Abstract
The Itakpe abandoned iron-ore mines constitute the largest iron-ore deposits in Nigeria with an estimated reserve of about three million metric tons of ore. The present effort is a part of a comprehensive study to estimate the environmental and radiological health hazards associated with previous mining operations in the study area. In this regard, heavy metals (Fe, Zn, Cu, Cd, Cr, Mn, Pb, Ni, Co and As) and natural radionuclides (U, Th and K) were measured in rock, soil and water samples collected at different locations within the mining sites. Atomic absorption and gamma-ray spectrometry were utilized for the measurements. Fe, Mn, Zn, Cu, Ni, Cd, Cr, Co Pb and As were detected at varying concentrations in rock and soil samples. Cd, Cr, Pb and As were not detected in water samples. The concentrations of heavy metals vary according to the following pattern; rock ˃ soil ˃ water. The mean elemental concentrations of K, U and Th are 2.9%, 0.8 and 1.2 ppm and 1.3%, 0.7 and 1.7 ppm, respectively, for rock and soil samples. Pearson correlation analyses of the results indicate that the heavy metals are mostly negatively correlated with natural radionuclides in the study area. Cancer and non-cancer risks due to heavy metals and radiological hazards due to natural radionuclides to the population living within the vicinity of the abandoned mines are lower than acceptable limits. It can, therefore, be concluded that no significant environmental or radiological health hazard is envisaged.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nigeria is generally endowed with different solid minerals (e.g., iron, tin, tantalite, marble, quart, gem stones, etc.) apart from oil and gas. Harnessing these mineral resources has contributed greatly to the socio-economic growth of the nation (Gbadebo and Ekwue 2014). Associated with the exploration, mining and processing of the minerals is the widespread dispersal and distributions of contaminants into the environment. Prominent among these contaminants are heavy metals (Fe, Mn, Zn, Cu, Ni, Cd, Cr, Co Pb and As) and radioactive elements (U, Th and K). Mining activity is fingered as one of the most potentially harmful anthropogenic activities carried out in the world leading to severe disruption of the environment (Acosta et al. 2011; Antunes et al. 2017). The extraction of iron metal from mining ore generates tailings and large volumes of contaminated waste rocks, which are left as heaps of waste products within the vicinity of the mining environment posing radiological and ecological risks to human health and the environment. Potentially toxic elements may be transported from the mining areas through run-off to nearby streams and rivers or leached into the groundwater; thus, abandoned mines are one of the most important sources of contamination to the environment (Antunes et al. 2017).
Heavy metals are persistent and non-degradable constituent of the earth’s environment. Heavy metals generally refer to metals and metalloids with density greater than 5 g/cm3 (Chen et al. 2015). Heavy metal contamination in the mining environment may pose health risks and pollution hazards to humans and the ecosystem through direct derma contact, ingestion of dust particle from rock and soil, entry into the food chain via soil-to-plant transfer and drinking of contaminated ground- and surface water (Wuana and Okieimen 2011). Unlike organic contaminants, heavy metals do not undergo environmental, chemical or microbial degradations (Kirpichtchikova et al. 2006). Their concentrations persist in soil for a long period of time after their introduction (Adriano 2003; Wuana and Okieimen 20111). Heavy metal pollution of the environment depends on the toxicity and concentration of individual metal in different environmental matrices (Ajayi et al. 2009).
