Abstract
We review the variation of X–Z stretching frequency in the formation of Z-bonds (X–Z···Y, Z = H, or other main group element). Majority of hydrogen bonds (H-bonds) occur between a strongly polar X–H bond and an electron pair donor (H-bond acceptor) Y and show a red shift in the X–H stretch. In very weak H-bonds, there are several instances where the X–H bond is blue shifted. We show that X–H shifts span a continuum going from red through zero to blue; there is nothing improper or anti about H-bonds that are blue shifted. Other Z-bonds bring more factors in deciding the blue and red shift. When negative hyperconjugation in the uncomplexed monomer outweighs the charge transfer from incoming electron-rich species Y during the formation of X–Z···Y (Z = halogen, chalcogen, etc.), it leads to blue shifting X–Z bond. In the absence of negative hyperconjugation in the monomer, X–Z bond length elongation (red shift) is almost always observed on complexation with Y. In an X–Z···Y interaction, the detailed nature of the fragment X has the major influence on the way Z and Y control bond length elongation/contraction. The general trend in the magnitude of blue shift amongst various weak interactions was observed to be in the following order: halogen > chalcogen > hydrogen > pnicogen bonding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Our fascination with weak interactions was triggered by X-ray structures of complexes having two CH···π interactions with one CC triple bond (Fig. 1). It is felt that independent of the nature of interaction, the structure where each CHCl3 interacts with a different π bond (2) should be lower in energy than (1) where both the CHCl3 interacts with one π bond1. Computations indeed showed that 2 is more stable than 1 in the gas phase by a very small magnitude. However, something more interesting was observed: the CH bond of CHCl3 in 1 and 2 became shorter than that in isolated CHCl3. Here, we give a very brief review of the work that led from this observation. At that time contraction of X–H bond on H-bond formation was counterintuitive; any X–H···Y interaction is known to weaken the X–H bond and it must be longer in the complex than in the isolated molecule. A red shift in the X–H stretching frequency was anticipated in 1 and 2. Being very cautious, we sought some experimental support before publishing these results. At that time (around 1996), Dr Viswanathan at IGCAR, who was planning to build a super-cooled nozzle jet expansion set-up, promised to study C2H2···CHCl3 complexes using surface IR in a cold matrix. Though it took over 3 years, the results confirmed the blue shift of the C–H stretching frequency of CHCl3 in the complex, and we published this collaborative work2. However, by that time, a few publications appeared about the abnormal and anti-hydrogen bonds with blue shift in weak H-bonded complexes3, 4. We noticed that H-bond interactions involving C–H were known even earlier5,6,–7. A more detailed analysis was done on this CH···π interaction by us soon after; further effort in this line of research was not made by us for some time8.
Ternary complex of disubstituted acetylene with CHCl3 having C–H···π interaction, (R = PPh2Np; Np = Napthyl). The two CHCl3 moieties interact with same (1) and different (2) π bonds of C ≡ C bond 1.
However, during the next 5 years, large number of experimental and theoretical studies appeared in the literature, which gave a variety of examples of blue-shifted H-bonds. These H-bonds were described variously as abnormal-, improper-, and anti-, each providing a new explanation for the observation9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,–25. Most aspects of chemistry form a continuum, and we felt that blue and red shift cannot be an exception, prompting us to look at this question again so that an explanation of these observations which can be transferred from one chemical system to another can be evolved (we do not subscribe to the other school of thought, going back to the four fundamental forces of nature, that all forces in chemistry belongs to just one of them and there is no further modes of understanding needed. Also, see the article by Politzer in this special issue). A possible hypothesis is that forces that elongate and contract the X–H bond exist and that in strong X–H···Y bonds (vast majority of them fall in this category), the forces that elongate the X–H bonds dominate. In the very weak ones, these forces are more evenly balanced. This leads to the obvious corollary that there should be examples where there is no shift in the X–H bond stretch (zero shift), but with definite existence of H-bond. This appeared even more counterintuitive at that time: X–H forms an H-bond with Y as in X–H···Y, with no change in the X–H stretching frequency or bond length.
