Abstract
Development, validation, and testing of algorithms for artificial pancreas (AP) systems require mathematical models for the glucose–insulin dynamics inside the body. These physiological models have been extensively studied over the past decades. Two broad types of models are available in diabetic research, each with its own unique purpose: (i) minimal models, which are relatively simple but still manages to capture the macroscopic behavior of the glucose–insulin dynamics of the body, and (ii) high-fidelity models, which are complex and precisely describe the internal dynamics of the glucose–insulin interaction in the body. The minimal models are primarily utilized for control algorithm synthesis, whereas the high-fidelity models are used as platforms for testing and validating AP systems. The most well-known variants of these physiological models are discussed in detail. In addition to these systems, data-driven models such as the auto-regressive moving average with exogenous inputs (ARMAX) models are also used widely in control algorithm synthesis for AP systems. High-fidelity models are utilized for simulating virtual diabetic patients for in silico testing and validation of artificial pancreas systems. Two currently available high-fidelity models are reviewed in this paper for completeness, including the Type-1 diabetes mellitus (T1DM) simulator approved by the food and drug administration of USA. Models accounting for exercise and also glucagon infusion (for dual-hormone AP systems) are also included, which are essential in developing control algorithms with better autonomy and minimal risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Diabetes mellitus (DM) is a metabolic condition in which a person is unable to regulate the blood glucose (BG) levels in his/her body. The blood sugar in normal people ranges between 70 and 180 mg per deciliter (mg/dL). Since people with DM are unable to regulate their BG, they experience hyperglycemia (above 180 mg/dL) and hypoglycemia (below 70 mg/dL). Prolonged hyperglycemia can lead to several long-term health complications such as diabetic retinopathy, neuropathy, nephropathy, and many more. Hypoglycemia can lead to loss of consciousness, coma, and sometimes even death. There are three types of diabetes that people are affected by, namely (i) Type-1 diabetes (T1DM), where no insulin secretion takes place in the pancreas; (ii) Type-2 diabetes (which affects the majority of diabetics in the world), where the amount of insulin secreted by the pancreas is not sufficient to regulate the blood sugar levels because of insulin resistance; and (iii) gestational diabetes which is generally caused by insulin-blocking hormones produced during pregnancy. According to the Diabetes Atlas by the International Diabetes Federation (IDF), approximately 537 million adults are affected by diabetes. This number is predicted to increase to 643 million by 2030 and 784 million by 20451. Approximately \(10\%\) of all diabetes are Type-1 while roughly \(90\%\) are Type-22. Gestational diabetes is temporary and goes away after pregnancy in most cases. However, it increases the risk of becoming Type-2 later in life2.
Since Type-1 Diabetic patients cannot produce insulin naturally, they depend on external insulin infusion (typically in the form of multiple injections on a daily basis). Insulin doses are typically delivered in two ways: (i) Basal insulin, which is the insulin dose given to maintain steady-state glucose levels at all time, and (ii) Bolus insulin, which is the insulin dose given to compensate for glucose rise whenever the patient consumes a main meal. Basal insulin is typically a long-acting insulin that is given once a day, the dose of which mainly depends on the body mass index (BMI) of the patient. The bolus insulin, on the other hand, is typically a short-acting insulin, the amount of which is calculated depending on the carbohydrate content of the food intake. Computation of the bolus amount is expected to be done by the patient based on a long chart of food items. Most of these patients also keep track of their glucose levels a few times a day using a standard self-monitoring blood glucose (BG) meter. However, in such an approach, the patients are more prone to significant health complications due to BG excursions beyond safe limits, both due to incorrect doses and also due to the sparsity of glucose monitoring.
Over the last few decades, significant technological advancements have enabled glucose-measuring devices that are more continuous and less intrusive in nature. Similarly, more accurate and convenient to use insulin pumps are now available. Popular devices that are fairly widely used by T1DM patients to monitor the BG level and deliver insulin are continuous glucose monitoring (CGM) sensors and insulin pumps, respectively. However, although insulin pumps are more convenient to use and are helpful in ensuring lesser excursions of BG beyond the safe limits provided the patient is smart enough to keep changing the basal and bolus set points appropriately, they are not a true alternative for the natural pancreas. This is because through such a mechanism it is impossible for a human being to keep changing the doses and select right amount of insulin infusion at the right time.
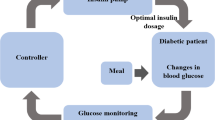
To address this strong limitation, taking the help of fairly continuous measurement of BG through CGM followed by smart control algorithms, efforts are being made to automate this glucose regulatory process. The resulting system, which is known as the Artificial Pancreas (AP) system, is a closed-loop biomedical system, designed to serve as close to the natural pancreas system as possible with no/minimal manual intervention by the patient. The insulin to be delivered is calculated using sophisticated control algorithms. The philosophy of the AP system was first introduced in 19743. Since then, several research efforts have been made to design control algorithms that mimic the metabolic control action of the natural pancreas. Note that artificial pancreas is a safety-critical control system, failure of which can result in significant health complications.