Radioactive materials are natural components of the environment in which man lives. In the terrestrial environment, radioactivity can be natural or artificial in origin. Natural radioactivity includes radionuclides that are formed at the creation of the earth known as primordial radionuclides or those formed through the interactions of cosmic rays with atmospheric substances known as cosmogenic radionuclides. Primordial radionuclides are naturally occurring radioactive elements contained in the uranium, thorium and actinium decay series together with the singly occurring radioactive isotope of potassium. The concentration levels of both primordial and cosmogenic radionuclides in the earth’s environment depend on several factors which include altitude, weathering processes, geography and general geology of the specific environment. Artificial radionuclides are those produced through human activities. Terrestrial gamma radiation from natural radionuclides accounts for ~ 85% of the total global annual average ionizing radiation (UNSCEAR 2001; Wang et al. 2017). Long-term exposure to and inhalation of heavy metals and natural radionuclides could cause many health problems, such as acute leukemia, anemia, cataracts of the eye as well as different types of cancer (Taskin et al. 2009; Qureshi et al. 2014; Wang et al. 2017). Several researchers have made efforts to correlate heavy metal contamination with natural radioactivity in soil and water (Baykara and Dogru 2010; Meindinyo and Agbalagba 2012; Faisal et al. 2015; Milenkovic et al. 2015; Chen et al. 2015), aquatic environment (Szarlowicz et al. 2013; Aytas et al. 2014; Goher et al. 2014; El-Amier et al. 2017), crude oil (Ajayi et al. 2009), fertilizer (El-Taher and Althoyaib 2012), uranium and phosphate mining areas (Atta et al. 2016; Bai et al. 2017), other mining environment (Mileusnic et al. 2014), etc. Information on such efforts concerning iron-ore mines is very scanty. The aim of this research work, therefore, is to measure the elemental concentrations of some heavy metals (Fe, Zn, Cu, Cd, Cr, Mn, Pb, Ni, Co and As) and naturally occurring radionuclides (U, Th and K) in different rock types, soil, tailings and water samples collected from abandoned mines of Itakpe iron-ore mining environment in order to estimate the ecological and radiological hazards due to the previous mining activities in the area. Atomic absorption and gamma spectrometry methods were utilized for the analysis of heavy metals and natural radionuclides, respectively.
2 Materials and Methods
2.1 Sample Collection
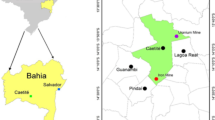
Representative rock, soil, tailing and water samples were collected randomly from Itakpe abandoned iron-ore mines and the adjoining environment for atomic absorption and gamma spectrometric analysis. The location of the study area is shown in Fig. 1. A total of 13 rock samples were collected representing the major rock types within the mining area with the aid of chisel and hammer. Also, ten soil samples consisting of soil mixed with tailings were collected around the mining sites. The soil samples were collected at the depth of 0–25 cm after the upper layer debris was removed. Water samples were collected from ten different locations consisting of streams, incubating pool and tailing effluent drains. The rock and soil samples were air dried in the open laboratory for about 4 weeks, pulverized, sieved through a 600 µm mesh and then stored in well-labeled plastic bags prior to laboratory analysis.
2.2 Sample Measurements for Atomic Absorption Spectrometry (AAS)
2.2.1 Digestion Procedure for Rock and Soil Samples
Determination of heavy metal contents of rock, soil and water samples was done at the AAS laboratory of Centre for Energy Research and Development, Obafemi Awolowo University, Ile Ife, Nigeria using a PG990 atomic absorption spectrometer. For the digestion of the samples, 1 g each of the pulverized rock and soil samples was weighed into a clean Teflon beaker, adding 20 ml of Hydrofluoric acid (HF) and heated to near dryness before 15 ml of Nitric acid (HNO3) was added. Further heating was done to near dryness in order to mop up the residue. After cooling for some minutes, 20 ml of distilled water was added to boil off the acid. After boiling to one-third its volume, the sample solution was allowed to cool and filtered. The digested solutions were made to 200 ml mark of the standard flask with distilled water and kept refrigerated pending analysis (Christoforidis and Stamatis 2009). Blank solutions without samples were prepared following the same procedure.
2.2.2 Digestion Procedure for Water Samples
25 ml of each of the filtered sample was accurately measured into clean conical flasks. 10 ml freshly prepared aqua-regia [HNO3:HCl (1:3)] was gently added through the side, and the mixture was gently swirled to achieve mixing. The flask was then heated gently to one-third its original volume; this was allowed to cool and filtered. The filtrate was then made up to mark in a 250 ml STD flask with double distilled water and transferred into clean and labeled sample bottles. The sample bottles were kept refrigerated pending AAS analysis.
2.2.3 Sample Measurement
Concentrations of ten heavy metals (Fe, Mn, Zn, Cu, Ni, Cd, Cr, Co Pb and As) were determined by atomic absorption spectrometry using a PG990 spectrometer. The wavelength and limits of detection of the spectrometer for the analyzed heavy metals are given in Table 1. Calibration curve was generated for each metal from the measured absorbance value for blank and working standard solution in order to evaluate the concentrations of heavy metals in the digested sample solution. The concentrations of heavy metals in mg/kg in the rock and soil samples were obtained from the reading of AAS using Eq. (1), while those in water samples were determined through Eq. (2):
For quality control, the samples were carefully handled and all the glass wares and digestion vessels utilized were thoroughly washed before use, cleansed and rinsed with deionized water. Accuracy and precision of the measuring procedures were ensured through the preparation of reagent blanks and duplicate samples (IAEA 2008).
