is the most homogenous since it presents lower fluoride concentrations as juxtaposed to the first and third clusters and its lower limit is only slightly higher than the WHO guideline value of 1.5 mg/L (Fig. 7).
Nonetheless, in the Sekyere South district, the HCA revealed only one cluster involving all the studied communities (Fig. 8). This cluster comprises communities with fluoride concentrations in their groundwater lower than 0.9 mg/L but greater than 0.2 mg/L, which are still below the WHO guideline of 1.5 mg/L (Fig. 8). Therefore, the cluster analysis is consistent with the mineralogical analysis which suggests that the elevated fluoride concentrations in the groundwaters of the Bongo district may have been as a result of dissolution of the Bongo Granites where the biotite peradventure released hydroxyl ions (OH−) and fluoride ions (F−) into the groundwater sourced from the Bongo district whilst the lower concentrations observed in groundwaters from Sekyere South district may have been the result of low biotite content in the host rocks and extremely low dissolution of muscovite.
In all, the mineralogical analysis and the fluoride levels in the studied samples indicate that the geological source of fluoride in groundwater is related to leaching of minerals from rocks thus metamorphic (gneissic) rocks and granitoids (Bongo Granites) which are rich in mica (biotite, muscovite and sericite), quartz as well as microcline and accessory apatite. Hence, the high fluoride concentrations in the groundwater of the Bongo district are more likely to result from the dissolution of micas (biotite and muscovite) and fluorapatite. But the low concentration of fluoride in groundwater samples in the Sekyere South district in Southern Ghana might be related to the low biotite content in the underlying rocks. Isotopic analysis of the rocks and water samples in the area is envisaged to be a robust method for further determining the source of the fluoride, therefore, this research is only a preliminary approach and would be followed up with a comprehensive isotopic analysis.
6 Conclusions
The fluoride concentrations in groundwater samples collected from Bongo and Sekyere South districts in the Northern and Southern parts of Ghana, respectively, have been evaluated and compared with each other. The fluoride concentration in Bongo district varies from 1.71 to 4.0 mg/L higher than the acceptable limit of the World Health Organization (WHO; 1.5 mg/L). The current situation of the fluoride distribution in this part of the country is such that no clear demarcation can be made of areas deficient in fluoride since some areas have groundwater sources in which the fluoride ion concentrations are higher (4.0 mg/L) than the WHO recommended limit for drinking water. This high concentrations of fluoride in the groundwater in Bongo may lead to dental fluorosis and skeletal fluorosis though the latter has not yet been recorded in the area. For the Sekyere South district in Southern Ghana, relatively low fluoride concentration of 0.3–0.8 mg/L was observed in the groundwater precluding the effects of dental fluorosis and skeletal fluorosis as well as other health-related issues on the inhabitants. The low fluoride ion concentrations in groundwater in the Sekyere South district indicate that fluoride levels in the groundwater is currently not a national problem.
The mineralogical results reveal that biotite is the abundant fluoride-bearing mineral which is statistically significant (R2 = 0.9407). From the foregoing background, the high biotite percentage in the Bongo district may account for the high fluoride concentration in the groundwater (borehole) samples collected for this study whereas the low biotite percentage recorded in rock samples in the Sekyere South district can be associated with the low fluoride concentration. This suggests that groundwater in the Sekyere South district may have had little or no interaction with the underlying granitic rocks as compared to the Bongo district in Northern Ghana. Many groundwater sources in the Northern part of Ghana, therefore, are in dire need of partial de-fluoridation to the optimum level of fluoride concentration safe for drinking.