Control algorithms are primarily of two types: (i) model-based and (ii) data-driven. Whenever possible, a model-based approach is preferred as it is based on the understanding of the underlying system dynamics. However, such an approach requires a mathematical model that describes the dynamics of the system as accurately as possible. The model mainly serves two purposes: (i) to compute the required control input by exploiting the model and (ii) to conduct in silico (computer simulation) trials in order to validate and gain confidence in the designed control system. Mathematical modeling plays a vital role in the development, testing, and validation of artificial pancreas systems as well. The two types of models that are extensively used for artificial pancreas are (i) minimal models: These models are relatively simple and only capture essential components of the glucose–insulin dynamics in the body. These models are also called as control-oriented minimal models as they are primarily utilized for control algorithm synthesis for blood glucose regulation. (ii) High-fidelity models: these models accurately mimic the internal dynamics of the body, including processes such as liver function, glucose production as well as glucagon production. The high-fidelity models are primarily used to verify and test AP algorithms as a precursor to clinical trials. Note that the FDA-approved high-fidelity T1DM model has sufficient fidelity to eliminate the requirement for animal testing of AP systems prior to the clinical trials in case one follows the same inclusion criteria4. The main purpose of this article is to review various minimal and high-fidelity models used for artificial pancreas development worldwide.
Before concluding this section, the authors of this paper would like to mention other similar works published on artificial pancreas and mathematical models associated with it. In the “The Artificial Pancreas: Current Situation and Future Directions”5 book, research activities carried out by multidisciplinary teams worldwide are outlined with a focus on the engineering and medical aspects of the AP research. A review of mathematical models from various research groups is also presented in the book. A review of simulators utilized for AP development was compiled by Hovorka et al. in Ref.6, where the focus is on high-fidelity models. Other notable review papers include the article by Boris Kovatchev7, where a history of glucose monitoring devices, mathematical models, and control algorithms is presented. However, the article does not present mathematical insight into models and algorithms. Skyler et al.8 discuss the advances in physical devices as well as control algorithms in AP systems.
The rest of the paper is organized as follows. Section 2 focuses on the mathematical modeling of the internal dynamics of glucose and insulin in the context of control synthesis. Section 3 focuses on high-fidelity models used in Type-1 Diabetic patient simulators. These models are primarily used to evaluate control algorithms for AP systems. The more recent advancements in modeling for AP systems include models for exercise and dual-hormone pumps. These are discussed in Sect. 4. Appropriate conclusions are derived in Sect. 5.
2 Minimal Models for Control Synthesis
As mentioned in Sect. 1, model-based control algorithms rely on a “plant-model”, in which a set of differential equations, the solution of which mimics the time response of the actual system. There are two kinds of models, namely: (i) physiological models that mathematically interpret the process of glucose–insulin interaction by modeling meal ingestion, insulin administration, and glucose regulation; and (ii) data-driven models that do not require a physiological interaction of the behavior, but utilize time-tagged historical data instead to establish an input–output relationship.
2.1 Physiological Minimal Models
In recent years, minimal models to approximately capture the internal glucose–insulin dynamics of the patient have been proposed to have a macroscopic understanding of the system behavior and to facilitate closed-loop control synthesis algorithms. The minimal model can be split into three primary subsections to describe the underlying physiological process, as described below.
2.1.1 Gluco-regulatory Models
Gluco-regulatory models describe the interaction between glucose and insulin in the bloodstream. This phenomenon has been studied for decades, and several mathematical models have been proposed. A few of the most relevant ones are included here.