With these ideas, we looked at many explanations that were offered for the counterintuitive blue-shifting H-bonds. The short-range Pauli repulsion19,20,–21, redistribution of electron density13, 14, 22, 23, effect of the electric field10, 15, 24 and rehybridization25 gained significant attention as explanations. These were inadequate to give a common explanation for the observation of either blue or red shift and to suggest the continuum nature of chemistry that may be manifested here as well. We decided to examine these observations in greater detail, only to find out that each of these individual explanation has a counter example26. The observed structures are a result of all of these factors.
In general, there is direct correlation between bond length change and vibrational shift; X–H bond elongation leading to red shift, whereas X–H bond contraction causes blue shift, given there is no vibrational mode mixing. These can be detected experimentally using FTIR spectroscopy.
2 Red, Blue and Zero Shift in H-bonds
We studied the details of H-bonded complexes computationally using a series of potential energy scans using a combination of X–H and Y, Fig. 2.26 For example, the distance between H and the center of benzene in the H3CH-Benzene complex, where the C–H bond is brought along the C6 axis of benzene, is decreased gradually from 6.0 Å. At each point rest of the structural parameters are optimized, maintaining the same symmetry. The optimal C–H distance at each point is plotted as a function of H···Y distance. Similarly total energy is also plotted as a function of H···Y distance (Fig. 2a). The C–H bond length decreases continuously, almost till the minimum energy structure is reached and then elongates. The observation of the two minima (in energy and the C–H distance) close to each other is pure coincidence in this example as seen further below. Though the effect of long-range correlation in changing the C–H bond length is obvious, this type of behavior is no guarantee that there will be blue shift whenever there is initial bond-shortening as seen in the next example, Fig. 2b. Here, a similar diagram is drawn for the interaction of H3CH with NH3 which is known to be a good H-bond acceptor. The C–H bond length decreases here as well as NH3 approaches closer, albeit not as steeply. The minimum in the C–H bond length is calculated approximately around 3.5 Å, much earlier than the minimum in energy. At the minimum energy point the C–H bond length is marginally longer than in the parent CH4. This results in red shift. We then changed the nature of the C–H bond and kept the H-bond acceptor the same. A similar potential energy scan of Cl3CH···C6H6 showed that the length of C–H bond decreases steeply as the distance between the two molecules is decreased, Fig. 2c. The minimum in the C–H distance occurs a little earlier than the minimum in energy. However, at the equilibrium distance, the C–H bond is elongated only slightly so that the distance is still shorter than that in isolated Cl3CH, exhibiting a blue shift. These results clearly indicate that there will be a continuum of behavior, going from blue to red through zero shift.
Variation of C–H bond length with total energy of the complex during the potential energy scan at MP2/6-31G* level of theory: a H3C–H···benzene, b H3C–H···NH3, c Cl3C–H···benzene 27.