References
Alfredo KA, Lawler DF, Katz LE (2014) Flouride contamination in the Bongo district of Ghana, West Africa: geogenic contamination and cultural complexities. Water Int 39(4):486–503
Anku YS, Banoeng-Yakubo B, Asiedu DK, Yidana SM (2009) Water quality analysis of groundwater in crystalline basement rocks, Northern Ghana. Environ Geol 58(5):989–997
Aoba T, Fejerskov O (2002) Dental fluorosis: chemistry and biology. Crit Rev Oral Biol Med 13(2):155–170
Apambire WB, Boyle DR, Michael FA (1997) Geochemistry, genesis and health implications of fluoriferous groundwater in the upper regions of Ghana. Environ Geol 35(1):13–24
Atipoka FA (2009) Water supply challenges in rural Ghana. Desalination 248(1–3):212–217
Ayenew T (2008) The distribution and hydrogeological controls of fluoride in the groundwater of central Ethiopian rift and adjacent highlands. Environ Geol 54:1313–1324
Banerjee A (2015) Groundwater fluoride contamination: a reappraisal. Geosci Front 6(2):277–284
Chae GT, Yun ST, Kwon MJ, Kim YS, Mayer B (2006) Batch dissolution of granite and biotite in water: implication for fluorine geochemistry in groundwater. Geochem J 40(1):95–102
Chilès JP, Delfiner P (1999) Geostatistics: modeling spatial uncertainty. Wiley, New York
Craig L, Lutz A, Berry KA, Yang W (2015) Recommendations for fluoride limits in drinking water based on estimated daily fluoride intake in the Upper East Region, Ghana. Sci Total Environ 532:127–137
Dean HT (1942) The investigation of physiological effects by the epidemiological method. In: Moulton FR (ed) Fluorine and Dental Health. American Association for the Advancement of Science, Washington, pp 23–31
Dibal HU, Dajilak WN, Lekmang IC, Nimze LW, Yenne EY (2017) Seasonal variation in fluoride content in groundwaters of Langtang area, Northcentral Nigeria. Contemp Trends Geosci 6(1):11–27
Dissanayake CB (1991) The fluoride problem in the ground water of Sri Lanka—environmental management and health. Int J Environ Stud 38(2–3):137–155
Edition F (2011) Guidelines for drinking-water quality. WHO Chron 38(4):104–108
Edmunds WM, Smedley PL (2013) Fluoride in natural waters. In: Essentials of medical geology. Springer, Dordrecht, pp 311–336
Elliott JC, Holcomb DW, Young RA (1985) Infrared determination of the degree of substitution of hydroxyl by carbonate ions in human dental enamel. Calcif Tissue Int 37(4):372–375
Fantong WY, Satake H, Ayonghe SN, Suh EC, Adelana SM, Fantong EBS, Banseka HS, Gwanfogbe CD, Woincham LN, Uehara Y, Zhang J (2010) Geochemical provenance and spatial distribution of fluoride in groundwater of Mayo Tsanaga River Basin, Far North Region, Cameroon: implications for incidence of fluorosis and optimal consumption dose. Environ Geochem Health 32(2):147–163
Firempong CK, Nsiah K, Awunyo-Vitor D, Dongsogo J (2013) Soluble fluoride levels in drinking water-a major risk factor of dental fluorosis among children in Bongo community of Ghana. Ghana Med J 47(1):16–23
Folk RL (1951) A comparison chart for visual percentage estimation. J Sediment Pet 21(1):32–33
Furi W, Razack M, Abiye TA, Ayenew T, Legesse D (2011) Fluoride enrichment mechanism and geospatial distribution in the volcanic aquifers of the Middle Awash basin, Northern Main Ethiopian rift. J Afr Earth Sci 60(5):315–327
Gaciri SJ, Davies TC (1993) The occurrence and geochemistry of fluoride in some natural waters of Kenya. J Hydrol 143:395–412
Ghosh A, Mukherjee K, Ghosh SK, Saha B (2013) Sources and toxicity of fluoride in the environment. Res Chem Intermed 39(7):2881–2915
Islam AT, Shen S, Haque MA, Bodrud-Doza M, Maw KW, Habib MA (2017) Assessing groundwater quality and its sustainability in Joypurhat district of Bangladesh using GIS and multivariate statistical approaches. Environ Dev Sustain:1–25. https://doi.org/10.1007/s10668-017-9971-3
Jabal MSA, Abustan I, Rozaimy MR, Al-Najar H (2014) Fluoride enrichment in groundwater of semi-arid urban area: Khan Younis city, southern Gaza Strip (Palestine). J Afr Earth Sci 100:259–266
Jacks G, Bhattacharya P, Chaudhary V, Singh KP (2005) Controls on the genesis of some high-fluoride groundwaters in India. Appl Geochem 20(2):221–228
Jeong CH, Kim CS, Kim SJ, Park SW (1996) Affinity of radioactive cesium and strontium for illite and smectite clay in the presence of groundwater ions. J Environ Sci Health Part A 31(9):2173–2192
Kanyerere T, Levy J, Xu Y, Saka J (2012) Assessment of microbial contamination of groundwater in upper Limphasa river catchment, located in a rural area of northern Malawi. Water SA 38(4):581–596
Krishnakumar P, Lakshumanan C, Kishore VP, Sundararajan M, Santhiya G, Chidambaram S (2014) Assessment of groundwater quality in and around Vedaraniyam, South India. Environ Earth Sci 71(5):2211–2225
Marshall E (1990) The fluoride debate: one more time. Science 247:276–277
Möller P, Rosenthal E, Inbar N, Magri F (2016) Hydrochemical considerations for identifying water from basaltic aquifers: the Israeli experience. J Hydrol Reg Stud 5:33–47
Naseem S, Rafique T, Bashir E, Bhanger MI, Laghari A, Usmani TH (2010) Lithological influences on occurrence of high-fluoride groundwater in Nagar Parker area, Thar desert, Pakistan. Chemosphere 78(11):1313–1321
