Abstract
Thermally-enhanced soil vapor extraction (T-SVE) combined with sand mixing is an alternative technology for remediating low-permeable soil polluted by organic contaminants. In this study, a T-SVE apparatus with a large heating cylinder was constructed for exploring removal mechanisms of typical petroleum hydrocarbons of n-C11 and C13−16 alkanes, and dynamics of heat propagation within soils during T-SVE operation was simulated by CMG-STARS software. After 6 days of T-SVE, most of the soil concentration-gradient curves of n-alkanes almost coincided with their isothermal contours, suggesting the crucial role of heat conductivity on T-SVE remediation efficiency. The instantaneous concentration of n-alkanes in extracted gas showed great fluctuations, and higher than 90% of spiked n-alkanes was removed from soils. n-alkanes were more easily eliminated from soil with lower organic matter due to the less retention ratio despite the trivial impact of organic matter on heat transfer. By contrast, the higher soil moisture adopted in the study retarded heat transfer in the initial 2 days due to thermal consumption induced by water evaporation. During the last 4 days, however, the average temperature of more humid soil was obviously elevated, especially in the zones receiving less energy input. It was attributed to the fact that soil temperature was mainly determined by heat conduction, and soil pores still occupied by water were more beneficial for heat conduction, leading to the promoted evaporation of n-alkane and therefore eventual remediation efficiency. Suitable original soil moisture is important for T-SVE remediating low-permeable soil combined with sand mixing.
Highlights
-
A T-SVE apparatus with a large heating cylinder was constructed.
-
Thermal conductivity was a crucial factor influencing remediation efficiency.
-
Volatilization of n-alkanes from heated soils showed an irregular pulsed mode.
-
Soil organic matter exhibited a trivial impact on heat transfer behavior in soils.
-
Higher soil moisture firstly retarded and then accelerated the removal of n-alkanes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil vapor extraction (SVE) is the most commonly used method for remediation of soil contaminated with VOCs (volatile organic compounds) and petroleum hydrocarbons (USEPA 1991). SVE has gained popularity due to its capable of treating large amounts of soil at relatively less cost, with estimate as low as 13$ per cubic meter (Frank and Barkley 1995). The successful application of SVE is based on adequate vapor flowing through all contaminated zone. However, the presence of low-permeable soil makes the contaminants with lower volatility at ambient temperature entrapped in strata without airflow (Kaleris and Croisé 1999). Consequently, the conventional SVE is limited by retardation of contaminants in-situ, especially in fine clay layers (Liu et al. 2024).
Thermally-enhanced soil vapor extraction (T-SVE) can accelerate the removal of recalcitrant compounds via promoted pore-partition and vaporization, and hence expand the spectrum of soils and contaminants treated. T-SVE originated from oil exploitation industry and its application in practical remediation since 1980s had promoted technological upgrades (Horst et al. 2021). Recent bibliometric analysis of academic research in the field further revealed the vigorous development and the continuous growth of attentions attracted over the past 20 years (Sun et al. 2024; Alamooti et al. 2023, 2024). Compounds with vapor pressure of greater than 70 Pa at 150 °C are candidates for T-SVE removal, and it is therefore expected that most of diesel components with 12–20 carbon bearing are removed by thermal desorpion (Poppendieck et al. 1999a). However, soil is a good insulator with higher heat capacity, and low-permeable strata will be heated mainly by thermal conduction. The fact implies that in-situ T-SVE operating on impermeable zones may not achieve cleanup standards even with long-term heating. Alternative procedures such as excavation, sand blending and then ex-situ T-SVE remediation might be desirable. It was reported that the total thermal conductivity of sandy soil was more than double that of loam and clay soils when the water volume fraction was 35% (Campbell and Norman 1998).