Bergman Minimal Model: The most well-known and widely used gluco-regulatory model proposed by Bergman et al.9 describes the disappearance of glucose from the blood and key physiological parameter such as insulin sensitivity. The three-compartmental model with appropriate variations describes the glucose-regulatory behavior of different patients with varying internal dynamics. The Bergman minimal model equations for Type-1 Diabetic patients are given in Eq. (1) where G, X represent the glucose in the bloodstream and insulin within the remote insulin compartment, respectively. Insulin sensitivity and glucose effectiveness are defined by \(S_I = p_3/p_2\) and \(p_1 (min^{-1})\), respectively. The term \(R_a\) describes the rate of appearance of glucose in the bloodstream due to the effect of a meal. The terms \( G_b\) and \(I_b\) denote the steady-state basal levels of glucose and insulin in the blood, respectively. Note that this model is augmented for healthy patients with another state that describes insulin levels:
Hovorka Gluco-regulatory Model: Hovorka et al. proposed a slightly higher fidelity model10 (as compared to the Bergman minimal model) by considering more complex interactions between glucose and insulin in the body. This model considers Endogenous Glucose Production (EGP) that is responsible for glucose production in the absence of a meal. The model equations are shown in Eq. (2), where the terms \(G_1\) and \(G_2\) represent the glucose levels in the accessible and non-accessible compartments, respectively. While the equations model the effect of insulin in glucose distribution, disposal, and production (\(x_1,x_2,x_3\), respectively), the Bergman minimal model only describes the interaction between glucose and insulin in the bloodstream. The equations describing the system dynamics are reproduced as follows:
Cobelli Gluco-regulatory Model: The model developed by Cobelli et al.11 also has a higher fidelity compared to the Bergman minimal model. This gluco-regulatory model is included in the FDA-approved high-fidelity T1DM simulator due to its ability to describe details such as EGP, insulin-independent glucose utilization (\(U_{id}\)) and insulin-dependent glucose utilization (\(U_{ii})\). The states are indicated by \(G_p\) and \(G_t\), which correspond to glucose in the plasma and glucose in the tissue, respectively. Together they are used to calculate the glucose levels in the blood, G. The glucose concentration in the plasma is \(G(t) =\frac{G_{p}}{V_{G}}\), where \(V_{G}\) is the volume distribution. Although the model has a higher fidelity, note that the parameters need to be identified through extensive ‘tracer studies’, which are difficult to carry out in general. Further, \(R_a\) is the rate of glucose appearance as seen in the Bergman minimal model (Eq. (1)):
2.2 Meal Models
In addition to the gluco-regulatory model, meal models that describe the carbohydrate ingestion process from the meal and its transport from the stomach to the intestines have also been developed, which are briefly reviewed in this section.
Dalla Man Meal Model: One of the most important models that describe the meal ingestion process was developed by Dalla Man et al.11. The model equations are shown in Eq. (4), where \(Q_{sto_{1}} \text { and } Q_{sto_{2}}\) represent the solid and liquid phase of the carbohydrate content in the stomach, respectively. The terms \(Q_{gut}\) and \(k_{abs}(min^{-1})\) indicate the carbohydrate content in the intestine and its rate of absorption, respectively:
The gastric emptying rate \(k_{empty} (min^{-1})\) depends on the total amount of food in the stomach (\(Q_{sto} = Q_{sto_{1}}+Q_{sto_{2}}\)) and is a time-varying parameter. The representation of the glucose transfer rate from the stomach to the intestines brings a vital advantage to the Dalla Man model. The system of equations describing this phenomenon is given as follows:
Hovorka Meal Model: Hovorka et al.6 proposed a much simpler model for glucose absorption in which the gastrointestinal system is a compartmental model with two identical compartments and transfer rates. The term D(t) indicates the carbohydrate ingested in grams. The term Bio represents the effectiveness of the ingested carbohydrate absorption, i.e., the consumed carbohydrates proportion that will go into the circulatory system. The maximum absorption time for the carbohydrates is indicated by \(t_{max}\) that regulates the transfer speed between the two compartments:
However, note that this model does not consider the variable gastric emptying rate, as shown in the Dalla Man model (Eq. (5)).
2.3 Subcutaneous Insulin Kinetics Models
T1DM patients regulate glucose by insulin injections or insulin pumps that deliver insulin subcutaneously. This mechanism of insulin transport from the subcutaneous layer to the bloodstream has been modeled by several studies. The prominent ones include the following12,13.
Dalla Man Insulin Model: The equations of this model are given in Eq. (7). The three states \(I_{sc_1}, I_{sc_2}\) and \(I_p\) represent non-monomeric insulin, monomeric insulin (in the subcutaneous tissue layer) and plasma insulin, respectively. The rate of absorption of non-monomeric insulin from the subcutaneous layer to plasma and the rate of diffusion into the second compartment is indicated by \(k_{a_{1}} (min^{-1})\) and \(k_d (min^{-1})\), respectively. The insulin is finally absorbed into the plasma at a rate of \(k_{a_{2}}(min^{-1})\). There is a significant time delay between insulin infusion and its appearance in the subcutaneous layer. This delay is modeled as a pure time delay (\(u(t-\tau )\)):
The aforementioned equations represent a basic three-compartment linear model in which the insulin absorption and disappearance rates are assumed to be unaffected by other factors.
Subcutaneous Insulin Model for Computer Simulation: This model was developed by Wong et al.13 to describe plasma insulin kinetics after the subcutaneous administration of an insulin bolus and a continuous infusion of insulin lispro. The associated equations corresponding to this model are given in Eq. (8). The model accounts for two different pathways of insulin absorption. The first with two compartments and a rate constant \(k_{a_{1}}\) (per min) and the second with a single compartment with rate constant \(k_{a_{2}}\) (per min) along with the proportion parameter k (%) of insulin channeled through these pathways. A saturable local degradation at the injection site is considered for both paths. The parameters \(V_{max} \) (mU/min) and \(K_m \) (mU) represent the saturation level and the insulin mass at which insulin degradation equals half of its maximum value, respectively:
The minimal models described in the above sections form an integral part of the control algorithm development for blood glucose regulation. The authors have presented a system of equations which is presented in Ref.14 where the modified Bergman Minimal Model, the Dalla Man meal model, the Dalla Man insulin kinetics system were augmented to construct a minimal model. (Eqs. (1), (4), and (7)). For the benefit of the reader, a summary of the advantages and limitations of each subsystem is highlighted in the following table.