2.3 Sample Measurements for Gamma-Ray Spectrometry
The pulverized rock and soil samples were packed each into separate Marinelli cylindrical beakers, sealed and kept for a period of 40 days prior to gamma spectrometric analysis so as to ensure secular radioactive equilibrium. The same procedure was carried out for water samples. A low-level NaI(Tl) detector was used. The detector is a cylindrical well-type NaI(Tl) detector (Model 802) coupled to an 8 k PC based multi-channeled analyzer manufactured by Canberra Inc. The detector volume is a 3ʺ × 3ʺ crystal housed in ~ 5 cm block of cylindrical lead shield with moveable cover to reduce the effect of background radiation on the measurements. The linearity of the detector was tested and found to be okay. Energy resolution calibration was carried out with gamma sources consisting of 60Co, 137Cs, 241Am and 22Na sourced from International Atomic Energy Agency (IAEA), Vienna. This was achieved by obtaining spectral data from the standard sources with an energy range of 0.511–2.62 MeV (Ademola et al. 2015). The detection efficiency calibration was carried out using a standard reference material (IAEA-385), consisting of known activities of 40K (607 Bq/kg), 238U (29 Bq/kg) and 232Th (33.7 Bq/kg). The resolution of the detector at 662 keV of 137Cs is 7.5% full width at half maximum (FWHM). Background count was determined by obtaining the counts due to empty Marinelli beaker of the same geometry as the sample holders. The net count due to the sample was obtained by deducting the background count from the gross count (Isinkaye et al. 2013). The counting time was set at 24 h in order to obtain gamma spectrum with good statistics. The activity concentration of 238U was determined based on the 1.76 MeV gamma line of 214Bi, while the 232Th concentration was evaluated based on the 2.62 MeV gamma line of 208Tl and 40K concentration was assayed directly from its 1.46 MeV gamma line. The minimum detectable activity for 40K, 238U and 232Th was 0.0255 and 0.00737 Bq/kg, respectively, for the NaI(Tl) detector used in this study. Values below these numbers were expressed as below detection limit (BDL) in the results. The specific activities of 40K, 238U and 232Th were converted to massic elemental concentrations of potassium (%K), uranium (eU) and thorium (eTh) using the recommendations of IAEA technical report 309 (IAEA 1989), which give the conversion values as (Eq. 3):
2.4 Heavy Metal Human Health Impact Assessment
Human health impact assessment is the process of evaluating the nature and possibility of adverse health effects in humans who may be exposed to toxic materials in contaminated environmental media (NRC 1983; Li et al. 2014). The human health impacts were estimated using exposure, non-carcinogenic and carcinogenic risk assessments. To calculate the levels of human exposure to heavy metals in rock dust, soil and water samples, the average daily intake (ADI) due to ingestion, inhalation and dermal absorption was used. The models used for the calculation of ADI were recommended by US Environmental Protection Agency (USEPA).
For ingestion pathway, Eq. (4) (Li et al. 2014) was used:
where ADIingestion is the average daily intake of heavy metals from soil ingestion (mg/kg-day), Cij is the concentration of heavy metal i in medium j, IngRj is the ingestion rate of j medium particles (mg/day or L/day), EF is the exposure frequency (day/year), ED is the lifetime exposure duration (year), BW is body weight of the exposed individual and AT is time period over which the dose is averaged (day).
For inhalation pathway, Eq. (5) was used:
where PEF is the particle emission factor (m3/kg).
For dermal absorption pathway, Eq. (6) was used:
where ADIdermal is the average daily intake of heavy metal from dermal absorption (mg/kg-day), SA is the exposed skin surface area (cm2), AF is the adherence factor (mg/cm2-day) and ABS is the dermal absorption factor (unitless).