Several studies on application of T-SVE using different heating approaches such as steam enhanced extraction (SEE), electrical resistance heating (ERH), thermal conductive heating (TCH) and radio frequency heating (RFH) in laboratory and field scenarios have been carried out (Poppendieck et al. 1999b; Harmon et al. 2001; Roland et al. 2008, 2012; Liu et al. 2014; Yu et al. 2019; Davis et al. 2020; Mumford et al. 2021). Temperature, treatment time, soil characteristics, heating power and well laying have been identified as key factors of T-SVE remediation process (Lighty et al. 1988, 1990; Bucalá et al. 1994; Piña et al. 2002; Sun et al. 2023). Both the temperature increasing and the heating time extending effectively improved the energy input, and hence the binding between contaminant and soil particles was alleviated and the remediation efficiency was promoted. Merino and Bucalá (2007) found that evaporation and desorption governed the removal process of C16 alkane when soil was heated to 150–800 °C for 30 min without the occurrence of pyrolysis. The increment of soil temperature from 50 to 150 °C gave rise to three orders of magnitude increase in removal rate constants of C14−19 alkanes (Poppendieck et al. 1999a). Temperature of 175 °C is sufficient to remedy diesel polluted sandy and silty soils, whereas the higher temperature of 250 °C is needed for clays (Falciglia et al. 2011). Simultaneously, soil characteristics such as moisture content and organic matter composition were identified as key factors strongly affecting the thermal desorption of contaminant from soil. The contaminants were adsorbed by organic matter through mechanisms of hydrophobic interaction, π–π interaction, hydrogen bond, electrostatic interaction, etc. The hydroxyl, carbonyl and other functional groups on organic matter have significant contributions to contaminant retention (Sun et al. 2024). An increase in ground-water flow rate and heating power enhanced thermal convection and improved temperature uniformity (Sun et al. 2020), whereas contradictive views were also proposed because water decreased the soil permeability and hindered the mass transfer of contaminant molecules within and between soil particles (Wang et al. 2021).
The reported researches on T-SVE were mainly performed on laboratory and in-situ field scale, and recently numerical models such as TMVOC, TOUGH2 and COMSOL multi-physics software were developed to simulate the gas/aqueous transport, multicomponent migration and heat transfer (Tse and Lo 2002; Guo et al. 2023; Zheng et al. 2023). It was proved that T-SVE exhibited a less sensitivity to the parameters such as soil layers and distributions of non-aqueous phase liquids (NAPLs) based on three-dimensional fluid flow and heat transfer simulation (Webb and Phelan 1997). Overall, most of experiments and mathematical researches lacked comprehensive consideration of gas/aqueous phase change, stream migration, heat conduction as the temperature increased under the effects of moisture rise and soil organic matter deprivation during ex-situ remediation, and the concentration of contaminant in extracted gases was seldom intensively monitored to offer the insights into remediation performance. Furthermore, it is still needed to explore the removal mechanism of organic contaminants from bulk soil filled in a larger tank, which better match the actual ex-situ engineering process relative to that from soil packed in a tiny tube. In this work, we constructed a three-dimension T-SVE apparatus with a large heating cylinder. To improve fluid permeability, coarse quartz sand was incorporated into the tested low-permeable soils with different organic matter and moisture content contaminated by n-C11 and C13−16 alkanes. The prepared soils were packed using the same procedure, and real-time temperature was acquired by sensors and then transferred to computer per second. CMG-STAR software was used to simulate the temporal and spatial distribution of temperature within soil column considering the difficulty of obtaining global temperature information from a limited number of monitoring sensors. The concentrations of 5 n-alkanes in extracted gases were monitored, and their residues in soil were analyzed after T-SVE process. The aims of this study were: (1) to explore the roles of temperature and soil characteristics such as moisture and organic matter content playing in contaminant removal, (2) to acquire the kinetic characteristics of petroleum hydrocarbon volatilization from soil during T-SVE process, and (3) to evaluate the decontamination efficiency by T-SVE combined with sand mixing. The obtained results were expected to provide technical references for design of T-SVE remediating low-permeable soil polluted by diesel hydrocarbons.
Material and Methods
Reagents and Soil
n-C11, 13−16 alkanes were selected as representatively volatile and semi-volatile diesel hydrocarbons. The boiling points of five n-alkanes are ranged from 196 to 287 °C. All chemicals used in experiment were of analytical reagent quality purchased from Aladdin Bio-Chem Technology Co., LTD (Shanghai, China).