Taking a series of H-bonds obtained from appropriate combinations of X–H···Y26, we showed that each of the explanation provided earlier is partial and a counter example can be found that require additional factors. This also enabled us to categorize H-bond donors which will always show red shift, which will show red or blue shift depending on the H-bond acceptor, and which will show only blue shift. Similar groups are made of the H-bond acceptors as well. This also led to examples with zero shift on H-bond formation. A schematic way of representing these qualitative ideas is given in Fig. 3. This is a schematic composite figure to explain the range of examples available in Z-bonds (Z = Hydrogen, Halogen, Chalcogen, and Pnicogen). Let us consider the schematic curve for H-bond. The X-axis corresponds to H-bond energy and Y-axis the difference in stretching frequency of X–H bond before and after the formation of H-bond. At the origin, there is no interaction between X–H and Y (i.e. infinite separation). Initially, large number of examples from experimental and computational results H-bonds was plotted. Most of the H-bonds have red-shifted X–H bonds and were above the X-axis and below the black line emanating from the origin. There were several points corresponding to blue-shifted H-bonds which were below the X-axis. An approximate curve was drawn to include all of them, shown as Hydrogen in Fig. 3. Thus, all the known H-bonds are seen within the area above this curve and below the black line emanating from the origin. This curve has many interesting ramifications. For example, the common notion that as the interaction energy increases, X–H frequency shifts from blue to red is not true for the very weak X–H bonds. There are examples on the left side of the minimum in this curve where the interaction energy may increase with an increase in the blue shift. Then, the blue shift bottoms out and moves on with further increase in the interaction energy to less blue shift and then to the red shift regime. A zeroth order explanation of these observations is the following. In normal (strong) hydrogen bonds, the X–H bond is highly polar. The electrostatic interaction between Hδ+ and the Y lone pair on the electronegative group is dominant. The interaction becomes stronger if the charge on the H is increased. This is achieved by greater polarization of the X–H bond as Y approaches which makes the X–H bond longer. If we now imagine a situation where the X–H bond is not at all polar, say in C–H which forms very weak H-bonds, the electron distribution corresponding to the bond will be more even around the X–H bond. The lone pairs on Y would still have a long-range polarization effect which would push the electrons of the X–H bond away from Y. This would increase the electron density between carbon and hydrogen of the C–H bond and bond length is decreased. It is possible to visualize examples where the polarity of C–H bond is controlled by the other substituents so that this effect can be controlled, explaining the lower part of the curve (Fig. 3). The more recent extensive definition of H-bond brings in a variety of examples all of which are expected to be points in this manifold27.
A qualitative picture of the continuum of X–Z bond, X–Z···Y, Z = hydrogen, halogen, chalcogen, pnicogen 40.
3 Red-, Blue- and Zero Shift in Z-bonds
While we were studying the unusual behavior of H-bonds, there were already several examples of weak interactions involving other main group elements. Though a similar behavior in terms of blue and red shift was anticipated among these, we did not study this at that time. About 5 years later, a new graduate student Jyothish Joy joined the group at IISER Thiruvananthapuram. We realized that there are varieties of weak interactions involving main group elements other than hydrogen (X–Z···Y, Z = main group element) such as halogen27,28,29,–30, chalcogen 31, pnicogen32, tetral 33,34,–35, boron 36, lithium37, 38 and beryllium39 studied computationally and experimentally. While the possibility for the formation of Z-bonds can be judged by examining the σ-hole available in X–Z30, we looked for the possibility of blue and red shifting on Z-bond formation40. It is found that the starting X–Z bond lengths in large measure decide the possibility of X–Z becoming a red-shifted or blue-shifted X–Z···Y40. Hydrogen with only one orbital and one electron, presents the simplest case of the weak interaction. The variations in the X–H bonds are limited by the nature of X. There is no further variable possible with hydrogen. In contrast, Z-bonds involve atoms having additional electrons and orbitals. The additional electrons on Z atoms in X–Z play a major role in reorganization of electron density in ways that are not possible in X–H. In addition, there is limited vibrational mode mixing in the X–H stretch. The linear relationship between the change in bond length and change in vibrational frequency is an unambiguous indicator of red/blue-shifted hydrogen bonds. In contrast, unavoidable vibrational mode mixing in X–Z stretching in X–Z···Y has forced researchers to switch to bond length change as a measure of red/blue/zero shift43. In addition, for a given X, the variation of the X–H distance in the parent molecule is minimal. In contrast, the X–Z bond length in the parent molecule (Z other than H) varies depending on the complexity of the group X.