Model variant | Advantages | Limitations |
|---|---|---|
Gluco-regulatory Model | ||
Bergman Model | Quantifies insulin sensitivity and glucose effectiveness, parameters easily identifiable | Does not account for endogenous glucose production (EGP), Interaction of glucose and insulin simplified |
Hovorka Gluco-regulatory Model | Higher fidelity, describes the effect of insulin on glucose production, disposal, and distribution | Number of states and parameters are higher than the other two models |
Cobelli Gluco-regulatory Model | Higher fidelity, model includes: EGP, insulin-dependent and insulin-independent glucose utilization | Identification of model parameters for individual patients requires tracer studies |
Meal Model | ||
Dalla Man Meal Model | Captures nonlinear effect of gastric emptying | Assumes rate of absorption of carbohydrate in the intestine is constant |
Hovorka Meal Model | Simpler two-compartment model | Assumes a constant gastric emptying rate |
Subcutaneous Insulin Kinetics Model | ||
Dalla Man Insulin Model | Accounts for the delay involved in insulin administered subcutaneously | Model only validated for 10.9 Units to 19.4 Units of fast acting insulin (Lispro) |
Jason et al. Insulin Model | Insulin injected appears proportionally via two pathways | Assumes a constant gastric emptying rate |
Even though physiological models are appealing, the major drawback of such models is the data requirements for parameter identification. To identify the model parameters, glucose and insulin data samples obtained from standard “tolerance tests” are used in parameter identification techniques. An alternative modeling method to avoid this problem is the “data-driven approach” that relies only on time-tagged historical glucose data (for a small finite window of time), which is relatively much easier to access using CGM sensors.
2.4 Data-Driven Insulin Customization
Since physiological model-driven control techniques rely on the identification of several customized parameters, there are often challenges associated with the observability of states and identification of physiological parameters. A possible alternative is to use lower order data-driven models. These techniques do not rely on an accurate mathematical model of the system for control synthesis. Instead, they rely on data to approximate the input–output relation to form second (or) third-order linear models that customize insulin doses according to past glucose data. A few popular data-driven modeling techniques are Auto-regressive Moving Average (ARMAX) and Nonlinear ARMAX (NARMAX) and their variants15. These models can be utilized with MPC by replacing the physiological minimal model, as they do not require extensive parameter identification. Detail discussions on these models are omitted here for brevity.
3 High-Fidelity T1DM Models
The internal dynamics of the glucose–insulin interaction in the body are quite complex. Although minimal models approximate this behavior, they do not account for multiple processes, such as liver function and glucagon secretion. Therefore, the minimal models cannot be utilized for simulating Type-1 patients’ behavior. High-fidelity models bridge this gap and capture the complexities of the internal dynamics of the body with a high degree of accuracy. These high-fidelity models can therefore be utilized for in silico testing and validating control algorithms. The subsystems of the high-fidelity model and two widely used simulators are discussed in this section.
3.1 Subsystems in High-Fidelity Models
In addition to the minimal model subsystems detailed in Sect. 2, the major subsystems/processes modeled include the conversion of carbohydrates into blood glucose, the kinetics of insulin from the subcutaneous layer to the bloodstream, and glucose production in the liver. This subsection will focus on aspects that are not accounted for in the control-oriented minimal model.
3.1.1 The Liver: Endogenous Glucose Production
Endogenous Glucose Production (EGP) is responsible for the rise of glucose in the bloodstream in the absence of food. In addition to the insulin-producing beta cells, the natural pancreas also has alpha cells, that produce a hormone called glucagon. Glucagon is responsible for increasing glucose concentration through endogenous glucose production.
The rate of glucose production (or) EGP in the liver is modeled as Eq. (9)16 and depends on the glucose concentration in the plasma (\(G_p(t)\)), delayed insulin action in the liver (\(X^{L}(t)\)), and delayed glucagon action in the liver (\(X^{H}(t)\)). The term \(\xi \) represents the change in glucose production in the liver due to the presence of glucagon. The delayed insulin action is dependent on the amount of insulin in the liver (\(I'(t)\)), and the glucagon action depends on the plasma glucagon concentration (H(t)). Glucagon action is modeled such that there is no glucagon secretion in the liver if its concentration is above the basal value \((H_b)\). Note that kp1 (mg/kg/min) is the endogenous glucose production rate at zero glucose and insulin, whereas, kp2, kH, ki are rate constants (min−1) that describe the delays between the compartments in the liver subsystem:
Finally, the endogenous glucose production can be modeled as \(EGP(t) = k_{p1}(t) - k_{p2}G_{p}(t) - k_{p3}(t)X^{L}(t) + \xi X^{H}(t)\).