Silty soil free of petroleum hydrocarbons was collected and sieved to pass 50 mesh (particle diameter of 0.30 mm) after air-dried. Organic matter was analyzed to be 1.19% using potassium dichromate oxidation spectrophotometric method. Soil moisture was 3.2% measured by oven-drying method. Soil texture was determined using hydrometer method, and percentage of three aggregate fractions corresponding to sand (particle diameter: > 0.05 mm), silt (particle diameter: 0.002–0.05 mm) and clay (particle diameter: < 0.002 mm) was 18.2, 62.1 and 19.7 wt.%, respectively. The surface area and micropore volume of soil was 2.02 m2/g and 0.026 mL/g, respectively which was determined by a Quantachrome NOVA 4000 analyzer.
Soil Contamination
About 27 kg of silty soil was artificially contaminated with around 0.3 L mixture of n-C11 and C13−16 alkanes dissolved in 11.7 L of acetone. Soil and contaminant solution was mixed uniformly and then vented in outdoor with constant stirring until complete removal of acetone. The spiked soil was kept in several self-sealing pockets and stored in a refrigerator for 7 days. To obtain a homogeneous powdered matrix, the polluted soils was ground and sieved in a 50 mesh again. Quartz sand (particle diameter of 0.355 − 0.425 mm and bulk density of 1.75 g/cm3) was mixed with the prepared soil at mass ratio of 1: 1 in an agitator running for 8 h in order to improve fluid permeability. The obtained sandy soil was designated as sand-modified soil (SS) in the study.
Aiming to evaluate the effect of soil organic matter on removal of n-alkanes from soil by T-SVE, another set of experiment was carried out on sandy soil with less organic matter (SS-LOM), which was obtained by heating original silty soil at 300 °C for 1 h in a muffle furnace firstly, and then being spiked with n-alkanes following the same procedure with preparation of SS. The organic matter of SS-LOM was measured to be 0.69%, which was about 42% lower than that of SS. For the purpose of investigating impact of soil moisture on remediation efficiency of T-SVE, the prepared SS was further mixed with deionized water and homogenized to simulate sandy soil with higher moisture (SS-HM). The moisture of SS-HM soil was determined to be 18.5%.
Before thermal treatment of SS, SS-HM and SS-LOM, point-centered quarter sampling method was adopted and 5–6 samples for each type of soil were analyzed by n-hexane extraction and subsequently gas chromatography (Agilent Technologies 7820A) equipped with a flame ionization detector (GC-FID) for measurement of n-C11, 13−16 alkanes levels in soils. The average concentrations of C11, C13, C14, C15 and C16 in SS were determined to be 65.4, 95.5, 133.3, 311.3 and 380.4 mg/kg, respectively; in SS-HM 81.9, 92.79, 129.2, 275.1 and 380.4 mg/kg, respectively; and in SS-LOM 89.7, 87.3, 134.8, 288.6 and 393.1 mg/kg, respectively.
T-SVE Apparatus and Soil Packing
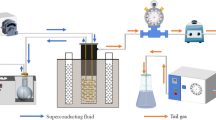
Three kinds of polluted soils were treated simulating ex-situ thermal process using a pilot-scale apparatus which mainly consisted of vacuum extraction system, extraction well, soil tank, oil bath, temperature controller, temperature sensors, signal acquisition and gas sampling systems (Fig. 1). The soil tank made of stainless steel was a large cylinder with internal diameter of 40 cm and depth of 30 cm. The bottom and side walls of the cylinder were nearest to oil bath whose temperature was set by a controller. A high-power magnetic stirrer was placed under the bottom wall of oil bath in order to promote heat propagation. Extracted vapor driven by a vacuum pump was joined to an extraction well (internal diameter = 3 cm) which was axially inserted up to the middle of cylinder. The under part of well was uniformly screened with vertical length of screened zone of 2 cm. The off-gases extracted by well were trapped and condensed in a glass bottle at 0 ℃ (ice bath). Non-condensable gas was led to a fume hood for the sake of safety.