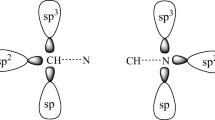
The most common explanation for the observed X–Z bond length in the parent system is negative hyperconjugation41,42. This refers to the transfer of electron density from the filled lone pair or the p orbital of the group X to the neighboring X–Z σ* antibonding molecular orbital (ABMO) of the parent X–Z molecule41, 42. This donation of electron density leads to X–Z bond elongation in the monomer due to partial occupation of X–Z σ* ABMO for the halogens, (Fig. 4a). There is also the reverse possibility where the lone pair of electrons on Z is donated to the σ* antibonding orbital of the X group, leading to a decrease in the X–Z bond length, Fig. 4b. The starting X–Z distance largely controls the change in the X–Z bond length on X–Z···Y formation. The exchange repulsion caused by the electrons of the approaching Y can shift electron density from the Z atom to X, either by pushing electron density from the σ* back to the X group (reversing negative hyperconjugation (Fig. 4a) or enhancing the negative hyperconjugation (Fig. 4b). In either case, there is possibility for blue shift in the X–Z stretch. If the effect of negative hyperconjugation is minimal, the charge transfer from Y to the X–Z σ* produces a red shift. What happens in a given case depends on the net effect of these. In other words, it is the competition between negative hyperconjugation in monomer X–Z and charge transfer from incoming Y which decides the nature of the shift. Though this mechanism of electron density redistribution may be debated, we have shown that by carefully selecting the X and Y in X–Z···Y interaction, continuum in Z bonds from red-, zero- to blue shifts can be achieved, adding on to the Fig. 3. Analysis of several examples of X–Z···Y involving Z = halogens, chalcogens, and pnicogens, supported the qualitative curves of Fig. 3. Similar schematic curves will evolve for other Z-bonds when more examples become available.
Overall, the continuum nature of weak interactions is mainly governed by the nature of X-group having a unique property of negative hyperconjugation. To have a reasonable interaction energy with optimum blue-shift X-group should be strong enough to create a σ-hole over the Z atom and the electronegativity of X atom which is directly connected to Z atom should be less than Z atom. This has led us to another question: why are there no Z-bonds where Z is an 18 electron transition metal, and this is discussed elsewhere43.
4 Conclusions
In general, all red-shifting HB donors are characterized by relatively highly polar, electron-rich, and short X–H bonds which can only show elongation with all reasonable Y’s. On the contrary, blue-shifting HB donor has less polar X–H bonds which show contraction in the presence of Y. This leads to a continuum of behavior where with appropriate X–H and Y combination, an X–H bond may be formed where the X–H may not elongate or contract, something between the blue shift and the red shift resulting in X–H···Y interaction with zero shift and non-zero interaction energy.
The continuum nature of weak interactions from red to blue through zero shift found in hydrogen bonding is also prevalent in all the other forms of weak interactions such as halogen, chalcogen, pnicogen and tetral bonds. The extend of negative hyperconjugation and consequent variation in the bond length in the parent X–Z molecule largely decides the nature of the Z-bond. The balance between the flow of electron density in the σ* X–Z antibonding molecular orbital from the negative hyperconjugation back to the X group and the charge transfer from Y group to σ* X–Z antibonding molecular orbital results in blue or red shift of X–Z bond in X–Z···Y complex. Thus, by controlling the nature of X and Y moieties of the weak interaction X–Z···Y, one can control these interactions which are responsible for maintaining the framework of supramolecular architectures. Though these interactions are small, the nature of blue and red shift must receive the attention of developers of forcefields for classical dynamics simulations.