3.1.2 Glucagon Secretion Model
It is mentioned in the previous subsection that glucose production in the liver is a function of insulin and glucagon hormones (\(X^L(t)\) and \(X^H(t)\), respectively). Further, the delayed glucagon action was shown to be a function of glucagon concentration in the plasma (H(t)). Therefore, the secretion of glucagon hormone from the pancreas, which subsequently appears in plasma (as H(t)), is simulated in the glucagon secretion model. The equations that mimic the secretion of the glucagon hormone in the body are shown in Eq. (10)16. The secretion of glucagon is modeled as a single-compartment linear model where n is the rate of clearance of glucagon from the plasma and \(SR_{H}\) is the secretion of glucagon in the alpha cells of the pancreas. Glucagon administered externally (in the case of dual-hormone pumps) also contributes to glucagon update. The effect of external glucagon administration is indicated by \(Ra_{H}(t)\):
The rate of secretion of glucagon cells \((SR_{H}(t))\) is given as a sum of dynamic and static secretion. The dynamic secretion \(SR^{d}_{H}(t)\) is a function of the rate of change of glucose levels (\(\delta \times \max ( -\frac{dG(t)}{dt},0 )\)), which implies that dynamic glucagon secretion occurs only when the glucose concentration in the bloodstream is decreasing:
The static rate of secretion \((\dot{SR}^{s}_{H}(t))\) depends on the difference between the current glucagon secretion and basal glucagon concentration in the body. In the event that glucose concentration in the blood is lower than the basal level (i.e., \([G(t) - G_b]<0\)), the rate of secretion also depends on the insulin level in the blood (I(t)) as shown in Eq. (12):
3.1.3 Insulin Delivery Models
In AP systems, insulin is administered through the subcutaneous layer using an insulin pump. The kinetic model of insulin from the subcutaneous layer to the bloodstream is included in the T1DM simulator and discussed in detail, along with other subcutaneous insulin kinetic models in Sect. 2. In addition, insulin can be delivered via intradermal injection and also be inhaled. Although these models are clinically validated, they are not discussed in this review article as they are typically not used in AP systems.
3.2 Advantages and Drawbacks of High-Fidelity Models
In addition to capturing the complex dynamics, the high-fidelity models account for the day-to-day variation parametric of the T1DM patient (intra-patient variability) and inter-patient variability of virtual patient populations as well. Besides giving a better insight to the underlying phenomenon, high-fidelity models are essential in control algorithm development for AP systems as they capture the natural physiological phenomena that mimic the internal dynamics of a diabetic patients, and hence can be used for the purpose of validation.
Although a comprehensive model such as the T1DM simulator accurately mimics the internal dynamics, they come with certain drawbacks as well. The parameter identifiability is one of the key drawbacks of a high-fidelity model. Conducting tracer studies become necessary to identify the parameters of all the models in the simulator. Due to many states and associated model parameters, the complexity of high-fidelity models prohibits them from being used for control algorithm development. Another critical behavior that alters blood glucose levels and the internal dynamics of the glucose–insulin system is the effect of exercise/physical activity. Note that many ‘high-fidelity models’ do not incorporate the effect of exercise into the simulation yet (this is a topic of ongoing research).
3.3 Currently Available High-Fidelity Models
Several research groups have developed high-fidelity models for simulating virtual T1DM populations. This section discusses the most widely used simulators for verifying the performance of AP algorithms and their features.
3.3.1 T1DM Simulator
A well-known Diabetic patient simulator is the T1DM Simulator16 that is jointly developed by the University of Virginia and the University of Padova. The T1DM simulator also called the UVA/Padova Simulator, is approved by the Food and Drug Administration (FDA) as a substitute for preclinical testing of insulin treatments17.
The various subsystems of the simulator primarily utilize the models developed by Dalla Man et al., including (i) The Gluco-regulatory model11 (ii) the Meal ingestion model11 (iii) The Subcutaneous Insulin Kinetics model12. Moreover, the effect of Endogenous Glucose Production and the effects of glucagon (see Sects. 3.1.1 and 3.1.2) are incorporated into the T1DM simulator. In addition to these physiological phenomena, the simulator includes models for Sensor noise and insulin pumps.