Nordstrom DK, Ball JW, Donahoe RJ, Whittemore D (1989) Groundwater chemistry and water-rock interactions at Stripa. Geochim Cosmochim Acta 53:1727–1740
Ozsvath DL (2006) Fluoride concentration in a crystalline bedrock aquifer Marathon County, Wisconsin. Environ Geol 50:132–138
Ozsvath DL (2009) Fluoride and environmental health: a review. Rev Environ Sci BioTechnol 8(1):59–79
Pickering WF (1985) The mobility of soluble fluoride in soils. Environ Pollut Ser B Chem Phys 9:281–308
Pilz J, Spöck G (2008) Why do we need and how should we implement Bayesian kriging methods. Stoch Env Res Risk Assess 22(5):621–632
Raj D, Shaji E (2017) Fluoride contamination in groundwater resources of Alleppey, southern India. Geosci Front 8(1):117–124
Rango T, Bianchini G, Beccaluva L, Tassinari R (2010) Geochemistry and water quality assessment of central Main Ethiopian Rift natural waters with emphasis on source and occurrence of fluoride and arsenic. J Afr Earth Sci 57(5):479–491
Rashed M, Niyazi B (2017) Environmental impact assessment of the former Al-Musk lake wastewater dumpsite using electromagnetic induction technique. Earth Syst Environ 1(1):10
Rossiter HMA, Owusu PA, Awuah E, MacDonald AM, Schäfer AI (2010) Chemical drinking water quality in Ghana: water costs and scope for advanced treatment. Sci Total Environ 408:2378–2386
Rozalen M, Huertas FJ (2013) Comparative effect of chrysotile leaching in nitric, sulfuric and oxalic acids at room temperature. Chem Geol 352:134–142
Sarma DRR, Rao SLN (1997) Fluoride concentrations in groundwaters of Visakhapatnam, India. J Environ Contam Toxicol 58:241–247
Saxena V, Ahmed S (2001) Dissolution of fluoride in groundwater: a water–rock interaction study. Environ Geol 40(9):1084–1087
Shah MT, Danishwar S (2003) Potential fluoride contamination in the drinking water of Naranji area, northwest frontier province, Pakistan. Environ Geochem Health 25:475–481
Simon D, Billica J, Gertig K, Stone S (2008) Fluoride tracer test planning and implementation to support water distribution model calibration and IDSE compliance. In: Water distribution systems analysis symposium 2006, pp 1–17
Smedley PL, Nicolli HB, Macdonald DMJ, Barros AJ, Tullio JO (2002) Hydrogeochemistry of arsenic and other inorganic constituents in groundwaters from La Pampa, Argentina. Appl Geochem 17:259–284
Sreedevi PD, Ahmed S, Made B, Ledoux E, Gandolfi JM (2006) Association of hydrogeological factors in the temporal variations of fluoride concentration in a crystalline aquifer India. Environ Geol 50(1):1–11
Sunkari ED, Danladi IB (2016) Assessment of trace elements in selected bottled drinking water in Ghana: a case study of Accra metropolis. Int J Water Resour Environ Eng 8(10):137–142. https://doi.org/10.5897/IJWREE2016.0685
Tekle-Haimanot R, Melaku Z, Kloos H, Reimann C, Fantaye W, Zerihun L, Bjorvatn K (2006) The geographic distribution of fluoride in surface and groundwater in Ethiopia with an emphasis on the Rift Valley. Sci Total Environ 367(1):182–190
Wenzel WW, Blum WEH (1992) Fluoride speciation and mobility in fluoride contaminated soil and minerals. Soil Sci 153:357–364
World Health Organization (1984) Fluorine and fluorides/published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization. Geneva: World Health Organization. Environmental health criteria 36: 136. http://www.who.int/iris/handle/10665/37288
World Health Organization (2011) Guidelines for drinking-water quality, 4th edn. Google Scholar
World Health Organization (WHO) (1970) Fluorides and human health. World Health Organization, Monogr Ser 59, Geneva
Wright J, Gundry S, Conroy R (2004) Household drinking water in developing countries: a systematic review of microbiological contamination between source and point-of-use. Trop Med Int Health 9(1):106–117
Yidana SM, Ophori D, Yakubo BB, Samed AA (2012) A factor model to explain the hydrochemistry and causes of fluoride enrichment in groundwater from the middle Voltaian sedimentary aquifers in the northern region, Ghana. ARPN J Eng Appl Sci 7(1):50–68
Acknowledgement
We wish to thank the staff of the Water Research Institute in Tamale for painstakingly running the water analysis for fluoride concentration of the samples and the department of geological engineering of the University of Mines and Technology, Tarkwa/Ghana for the mineralogical analysis. Iliya Bauchi DANLADI is acknowledged for his valuable contribution to this research. The first author also thanks the Scientific and Technological Research Council of Turkey (TÜBITAK) for the continuous financial support during the time of this research as a fellow of BIDEB 2215 Graduate Scholarship Program for International Students.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Sunkari, E.D., Zango, M.S. & Korboe, H.M. Comparative Analysis of Fluoride Concentrations in Groundwaters in Northern and Southern Ghana: Implications for the Contaminant Sources. Earth Syst Environ 2, 103–117 (2018). https://doi.org/10.1007/s41748-018-0044-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41748-018-0044-z