References
Müller TE, Mingos DMP, Williams DJ (1994) T-shaped intermolecular CH···π (C ≡ C) interactions in chloroform solvates of gold (I) ethyne complexes. J Chem Soc Chem Commun 15:1787–1788
Jemmis ED, Giju KT, Sundararajan K, Sankaran K, Vidya V, Viswanathan KS, Leszczynski J (1999) An ab initio and matrix isolation infrared study of the 1:1 C2H2–CHCl3 adduct. J Mol Struct 510:59–68
Hobza P, Špirko V, Selzle HL, Schlag EW (1998) Anti-hydrogen bond in the benzene dimer and other carbon proton donor complexes. J Phys Chem A 102(15):2501–2504
Boldeskul IE, Tsymbal IF, Ryltsev EV, Latajka Z, Barnes AJ (1997) Reversal of the usual ν(C-H/D) spectral shift of haloforms in some hydrogen-bonded complexes. J Mol Struct 436–437:167–171
Buděšinský M, Fiedler P, Arnold Z (1989) Triformylmethane: an efficient preparation, some derivatives, and spectra. Synthesis 1989(11):858–860
Jayaraman H (1964) Indian J Chem 2:94
Green RD (1974) Hydrogen bonding by C–H groups. Wiley, New York
Jemmis ED, Subramanian G, Nowek A, Gora RW, Sullivan RH, Leszczynski J (2000) CH···π interactions involving acetylene: an ab initio MO study. J Mol Struct 556:315–320
Caminati W, Melandri S, Moreschini P, Favero PG (1999) The C–F–···H–C ‘anti-hydrogen bond’ in the gas phase: microwave structure of the difluoromethane dimer. Angewandte Chem Int Ed 38:2924–2925
Hobza P, Havlas Z (1999) The fluoroform ethylene oxide exhibits a C–H···O anti-hydrogen bond. Chem Phys Lett 303:447–452
Hobza P, Havlas Z (2000) Blue-shifting hydrogen bonds. Chem Rev 100(11):4253–4264
Kryachko ES, Zeegers-Huyskens T (2001) Theoretical study of the CH···O interaction in fluoromethanes H2O and chloromethanes H2O complexes. J Phys Chem A 105(29):7118–7125
van der Veken BJ, Herrebout WA, Szostak R, Shchepkin DN, Havlas Z, Hobza P (2001) The nature of improper, blue-shifting hydrogen bonding verified experimentally. J Am Chem Soc 123(49):12290–12293
Hobza P (2001) The H-index unambiguously discriminates between hydrogen bonding and improper blue-shifting hydrogen bonding. Phys Chem Chem Phys 3:2555–2556
Delanoye SN, Herrebout WA, van der Veken BJ (2002) Improper or classical hydrogen bonding? A comparative cryosolutions infrared study of the complexes of HCCIF2, HCCI2F, and HCCI3 with dimethyl ether. J Am Chem Soc 124(25):7490–7498
Li X, Liu L, Schlege HB (2002) On the physical origin of blue shifted hydrogen bonds. J Am Chem Soc 124:9639–9647
Matsuura H, Yoshida H, Hieda M, Yamanaka SY, Harada T, Shin-Ya K, Ohno K (2003) Experimental evidence for intramolecular blue-shifting C–H···O hydrogen bonding by matrix-isolation infrared spectroscopy. J Am Chem Soc 125(46):13910–13911
Diana E, Stanghellini PL (2004) Proper and improper hydrogen bonds in metalloorganic crystal architecture: experimental evidence in [CoCp2]+ and [FeCp2]+ salts. J Am Chem Soc 126(24):7418–7419
Zierkiewicz W, Jurecka W, Hobza P (2005) On differences between hydrogen bonding and improper blue-shifting hydrogen bonding. ChemPhysChem 6:609–617
Hobza P, Špirko V, Havlas Z, Buchhold K, Reimann B, Barth HD, Brutschy B (1999) Anti-hydrogen bond between chloroform and fluorobenzene. Chem Phys Lett 299:180–186
McDowell SAC (2005) Blue and red shifts in Rg···HCN and Rg···HNC complexes (Rg = He, Ne, Ar, Kr). Mol Phys 103:2763–2768
Zierkiewicz W, Michalska D, Havlas Z, Hobza P (2002) Study of the nature of improper blue-shifting hydrogen bonding and standard hydrogen bonding in the X3CH···OH2 and XH···OH2 complexes (X = F, Cl, Br, I): a correlated ab initio study. ChemPhysChem 3:511–518
Hobza P, Havlas Z (2002) Improper, blue-shifting hydrogen bond. Theoret Chem Acc 108:325–334
Masunov A, Dannenberg JJ, Contreras RH (2001) C–H bond-shortening upon hydrogen bond formation: influence of an electric field. J Phys Chem A 105:4737–4740