The non-carcinogenic health risk was assessed by the evaluation of the dimensionless hazard quotient (HQ) defined as the ratio of the chronic average daily intake to the toxicity threshold value, which is referred to as the reference dose (RfD) of a specific heavy metal (Li et al. 2014; Hiller et al. 2016) as given in Eq. (7):
where ADI is the average daily intake of a single toxic element and RfD is the chronic reference dose for the element (mg/kg-day) (USEPA 2001). The hazard index HI is defined as the total sum of HQ obtained for different pathways as shown in Eq. (8) (Xu et al. 2013, Bai et al. 2017):
The acceptable value of HI is ≤ 1.0. If HI > 1.0, non-carcinogenic health risk is probable and the risk increases with increase in HI (Bleam 2016; Bai et al. 2017).
The carcinogenic risk is used to assess the possibility of an individual developing any type of cancer due to exposure to cancer-causing agents during a lifetime. This was calculated using Eq. (9) (Li et al. 2014):
where SF is the carcinogenic slope factor (per mg/kg-day). Cancer risk values higher than 1 × 10−4 are regarded as unacceptable, while those below 1 × 10−6 are not considered to pose any significant cancer risks. The acceptable range falls within 1 × 10−4 and 1 × 10−6 depending on the circumstance of exposure (Hu et al. 2012; Li et al. 2014). The parameters used for the evaluation of exposure risks of heavy metals in rock dust and soil are presented in Table 2.
2.5 Radiological Health Risks Assessment
2.5.1 Gamma Dose Rates
Gamma dose rates were estimated for the rock and soil samples at a distance of 1 m height in order to assess the radiological health risks associated with the specific activities of natural radionuclides in the study area (Isinkaye et al. 2013). The formula for the calculation is given as (IAEA 2003):
where CK, CU and CTh are the mass concentration of potassium (%), uranium (ppm) and thorium (ppm), respectively. The formula is based on the assumption that 1% K produces gamma dose rates of 13.078 nGy/h, 1 ppm eU equals 5.675 nGy/h and 1 ppm eTh gives 2.294 nGy/h.
To convert the external dose rates due to natural radionuclides in rock and soil samples to annual effective dose equivalent (AEDE), Eq. (11) was used (UNSCEAR 2011):
where D is the dose rate obtained from the mass concentration of K, U and Th in (nGy/h), 0.2 is a factor that gives the percentage of time spend outdoors and 0.7 is the factor that converts absorbed dose in air outdoor to effective dose received by individual adults.
2.5.2 Excess Life-Time Cancer Risk
The excess lifetime cancer risk due to external gamma dose exposure in the mining environment was calculated using Eq. (12) (Qureshi et al. 2014):
where LE is the life expectancy (70 year) and RF is the fatal risk factor per Sievet, which is 0.05 as reported in ICRP-60.
3 Results and Discussion
3.1 Heavy Metals
The summaries of the basic descriptive statistics for metal concentrations of Fe, Zn, Cu, Cd, Cr, Mn, Pb, Ni, Co and As in rock and soil samples from Itakpe iron-ore mines are presented in Tables 3 and 4. As observed in Table 3, the arithmetic mean, geometric mean and 50th percentile (median) values for Cu, Zn, Ni, Cd, Cr, Co and As are higher in rock samples from Itakpe iron-ore mines than their corresponding average crustal values (ACVs) as obtained in Shaw et al. (1967), while Fe and Mn present arithmetic mean, geometric mean and 50th percentile values lower than their respective ACVs. Only Pb values are similar to its ACV. From Table 4, Fe, Mn, Ni, Cr and Pb present values lower than their ACVs. Of note is the concentration of Cd in rock and soil samples which is about 404 and 62.7 times, respectively, higher than the average crustal concentration. The average concentration of heavy metals in rock samples decreases according to the following order: Fe > Zn > Cu > Mn > (Cr=Co) > Cd > Ni > As > Pb, while those of soil samples follow the following decrease order: Fe > Zn > Co > Mn > Cu > Cr > Ni > Cd > As > Pb. Figures 2 and 3 show the box whisker plot for the distributions of heavy metals in the study area. It can be seen from the figures that only Fe, Zn and Cu show moderately wide distribution, while others fall within narrow ranges indicating that the distributions of the heavy metals are influenced by a wide degree of variations both in rock and soil samples. The calculation of coefficient of variation also confirms the variability in the dispersion of the heavy metals in the study area. Coefficient of variation value lower than 20% indicates low variability, while value between 20 and 50% implies moderate variability, value greater than 50% but less or equal to 100% shows high variability and coefficient of variation value greater than 100% is regarded as exceptionally high variability (Karim et al. 2015). From the results, all the heavy metals show low to moderate variability in the rock, soil and water samples. The statistical description of heavy metal concentrations for water samples is summarized and presented in Table 5. The heavy metals show average concentration of 5.5 ± 2.5, 1.8 ± 0.8, 8.5 ± 3.0, 5.8 ± 1.0, 0.6 ± 0.2 and 0.4 ± 0.2 mg/l for Fe, Mn, Cu, Zn, Ni and Co, respectively. Cd, Cr, Pb and As were not detected in all the water samples.