The three prepared soils were equally divided into 4 layers, and then poured into the cylinder layer by layer. The thickness of each layer was kept 4 cm, and the total height of loaded soil was 16 cm. The surface of top layer was covered by putty powder (200 − 300 mesh) with thickness of 4 cm to avoid the occurrence of preferential air flow.
The real-time soil temperature evolution was monitored by 16 temperature sensors (4 sensors per layer) located at a cruciform rack above the surface of putty powder. The sensors were arranged in a shape of “V” and designated by A1 − A8 and B9 − B16 in A and B profiles, respectively, as shown in Fig. 1. These sensors were controlled by NI 9216 8-ch PT100 RTD (24 bit) input module, and the temperature data was recorded per second. The data acquisition program was coded by Labview software purchased from National Instruments Corporation (Austin, USA).
T-SVE Process
In each of T-SVE remediation, the apparatus was operated for 6 days. During the first 5 days, soil was heated for 5 h but vapor extraction was lasted for 10 h. At the final day, the heating time was 2 h and vapor extraction was continued for another 2 h. Therefore, the total experiment duration was 54 h. The heating of oil bath and temperature data collection were started after airflow rate reached the steady state. The constant temperature of oil bath at 210 °C and airflow rate of 60.5 L/min was kept in the experiments, respectively.
Vapor Sampling and Analysis
Vapor sampling section was composed of low-flow rate sampling pump (LFS-113DC, Gilian, USA), pressure gage and mass flow meter (FMA 1700A/1800A, OMEGA, USA). The disposable syringe with a piece of polyurethane foam (PUF) absorber was connected to both vapor sampling section and the hose pipe at outlet of extraction well. Every half an hour the extracted vapor was sampled. The sampling lasted for 10 min, and about 150 cm3 of vapor was collected under the help of low-flow rate sampling pump. n-alkanes were captured by PUF absorber.
For quantification, 10 µL of n-C12 alkane was spiked in PUF as internal standard. All the n-alkanes in PUF were extracted by n-hexane for 5 times in a ultrasonic extractor, and all the extracts were combined and then concentrated to 0.5 mL by nitrogen evaporator, subjecting to GC-FID with a capillary column (ADB-5 silica, 30 m length × 0.25 mm i.d × 0.25 µm film thickness). The recoveries of n-C11 and C13−16 alkanes in vapor samples were in the range of 65.7–91.6%. The reported concentrations of n-C11, 13−16 alkanes in vapor samples were corrected by recovery of internal standard of n-C12 alkane.
Soil Sampling and Analysis
As T-SVE finished, putty powder was carefully removed and soil samples were collected layer by layer. There were 16 samples for each layer and therefore total 64 samples were obtained. The mass of each soil sample was about 20 g. Subsample of about 1.4 g soils was obtained using point-centered quarter sampling method for subsequent analysis.
Subsample was extracted with 5 mL n-hexane for 5 times under ultrasonic sound assistance and then centrifuged at 3000 r/min. All the extracts were combined and cleaned by passing a column packed with silica and anhydrous sodium sulfate. The effluent was concentrated to 0.5 mL and then measured by GC-FID. The recoveries of n-C11, 13−16 alkanes in soil samples were in the range of 56.7–98.2%. The reported concentrations of n-C11, 13−16 alkanes in soil samples were corrected by the recovery of internal standard of n-C12 alkane.
Results and Discussions
Temperature Profiles Within Soil Column
Heat propagation was recorded by 16 temperature sensors located in 4 different layers during 6 consecutive days. Figure 2 shows the pulsed temperature variation of tested soils as a function of elapsed time. The temperatures ranged from above 180 °C at the bottom (Layer 4) to just above 100 °C near the surface (Layer 1) at steady state during heating. It was observed that the shapes of temperature curve for 3 tested soils within the same layer at the same day were almost similar. During the initial 5 days, the temperature curves of all layers could be generally divided into 3 stages: rapid increase, slow increase and decrease. At the sixth day, T-SVE only ran for 4 h instead of 10 h, which made the shapes of temperature curves changed greatly.