Alabugin IV, Manoharan M, Peabody S, Weinhold F (2003) Electronic basis of improper hydrogen bonding: a subtle balance of hyperconjugation and rehybridization. J Am Chem Soc 125:5973–5987
Joseph J, Jemmis ED (2007) Red-, blue-, or no-shift in hydrogen bonds: a unified explanation. J Am Chem Soc 129:4620–4632
Arunan E, Desiraju GR, Klein RA, Sadlej J, Scheiner S, Alkorta I, Clary DC, Crabtree RH, Dannenberg JJ, Hobza P (2011) Definition of the hydrogen bond (IUPAC Recommendations 2011). Pure Appl Chem 83:1637–1641
Amezaga NJM, Pamies SC, Peruchena NM, Sosa GL (2010) Halogen bonding: a study based on the electronic charge density. J Phys Chem A 114:552–562
Politzer P, Lane P, Concha MC, Ma Y, Murray JS (2007) An overview of halogen bonding. J Mol Model 13:305–311
Politzer P, Murray JS, Clark T (2013) Halogen bonding and other σ-hole interactions: a perspective. Phys Chem Chem Phys 15:11178–11189
Alikhani E, Fuster F, Madebene B, Grabowski SJ (2014) Topological reaction sites-very strong chalcogen bonds. Phys Chem Chem Phys 16:2430–2442
Zahn S, Frank R, Hey-Hawkins E, Kirchner B (2011) Pnicogen bonds: a new molecular linker? Chem A Eur J 17:6034–6038
Mani D, Arunan E (2013) The X-C···Y (X = O/F, Y = O/S/F/Cl/Br/N/P) ‘carbon bond’ and hydrophobic interactions. Phys Chem Chem Phys 15:14377–14383
Mani D, Arunan E (2014) The X-C···π (X = F, Cl, Br, CN) carbon bond. J Phys Chem A 118:10081–10089
Bundhun A, Ramasami P, Murray JS, Politzer P (2013) Trends in σ-hole strengths and interactions of F3MX molecules (M = C, Si, Ge and X = F, Cl, Br, I). J Mol Modell 19:2739–2746
Grabowski SJ (2014) Boron and other triel lewis acid centers: from hypovalency to hypervalency. ChemPhysChem 15:2985–2993
Shahi A, Arunan E (2014) Hydrogen bonding, halogen bonding and lithium bonding: an atoms in molecules and natural bond orbital perspective towards conservation of total bond order, inter- and intra-molecular bonding. Phys Chem Chem Phys 16:22935–22952
Esrafili MD, Juyban P, Solimannejad M (2014) Exploring lithium bonding interactions between noble-gas hydrides HXeY and LiX molecules (Y = H, CN, NC and X = H, CN, NC, OH, NH2, CH3): a theoretical study. Comput Theor Chem 1027:84–90
Villanueva EF, Mo O, Yanez M (2014) On the existence and characteristics of π-beryllium bonds. Phys Chem Chem Phys 16:17531–17536
Joy J, Jemmis ED, Vidya K (2015) Negative hyperconjugation and red-, blue- or zero-shift in X-Z···Y complexes. Faraday Discuss 177:33–50
McNaught AD, Wilkinson A (1997) Compendium of chemical terminology: IUPAC recommendations, 2nd edn. Blackwell Science, Oxford England
Fleming I (2009) Molecular orbitals and organic chemical reactions. Wiley, Chichester
Joy J, Jemmis ED (2017) Contrasting behavior of the Z bonds in X-Z···Y weak interactions: Z = main group elements versus the transition metals. Inorg Chem 56:1132–1143
Acknowledgements
GK thanks the SERB-DST for a postdoctoral Fellowship. We thank SERC-IISc for computational facilities. EDJ thanks SERB-DST for funding through the Year of Science Chair Professorship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This contribution is for the special issue edited by Prof E. Arunan, slated for Jan 2020.
The article is a brief review of our work to understand the blue, zero and red shifts in weak interactions.
Rights and permissions
About this article
Cite this article
Karir, G., Jemmis, E.D. Continuum in H-bond and Other Weak Interactions (X–Z···Y): Shift in X–Z Stretch from Blue Through Zero to Red. J Indian Inst Sci 100, 127–133 (2020). https://doi.org/10.1007/s41745-019-00150-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41745-019-00150-8