The process of mining and extraction of the iron metal from the ore rocks involves crushing of the rocks, which generate huge rock and soil dusts in the mining environment. The dust generated can cause exposure to human through ingestion, inhalation and dermal contact. Human health risks associated with heavy metals in rock and soil samples were assessed through these three pathways on the assumption that rock and soil dust cause similar health risks. The results of human health risks assessment due to heavy metals in rocks and soils of the abandoned ire-ore mines are presented in Tables 6 and 7. From the tables, it can be seen that ingestion pathway plays a significant role in the average daily intake of heavy metals in the mining environment for non-cancer risks. The hazard quotients due to dermal contact are higher for Cu, Cr and Cd in rock samples than ingestion and inhalation pathways. For soil, the hazard quotients due to dermal contact are higher in Zn, Cu, Cr and Cd than other pathways. Only the hazard index (HI) due to Cd in dust from rock samples show values that are above the acceptable limit of HI equals unity. For non-cancer risk, the values of the hazard indices due to heavy metals rock samples are more pronounced than those for soil samples. For cancer risk, only Ni, Cr, Cd, Co and As were considered (Bai et al. 2017). The cancer risks obtained for the five heavy metals in rock and soil dusts are much lower than the acceptable upper limit of 10−4. From the results, it can be observed that non-cancer risks are more important than cancer risks in the study area. It can also be seen that both non-cancer and cancer risks for humans living in the vicinity of the abandoned iron-ore mines are all at acceptable levels.
3.2 Natural Radionuclides
The summary of the results of the elemental concentrations of K, eU and eTh for rock and soil samples is presented in Tables 8 and 9. The evaluated average concentrations of K, U and Th in rock samples are as follows: 2.9%, 0.8 and 1.2 ppm with a range of 0.5–6.2%, 0.2–2.1 and 0.4–2.1 ppm, respectively. In soil samples, the average concentrations are 1.3%, 0.7 and 1.7 ppm with a range of 0.2–2.2%, ND to 1.5 and ND to 4.4 ppm, for K, U and Th, respectively. The world mean crustal values of K, U and Th are 1.3%, 2.7 and 11.1 ppm, respectively (UNSCEAR 2008) and the mean values obtained for rock and soil samples in the abandoned mines were far lower than these mean values except for K which falls within the same range. The air-absorbed gamma dose rates, annual effective dose and excess life cancer risks calculated from elemental concentrations of radionuclides in rock and soil samples are shown in Tables 8 and 9, respectively. The mean absorbed dose rate in rock samples is 46.0 nGy/h, while that of soil sample is 26.0 nGy/h. The mean absorbed gamma dose rate obtained for both rock and soil samples is lower than the worldwide population weighted mean of 60 nGy/h as reported in UNSCEAR (2008). The mean annual effective doses in rock and soil samples are 56.5 and 31.9 µSv/y, which are both lower than the world average value of 70 µSv/y. The average excess lifetime cancer risks due to the levels of radionuclides in rock and soil samples are 1.98 × 10−4 and 1.12 × 10−4, respectively. The values are lower than the world average figure of 0.29 × 10−3 for soil and 0.05 for low-level radiation exposures as recommended by UNSCEAR and ICRP (Kolo et al. 2015).