In our previous work (Yu et al. 2019), dynamics of heat propagation during T-SVE operation had been successfully simulated by CMG-STARS (Computer Modeling Group Ltd., Canada) based on hypothesis of homogeneous porosity and permeability of soil. Here the software was used again to exhibit temperature profiles within SS soil column with time (from 50 to 500 min) in each day of the first five T-SVE operations, as shown in Fig. 3. The variations in soil temperature profiles of SS-LOM and SS-HM during each heating and cooling cycle were similar to those of SS, and thus SS was chosen as a representative soil and simulated by CMG-STARS.
As demonstrated in Fig. 3, the temperature demonstrated the decreasing tendency from extraction well to upper side and top of soil column since the bottom and side walls of container were next to oil bath. From 100 min on, an approximately hemispheric shape of lower temperature zone was formed. At 150 min, about a half of soil was heated to above 100 °C. After 200 min of heating, Layer 4 nearest to the bottom wall exhibited a relatively even temperature distribution. At 350 min, the temperature of greater than 65% of soil matrix was above 160 °C. Due to the fact that sandy soil was a material with poor heat conductivity, soil temperature still gradually increased until 350 min even though oil bath had been powered off at 300 min.
Influence of Soil Organic Matter and Moisture Content on Heat Transfer
The temperature variation trend of different layers for SS-LOM (organic matter content: 0.69%) were almost consistent with those for SS (organic matter content: 1.19%) (Fig. 2), and most of the temperature average for the two soils at the same day was quite close, except for the middle layers at the first two days (Fig. 4). The above observations indicated the trivial impact of organic matter on heat transfer behavior within soils.
On the contrary, soil moisture exhibited the remarkable influence. In the first 2 days, the average temperature of Layers 2 − 4 for SS-HM (moisture content: 18.5%) was much lower than that for SS (moisture content: 3.2%), but the comparison between the two soils was reversed from the third day on because of the obviously higher average temperature of Layers 1 − 3 for SS-HM relative to that for SS (Fig. 4). These results suggested that under the condition of higher soil moisture, energy propagation was firstly retarded at the beginning of 2 days and then accelerated at the next following 4 days. The lower temperature of SS-HM on Day 1 and 2 should be due to the thermal energy consumption required by much water evaporation from SS-HM since water has a high specific heat capacity and latent heat. In Layer 4 nearest to the oil bath, soil pores occupied by water were mostly displaced by air with the continuous operation of T-SVE, and since the third day the almost same average temperature of SS-HM with that of the other two soils was reached attributed to similar thermal conductivities (0.2–0.25 W/m K) of different soils under dry condition (Wu et al. 2022), leading to the enhanced temperature increasing rate of SS-HM in the initial stage of heating. During the last 4 days the obviously higher average temperature of Layers 1 − 3 for SS-HM was presumably attributed to the water still retained and redistributed during extraction. The thermal conductivity of water was higher than that of air by one to two magnitudes which made soil pores filled by water instead of air more beneficial for heat conduction. Soil temperature was principally determined by heat conduction rather than convection except for the case of extremely porous medium, and therefore soil humid was more favorable for heat transferred further during extraction. At the early period of T-SVE the certain level of soil moisture was recommended (Xu et al. 2022).
Volatilization and Kinetic Characteristics of n-Alkane During T-SVE Operation
Through volatilization and desorption, n-alkanes were partitioned from treated soil into extracted gases when T-SVE was running. Figure 5A presents the variations in concentrations of 5 n-alkanes in extracted gases with elapsed time in 6 consecutive days. In general, the instantaneous gas-phase concentrations of n-alkanes showed great fluctuations over time, especially for contaminants with longer carbon chain (n-C15 and n-C16). Sometimes the gas-phase concentrations of n-alkanes increased rapidly by 1–2 orders of magnitudes and then quickly decreased, and there were also plenty of lower peaks up and down. This result indicated a pulsed type of mass transfer of n-alkanes transported from heating zones to extraction well. Related to the change of temperature with time as shown in Fig. 3, the kinetic release of n-alkanes was mainly attributed to the temporal and spatial variation of soil temperature and then delayed evaporation of contaminants.