The validity of the T1DM simulator was established by comparing the simulations with the results of T1DM patients in clinical studies. The simulator also incorporates phenomenon that mimics real-world conditions such as (i) The dawn phenomenon: rise in glucose levels in T1DM subjects during the early morning hours (between 3:00 AM and 7:00 AM. (ii) The intra-variability of insulin sensitivity (\(S_I\)). Further, insulin sensitivity is a crucial parameter in T1DM patients that determines the effectiveness of a given quantity of insulin in lowering blood sugar levels. Insulin sensitivity does not remain constant and may vary from meal to meal. The variation of this parameter is also modeled in the T1DM simulator. The simulator has a virtual population of over 300 subjects (constituting adults, adolescents, and children) that can help to evaluate the robustness of control algorithms for a wide range of physiological characteristics. In addition, the simulator incorporates models to simulate Continuous glucose Monitors (CGM). CGM sensors have a significant delay in measurement and are prone to measurement noise. The details of the mathematical models, model parameters, and physiological interpretation of the models in the T1DM simulator are documented in Ref.16. A visual representation of the various subsections and the corresponding models within the simulator is shown in Fig. 1.
T1DM simulator functional block diagram (Ref:16).
3.3.2 Hovorka’s High-Fidelity Model
Hovorka et al. have also proposed a high-fidelity model consisting of a virtual population of 18 T1DM subjects18, which served as a simulator that was utilized for evaluating closed-loop algorithms. The various physiological phenomenon modeled in the simulator include Meal absorption, insulin action, glucose kinetics, insulin absorption, and Insulin kinetics. The equations for Hovorka’s model are included in Ref.19. In addition to the internal dynamics, the simulator incorporates models to simulate glucose measurements using a CGM and insulin delivery via an insulin pump. Further, the model parameters were validated in a clinical study with 12 T1DM patients6. In order to account for intra-subject variability, sinusoidal oscillations of model parameters are added to the simulator. The Hovorka group used this simulator to develop an AP system for children and adolescents affected by T1DM and was supported by the Juvenile Diabetic Research Foundation (JDRF)20.
4 Current and Future Trends
There are sufficient clinical studies that have shown significant improvement in the overall health and lifestyle of the patients using the AP systems. However, several aspects are still the subject of research, such as automatic meal detection and exercise compensation. More recently, dual-hormone pumps have been extensively studied. These futuristic aspects of AP research are discussed briefly in this section.
4.1 Dual-Hormone Models
Most AP approaches follow a uni-hormonal approach in which glucose levels are regulated using only insulin. However, it is to be noted that insulin can only decrease blood glucose levels. Therefore, it is considered to be a “one-sided control action”. More recently, bi-hormonal/dual-hormone AP systems and insulin pumps have been developed where insulin and glucagon are used to provide a “two-sided control action” for effectively regulating glucose levels21. Although the dual-hormone AP systems offer better control during exercise and prevent hypoglycemia22, there are certain disadvantages of using glucagon. In practice, it is challenging to use native glucagon as it is unstable23. The current research is focused on stabilizing glucagon and using it for AP Systems. Although the T1DM simulator contains a subsystem describing the effect of glucagon on endogenous glucose production, it has to be administered externally (via subcutaneous injections). A model capturing the external administration of glucagon is given below.
Subcutaneous Glucagon Kinetics: The kinetics of subcutaneous glucagon from administration to final appearance in the bloodstream was presented by Farhy et al.24. A two-compartmental model, as shown in Eq. (13), was proposed. The external insulin infused (\(H_{inf}(t)\)) subcutaneously appears in the first compartment (\(H_{sc1}\)) and diffuses into the second subcutaneous layer \(H_{sc_{2}}\) with a rate \(k_{h_{1}} (min^{-1})\). The glucagon finally appears in the bloodstream (\(Ra_{H}(t)\)) from the second compartment at a rate \(k_{h_{2}}(min^{-1})\) as is indicated in Eq. (10):
Finally, the externally infused glucagon appears in the glucagon secretion model as \(Ra_{H}(t) = k_{h_{3}}H_{sc_{2}}(t)\).
4.2 Models for Exercise
In addition to the glucose variation due to uncertainty in announced and unannounced meals, the patient’s internal-glucose dynamics also vary significantly during physical activity. It is well known that an increase in physical activity directly relates to faster-than-normal glucose decay in the bloodstream25. Therefore, an exercise model is necessary to compensate for the exercise-induced blood glucose degradation.