3.3 Correlation Analysis
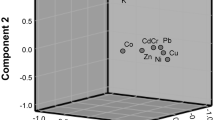
Pearson correlations between the obtained data were performed utilizing STATISTICA (Release 7) software package in order to determine the relationships between heavy metals and natural radionuclides. The correlation coefficients at p ≤ 0.05 for heavy metal and natural radionuclides in rock and soil samples are presented in Tables 10 and 11. In the rock samples, none of the three radionuclides show significant relationship with heavy metals. Fe has a strong positive correlation with Mn, Cu and Cr but negatively correlated with Zn, Co, Pb, K, U and Th. Mn is significantly correlated with Cr and As. Cu is strongly and positively correlated with Zn, Ni Cd, Cr and As. Zn has a significant positive relationship with Ni. Cd is strongly and positively related with Cr, Co and As, while Cr is strongly correlated with As. There also exists a positive significant relationship between Co and Pb. For soil samples, K, U and Th show negative correlation with most of the heavy metals. Some of the heavy metals show significant positive correlations in soil sample, e.g., Zn with Fe, Cd with Mn, Cu, and Ni, Cr with Mn, Cu and Cd, Cu with Mn, and As with Mn, Cu, Cr, and Co. The relationship between U and K is positive and significant so also between Th and U. Strong positive correlations between heavy metals or radionuclides suggest similarity in origin, transfer mechanism or behavior in the environment. The absence of correlations implies that the heavy metals or radionuclides are mutually independent or differ in behavior (Bai et al. 2017).
4 Conclusion
Heavy metals (Fe, Mn, Zn, Cu, Ni, Cd, Cr, Co Pb and As) and natural radionuclides (K, U and Th) were measured in rock, soil and water samples from an abandoned iron-ore mines located in the north central region of Nigeria. All the metals considered in this study were detected at varying concentrations in rock and soil samples. Cd, Cr, Pb and As were not detected in water samples. The concentrations of heavy metals vary according to the following pattern; rock > soil > water. It can then be concluded that heavy metals in the study area are derived predominantly from the rocks through weathering and other geological and geographical processes. Cancer and non-cancer risks due to potentially toxic heavy metals were estimated for adult population living in the vicinity of the abandoned mines. Values obtained for rock and soil samples were lower than the acceptable limits. The risks of heavy metals are higher in rock than soil samples. The average elemental concentrations of the three radionuclides are lower than their respective worldwide mean values. The mean annual effective doses in rock and soil samples are lower than the world mean value of 70 µSv/y as reported in UNSCEAR Reports. Various levels of correlations were observed among the heavy metals and natural radionuclides for rock and soil samples. Natural radionuclides were negatively correlated with most of heavy metals in rock and soil samples indicating that their origins and behaviors are different.
References
Acosta JA, Faz A, Martínez-Martínez S, Zornoza R, Carmona DM, Kabas S (2011) Multivariate statistical and GIS-based approach to evaluate heavy metals behavior in mine sites for future reclamation. J Geochem Explor 109(1):8–17
Ademola AK, Olaoye MA, Abodunrin PO (2015) Radiological safety assessment and determination of heavy metals in soil samples from some waste dumpsites in Lagos and Ogun state, south-western, Nigeria. J Radiat Res Appl Sci 8(1):148–153
Adriano DC (2003) Trace elements in terrestrial environments: biogeochemistry, bioavailability and risks of metals, 2nd edn. Springer, New York
Ajayi TR, Torto N, Tchokossa P, Akinlua A (2009) Natural radioactivity and trace metals in crude oils: implication for health. Environ Geochem Health 31(1):61–69
Antunes IMHR, Neiva AMR, Albuquerque MTD, Carvalho PCS, Santos ACT, Cunha PP (2017) Potential toxic elements in stream sediments, soils and waters in an abandoned radium mine (central Portugal). Environ Geochem Health. https://doi.org/10.1007/s10653-017-9945-2