Influence of Soil Organic Matter and Moisture Content on n-Alkane Volatilization
To further clarify the variation of volatilized n-alkanes with soil organic matter and moisture content, the average concentrations of n-alkane in extracted gases were calculated. As shown in Fig. 5B, both soil organic matter and moisture content exhibited the significant impact on evaporation of n-alkanes. The lower soil organic matter content resulted in an accelerated volatilization of n-alkanes, especially for n-C14-16 alkanes with higher boiling point. On the first day of thermal remediation, the average concentration of total n-alkanes (∑n-C11, 13–16) in extracted gas from SS-LOM was 166.8 µg/L, which was obviously higher than that from SS (105.7 µg/L). It was reported that the volatilization rate and desorption efficiency was negatively correlated with soil organic matter, and contaminant required more energy input to overcome activation energy barrier and desorb from soil with higher organic matter (Luo et al. 2019).
At the early stage of T-SVE, the higher moisture content restrained the volatilization of n-alkanes, especially for n-C11, 13 alkanes with lower boiling point. On Day1, 2 and 3, the average concentration of ∑n-C11, 13–16 in extracted gases from SS-HM was 75.6, 94.5 and 148.6 µg/L, respectively, which was remarkably lower than that from SS (105.7, 122.0 and 205.6 µg/L, respectively). Presumably, the declined gas-phase of n-alkanes from SS-HM was attributed to the delayed temperature increment (Fig. 2). During the last 3 days, however, the higher soil moisture was beneficial for evaporation of n-alkanes. The average concentration of ∑n-C11, 13–16 in extracted gases from SS-HM was peaked on Day 4 which was obviously higher than that from SS observed on Day 3. The accelerated volatilization of n-alkanes from SS-HM at the later stage of T-SVE could be explained by the higher average soil temperature in top three layers as well as the formation and propagation of gas-filled fractures during boiling and subsequently enhanced steam-stripping of n-alkanes from soil particles. Steam production by boiling of pore water had been identified as one of the major mechanisms for overcoming mass-transfer limitations. Moreover, water molecules were easier to adsorb on the surface of soil medium than petroleum hydrocarbons due to their greater polarity which was favorable for the release of contaminants (Shi et al. 2021). It was reported that 1, 2-dichloroethane (DCA) concentration in the clay were reduced by two orders of magnitude after roughly half the pore water was removed (Liu et al. 2014). The gas-phase permeability was greatly promoted when gas bubbles coalesced during heating, providing more preferential pathways for transport of VOCs in vapor-phase (Liu et al. 2014).
Residue Characteristics of n-Alkanes in Soil After T-SVE Operation
The contour of remained n-alkanes vs. soil depth together with distance from well was obtained by interpolation method (Fig. 6). The zones close to oil bath and punched portion of well were cleaned to very low residual levels of n-alkanes compared with the other zones. The difference in concentration of n-alkanes between the two zones was amplified with the increasing of carbon chain of n-alkanes. Most of the soil concentration-gradient curves of tested n-alkanes almost coincided with their isothermal contours (Fig. 2), indicating the key impact of thermal drive force on contaminant transfer which was in agreement with the simulation of a developed second-order polynomial model for remediation of sandy soil contaminated with VOCs using T-SVE method (Sabour et al. 2017). Since the unusually maximum concentrations of remained n-alkanes were always measured at the colder zone (Layer1 or Layer 2), especially for hydrocarbons with longer carbon chain, it is important to reduce the soil volume with lower temperature in order for improving the remediation efficiency of T-SVE.
Influence of Soil Organic Matter and Moisture Content on Remediation Efficiency
The remediation efficiency of T-SVE for three artificially contaminated soils was evaluated by comparing the original concentrations vs. average residual concentrations of n-alkanes. The average residual concentration was calculated by averaging the remained concentrations of each n-alkane in SS, SS-LOM and SS-HM. As shown in Fig. 7, T-SVE process achieved the higher remediation efficiency for SS-LOM polluted by n-alkanes. The average residual concentrations of 5 n-alkanes in SS-LOM (organic matter content: 0.69%) were all obviously lower than those in SS (organic matter content: 1.19%). After 6 consecutive days of T-SVE, 94.1% of ∑n-C11, 13–16 was removed from SS-LOM, whereas from SS the eliminated ratio of ∑n-C11, 13–16 was 90.4%. It was reported that humic substances (80% of soil organic matter) were closely correlated with the adsorption of organic pollutants (Ussiri and Johnson 2003). Different humus types of organic matter thermally degraded in the temperature range of 200–650 ℃ (Hempfling and Schulten 1990; Schulten 1987). Considering that the temperature of most soil samples ever reached were below 200 ℃ when T-SVE was running, there was still abundant adsorption sites contributed by organic matter. Therefore, less soil organic matter content led to the lower retention and thus the greater removal of n-alkanes from SS-LOM.