4.2.1 High-Fidelity Exercise Model
The critical component missing from the T1DM simulator is the effect of exercise. Exercise plays a vital role in the rate of glucose decay as well as insulin effectiveness. Insulin overdose during and after a strenuous activity/exercise can lead to hypoglycemia. The exercise effect on glucose was captured successfully at the Oregon Health and Science University, and a high-fidelity model was proposed with a statistical virtual patient population (VPP)26. The proposed mathematical patient models included (i) A Dual-Hormone VPP, which includes insulin and glucagon input, and (ii) A Single-Hormone VPP, which only takes insulin input. The models presented were validated via clinical testing, in which 20 patients were given known meals and participated in aerobic exercises for 45 min (two hours post-food intake). Although physical exercise is recommended by the American Diabetic Association (27), most Type-1 Diabetic patients (around \(63\%\)) do not exercise regularly as it may lead to hypoglycemia28. Although the effect of exercise on human physiology has been well studied, very few mathematical models exist that accurately represent the effect of exercise on glucose and insulin dynamics of the human body (particularly in T1DM patients)
4.2.2 Control Oriented Exercise Model
The high-fidelity exercise model discussed above is primarily utilized for simulating the exercise effect in T1DM patients but cannot be used for control algorithm synthesis due to a large number of parameters and states. Therefore, just as in the case of the minimal model, a control-oriented model for capturing exercise is required. According to Dalla Man et al.29, exercise changes the rate of disappearance of glucose in two ways: (i) insulin-independent rate of disappearance (which is given by the \(p_1\) parameter in the gluco-regulatory model in Eq. 1 and (ii) insulin-dependent rate of disappearance given by \(S_I\), that is also the insulin sensitivity parameter. In the same study, six models were evaluated. In addition, it was found that exercise had an immediate effect on the insulin-independent rate of disappearance and a delayed effect on the insulin-dependent rate of disappearance of glucose. The effect of exercise can be modeled as a variation of the parameters of the gluco-regulatory model as given in Eq. (14):
Here, E(t) is the intensity of the exercise level that varies from 0 to 1 and \(E_a(t)\) is the delayed signal of exercise that is given by \(\dot{E}_a(t) = -p_4 E_a(t) + p_4 E(t)\). The terms \(p_5\) and \(p_6\) indicate the insulin-independent and insulin-dependent effects of exercise on the glucose disappearance rate, respectively. The intensity (or) level of exercise is either obtained from oxygen consumption (as detailed by Ref.29) or using the elevation in heart rate (HR) as proposed by Ref.30.
5 Conclusion
There has been significant progress in developing mathematical models for Diabetic research over the past few decades. The impact of this work has led to (i) a better understanding of the internal dynamics of Type-1 diabetics as well as (ii) the development and testing of several Artificial Pancreas systems which improve the health and lifestyles of patients significantly.
Although there are a large number of mathematical models proposed by different groups, this review summarizes the key facets of some of the most widely used models. Two types of models, namely (i) control-oriented minimal models and (ii) high-fidelity models, are discussed with necessary details. The major section of the paper discussed the different subsystems in the minimal model which is primarily utilized for control algorithm development. The various subsystems of the blood glucose regulation process have been modeled extensively by different research groups. The advantages and limitations of each variant were summarized in this paper. In addition to the minimal model, the need for high-fidelity models and the features that these models offer is presented in this article. The novel features of two widely used high-fidelity models (i) the FDA-approved T1DM simulator and (ii) the Hovorka simulator were presented along with their drawbacks.
Although these high-fidelity models and minimal models are widely used in diabetes research and AP development, they do not account for the effects of exercise and physical activity. Research is being carried out to include those. Another field of research of current interest is the application of dual-hormone pumps. Glucagon and Insulin are both used in these systems for better glucose control. Models that describe the effect of glucagon secretion and the subcutaneous kinetics of glucagon administration are briefly discussed. These models are currently being utilized in the development of dual-hormone pumps and AP systems.
References
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JC, Mbanya JC et al (2022) Idf diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Practice 183:109119
CDC: Center for Disease Control. https://www.cdc.gov/diabetes/basics/diabetes.html
Albisser A, Leibel B, Ewart T, Davidovac Z, Botz C, Zingg W (1974) An artificial endocrine pancreas. Diabetes 23(5):389–396
Kovatchev BP, Breton M, Dalla Man C, Cobelli C (2009) In silico preclinical trials: a proof of concept in closed-loop control of type 1 diabetes. SAGE Publications Sage CA, Los Angeles
Sanchez EN (2019) The artificial pancreas: current situation and future directions. https://doi.org/10.1016/C2017-0-02120-1