Atta ER, Zakaria KM, Ibrahim MS (2016) Assessment of the heavy metals and natural radioactivity in phosphate mines and occupational health effects at some Egyptian regions. J Environ Prot 7(11):1657
Aytas S, Erenturk S, Aslani MA, Yusan S, Turkozu DA, Gok C, Karali T, Gokce M, Oguz KF (2014) Determination and evaluation of natural radioactivity and heavy metal levels in the aquatic environment of trans-boundary rivers: Maritza, Tundja and Arda. J Radioanal Nucl Chem 300(3):933–945
Bai H, Hu B, Wang C, Bao S, Sai G, Xu X, Zhang S, Li Y (2017) Assessment of radioactive materials and heavy metals in the surface soil around the Bayanwula prospective uranium mining area in China. Int J Environ Res Public Health 14(3):300
Baykara O, Doğru M (2010) Assessment of natural radioactivity and heavy metals in water and soil around seismically active area. J Radioanal Nucl Chem 283(3):547–553
Bleam WF (2016) Soil and environmental chemistry. Academic Press, Cambridge
Chen H, Teng Y, Lu S, Wang Y, Wang J (2015) Contamination features and health risk of soil heavy metals in China. Sci Total Environ 512:143–153
Christoforidis A, Stamatis N (2009) Heavy metal contamination in street dust and roadside soil along the major national road in Kavala’s region, Greece. Geoderma 151(3):257–263
El-Amier YA, Elnaggar AA, El-Alfy MA (2017) Evaluation and mapping spatial distribution of bottom sediment heavy metal contamination in Burullus Lake, Egypt. Egypt J Basic Appl Sci 4(1):55–66
El-Taher A, Althoyaib SS (2012) Natural radioactivity levels and heavy metals in chemical and organic fertilizers used in Kingdom of Saudi Arabia. Appl Radiat Isot 70(1):290–295
Faisal BMR, Majumder RK, Uddin MJ, Deeba F, Paul D, Haydar MA, Ali MI (2015) Assessment of heavy metals pollution and natural radioactivity in topsoil of Savar industrial area, Bangladesh. Int J Environ Sci 5(5):964
Ferreira-Baptista L, De Miguel E (2005) Geochemistry and risk assessment of street dust in Luanda, Angola: a tropical urban environment. Atmos Environ 39:4501–4512
Gbadebo AM, Ekwue YA (2014) Heavy metal contamination in tailings and rocksamples from an abandoned goldminein southwestern Nigeria. Environ Monit Assess 186(1):165–174
Goher ME, Farhat HI, Abdo MH, Salem SG (2014) Metal pollution assessment in the surface sediment of Lake Nasser, Egypt. Egypt J Aquat Res 40(3):213–224
Hiller E, Lachká L, Jurkovič Ľ, Ďurža O, Fajčíková K, Vozár J (2016) Occurrence and distribution of selected potentially toxic elements in soils of playing sites: a case study from Bratislava, the capital of Slovakia. Environ Earth Sci 75(20):1390
Hu X, Zhang Y, Ding Z, Wang T, Lian H, Sun Y, Wu J (2012) Bioaccessibility and health risk of arsenic and heavy metals (Cd Co, Cr, Cu, Ni, Pb, Zn and Mn) in TSP and PM2. 5 in Nanjing, China. Atmos Environ 57:146–152
IAEA (1989) Construction and use of calibration facilities for radiometric field equipment, Technical Report Series No. 309, IAEA Vienna, Austria
IAEA (2003) Guidelines for radioelement mapping using gamma ray spectrometry data. IAEA-TECDOC-1363. IAEA,Vienna, Austria
IAEA (2008) Quality control procedures applied to nuclear instruments. IAEA-TECDOC 1599, IAEA, Vienna, Austria
Isinkaye MO, Shitta MO, Oderinde OM (2013) Determination of radionuclides and elemental composition of clay soils by gamma-and X-ray spectrometry. SpringerPlus 2(1):74
Karim Z, Qureshi BAL, Mumtaz M (2015) Geochemical baseline determination and pollution assessment of heavy metals in urban soils of Karachi, Pakistan. Ecol Indic 48:358–364
Kirpichtchikova TA, Manceau A, Spadini L, Panfili F, Marcus MA, Jacquet T (2006) Speciation and solubility of heavy metals in contaminated soil using X-ray microfluorescence, EXAFS spectroscopy, chemical extraction, and thermodynamic modeling. Geochim Cosmochim Acta 70(9):2163–2190
Kolo MT, Aziz SABA, Khandaker MU, Asaduzzaman K, Amin YM (2015) Evaluation of radiological risks due to natural radioactivity around Lynas Advanced Material Plant environment, Kuantan, Pahang, Malaysia. Environ Sci Pollut Res 22(17):13127–13136
Li Z, Ma Z, van der Kuijp TJ, Yuan Z, Huang L (2014) A review of soil heavy metal pollution from mines in China: pollution and health risk assessment. Sci Total Environ 468:843–853
Meindinyo RK, Agbalagba EO (2012) Radioactivity concentration and heavy metal assessment of soil and water, in and around Imirigin oil field, Bayelsa state, Nigeria. J Environ Chem Ecotoxicol 4(2):29–34
Milenkovic B, Stajic JM, Gulan L, Zeremski T, Nikezic D (2015) Radioactivity levels and heavy metals in the urban soil of Central Serbia. Environ Sci Pollut Res 22(21):16732–16741
Mileusnić M, Mapani BS, Kamona AF, Ružičić S, Mapaure I, Chimwamurombe PM (2014) Assessment of agricultural soil contamination by potentially toxic metals dispersed from improperly disposed tailings, Kombat mine, Namibia. J Geochem Explor 144:409–420
National Research Council (NRC) (1983) Risk assessment in the federal government: managing the process. National Academies Press, Washington DC, USA
Qureshi AA, Tariq S, Din KUS, Calligaris C, Waheed A (2014) Evaluation of excessive lifetime cancer risk due to natural radioactivity in the rivers sediments of Northern Pakistan. J Radiat Res Appl Sci 7:438–447
Shaw DM, Reilly GA, Muysson JR, Pattenden GE, Campbell FE (1967) An estimate of the chemical composition of the canadian precambrian shield. Can J Earth Sci 4(5):829–853
Szarlowicz K, Reczynski W, Misiak R, Kubica B (2013) Radionuclides and heavy metal concentrations as complementary tools for studying the impact of industrialization on the environment. J Radioanal Nucl Chem 298(2):1323–1333
Taskin H, Karavus MAP, Topuzoglu A, Hindiroglu S, Karahan G (2009) Radionuclide concentrations in soil and lifetime cancer risk due to the gamma radioactivity in Kirklareli, Turkey. J Environ Radioact 100:49–53
United Nations Scientific Committee on the Effect of Atomic Radiation (UNSCEAR) (2001) Sources and effects of ionizing radiation. Report to general assembly with scientific annexes. United Nations, New York
UNSCEAR (2008) Sources and effects of ionizing radiation. United Nation Scientific Committee on Effects of Atomic Radiation (UNSCEAR) 2008 Report to the General Assembly, vol 1
UNSCEAR (2011) Sources and effects of ionizing radiation. United Nation Scientific Committee on Effects of Atomic Radiation (UNSCEAR) Report to the General Assembly
USEPA (1999) Cancer Risk Coefficients for Environmental Exposure to Radionuclides. Federal Guidance Report 13. Available at: https://nepis.epa.gov/Exe/ZyNET.exe/00000C9E.TXT?ZyActionD=ZyDocument&Client=EPA&Index=1995+Thru+1999&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A%5Czyfiles%5CIndex%20Data%5C95thru99%5CTxt%5C00000013%5C00000C9E.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1&SeekPage=x&ZyPURL. Accessed 18 Sept 2017
USEPA (2001) Supplemental guidance for developing soil screening levels for superfund sites. OSWER9355.4-24. Office of Solid Waste and Emergency Response. United States Environmental Protection Agency, Washington DC. http://www.epa.gov/superfund/resources/soil/ssgmarch01.pdf. Accessed 18 Sept 2017
Wang J, Du J, Bi Q (2017) Natural radioactivity assessment of surface sediments in the Yangtze Estuary. Mar Pollut Bull 114(1):602–608
Wuana RA, Okieimen FE (2011) Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. Int Sch Res Not Ecol 2011:1–20
Xu S, Zheng N, Liu J, Wang Y, Chang S (2013) Geochemistry and health risk assessment of arsenic exposure to street dust in the zinc smelting district, Northeast China. Environ Geochem Health 35(1):89–99
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Isinkaye, O.M. Distribution and Multivariate Pollution Risks Assessment of Heavy Metals and Natural Radionuclides Around Abandoned Iron-Ore Mines in North Central Nigeria. Earth Syst Environ 2, 331–343 (2018). https://doi.org/10.1007/s41748-018-0035-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41748-018-0035-0