Wilinska ME, Hovorka R (2008) Simulation models for in silico testing of closed-loop glucose controllers in type 1 diabetes. Drug Discov Today: Dis Models 5(4):289–298
Kovatchev B (2019) A century of diabetes technology: signals, models, and artificial pancreas control. Trends Endocrinol Metab 30(7):432–444
Peyser T, Dassau E, Breton M, Skyler JS (2014) The artificial pancreas: current status and future prospects in the management of diabetes. Ann NY Acad Sci 1311(1):102–123
Bergman RN, Phillips LS, Cobelli C et al (1981) Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Investig 68(6):1456–1467
Hovorka R, Shojaee-Moradie F, Carroll PV, Chassin LJ, Gowrie IJ, Jackson NC, Tudor RS, Umpleby AM, Jones RH (2002) Partitioning glucose distribution/transport, disposal, and endogenous production during ivgtt. Am J Physiol Endocrinol Metab 282(5):992–1007
Dalla Man C, Rizza RA, Cobelli C (2007) Meal simulation model of the glucose-insulin system. IEEE Trans Biomed Eng 54(10):1740–1749
Schiavon M, Dalla Man C, Cobelli C (2017) Modeling subcutaneous absorption of fast-acting insulin in type 1 diabetes. IEEE Trans Biomed Eng 65(9):2079–2086
Wong J, Chase JG, Hann CE, Shaw GM, Lotz TF, Lin J, Le Compte AJ (2008) A subcutaneous insulin pharmacokinetic model for computer simulation in a diabetes decision support role: model structure and parameter identification. J Diabetes Sci Technol 2(4):658–671
Nath A, Biradar S, Balan A, Dey R, Padhi R (2018) Physiological models and control for type 1 diabetes mellitus: a brief review. IFAC-PapersOnLine 51(1):289–294
Billings SA (2013) Nonlinear system identification: NARMAX methods in the time, frequency, and spatio-temporal domains. Wiley, New York
Man CD, Micheletto F, Lv D, Breton M, Kovatchev B, Cobelli C (2014) The uva/padova type 1 diabetes simulator: new features. J Diabetes Sci Technol 8(1):26–34
Visentin R, Dalla Man C, Kovatchev B, Cobelli C (2014) The university of virginia/padova type 1 diabetes simulator matches the glucose traces of a clinical trial. Diabetes Technol Ther 16(7):428–434
Wilinska ME, Chassin LJ, Acerini CL, Allen JM, Dunger DB, Hovorka R (2010) Simulation environment to evaluate closed-loop insulin delivery systems in type 1 diabetes. J Diabetes Sci Technol 4(1):132–144
Hovorka R, Canonico V, Chassin LJ, Haueter U, Massi-Benedetti M, Federici MO, Pieber TR, Schaller HC, Schaupp L, Vering T et al (2004) Nonlinear model predictive control of glucose concentration in subjects with type 1 diabetes. Physiol Meas 25(4):905
JDRF: Juvenile Diabetic Research Foundation. https://www.jdrf.org/blog/2011/02/09/artificial-pancreas-and-fda-the-latest/
Haidar A, Legault L, Dallaire M, Alkhateeb A, Coriati A, Messier V, Cheng P, Millette M, Boulet B, Rabasa-Lhoret R (2013) Glucose-responsive insulin and glucagon delivery (dual-hormone artificial pancreas) in adults with type 1 diabetes: a randomized crossover controlled trial. CMAJ 185(4):297–305
Taleb N, Emami A, Suppere C, Messier V, Legault L, Ladouceur M, Chiasson J-L, Haidar A, Rabasa-Lhoret R (2016) Efficacy of single-hormone and dual-hormone artificial pancreas during continuous and interval exercise in adult patients with type 1 diabetes: randomised controlled crossover trial. Diabetologia 59(12):2561–2571
Haidar A (2019) Insulin-and-glucagon artificial pancreas versus insulin-alone artificial pancreas: a short review. Diabetes Spectr 32(3):215–221
Lv D, Breton MD, Farhy LS (2013) Pharmacokinetics modeling of exogenous glucagon in type 1 diabetes mellitus patients. Diabetes Technol Ther 15(11):935–941
Garcia-Garcia F, Kumareswaran K, Hovorka R, Hernando ME (2015) Quantifying the acute changes in glucose with exercise in type 1 diabetes: a systematic review and meta-analysis. Sports Med 45(4):587–599
Resalat N, El Youssef J, Tyler N, Castle J, Jacobs PG (2019) A statistical virtual patient population for the glucoregulatory system in type 1 diabetes with integrated exercise model. PLoS ONE 14(7):0217301
Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, Horton ES, Castorino K, Tate DF (2016) Physical activity/exercise and diabetes: a position statement of the american diabetes association. Diabetes Care 39(11):2065–2079
Bohn B, Herbst A, Pfeifer M, Krakow D, Zimny S, Kopp F, Melmer A, Steinacker JM, Holl RW (2015) Impact of physical activity on glycemic control and prevalence of cardiovascular risk factors in adults with type 1 diabetes: a cross-sectional multicenter study of 18,028 patients. Diabetes Care 38(8):1536–1543
Romeres D, Schiavon M, Basu A, Cobelli C, Basu R, Dalla Man C (2021) Exercise effect on insulin-dependent and insulin-independent glucose utilization in healthy individuals and individuals with type 1 diabetes: a modeling study. Am J Physiol Endocrinol Metab 321(1):122–129
Rashid M, Samadi S, Sevil M, Hajizadeh I, Kolodziej P, Hobbs N, Maloney Z, Brandt R, Feng J, Park M et al (2019) Simulation software for assessment of nonlinear and adaptive multivariable control algorithms: glucose-insulin dynamics in type 1 diabetes. Comput Chem Eng 130:106565
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
On behalf of all the authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chandrasekhar, A., Padhi, R. Blood Glucose Regulation Models in Artificial Pancreas for Type-1 Diabetic Patients. J Indian Inst Sci 103, 353–364 (2023). https://doi.org/10.1007/s41745-023-00362-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41745-023-00362-z