Higher moisture gave rise to the enhanced remediation efficiency of T-SVE. The average residual concentrations of 5 n-alkanes in SS-HM (moisture content: 18.5%) were all significantly lower than those in SS (moisture content: 3.2%) (Fig. 7). After T-SVE decontamination process, the removal ratio of ∑n-C11, 13–16 for SS-HM was calculated to be 92.6%, which was remarkably higher than that for SS (90.4%). Although the volatilization of n-alkanes from SS-HM was suppressed in the initial 2 days, the final elimination rate was still enhanced which implied the positive influence of suitable moisture content on removal of petroleum hydrocarbons from soil during T-SVE operation.
Conclusions
T-SVE combined with sand mixing achieved higher remediation efficiency for petroleum hydrocarbon-contaminated soil. Above 90% of spiked n-alkanes was removed during 54 h of pulsed T-SVE running. Soil temperature and thermal conductivity of fluid within soil pores crucially affected the spatial distribution of residual n-alkanes and final remediation efficiency. The higher temperature soil zones were decontaminated to very low residual levels of n-alkanes. The instantaneous concentration of n-alkanes in extracted gas showed great fluctuations, and volatilization of n-alkanes from soil with lower organic matter content was accelerated despite the trivial impact of organic matter on energy propagation. By contrast, higher soil moisture firstly retarded and then promoted the heat transfer and thermal desorption of n-alkane as a function of time, characterized with finally enhanced remediation efficiency of T-SVE. In conclusion, reduction of low-temperature soil volume and maintenance of suitable original soil moisture are key factors for T-SVE remediating low-permeable soil combined with sand mixing.
Data availability
Data will be made available on request.
References
Alamooti A, Colombano S, Glabe ZA, Lion F, Davarzani D, Ahmadi-Sénichault A (2023) Remediation of multilayer soils contaminated by heavy chlorinated solvents using biopolymer-surfactant mixtures: two-dimensional flow experiments and simulations. Water Res 243:120305. https://doi.org/10.1016/j.watres.2023.120305
Alamooti A, Colombano S, Shoker A, Ahmadi-Sénichault A, Lion F, Cazaux D, Marion C, Lagron J, Sawadogo I, Davarzani D (2024) Enhancing remediation of residual DNAPL in multilayer aquifers: post-injection of alcohol-surfactant-polymer mixtures. Sci Total Environ 918:170680. https://doi.org/10.1016/j.scitotenv.2024.170680
Bucalá V, Saito H, Howard JB, Peters WA (1994) Thermal treatment of fuel oil-contaminated soils under rapid heating conditions. Environ Sci Technol 28(11):1801–1807. https://doi.org/10.1021/es00060a008
Campbell GS, Norman JM (1998) An Introduction to environmental btiophysics. Springer, New York. https://doi.org/10.1007/978-1-4612-1626-1
Davis RJ, Liljestrand HM, Katz LE (2020) Evidence for multiple removal pathways in low-temperature (200–400 ℃) thermal treatment of pentachlorophenol-laden soils. J Hazard Mater 400:122870. https://doi.org/10.1016/j.jhazmat.2020.122870
Falciglia PP, Giustra MG, Vagliasindi FGA (2011) Low-temperature thermal desorption of diesel polluted soil: Influence of temperature and soil texture on contaminant removal kinetics. J Hazard Mater 185:392–400. https://doi.org/10.1016/j.jhazmat.2010.09.046
Frank U, Barkley N (1995) Remediation of low permeability subsurface formations by fracturing enhancement of soil vapor extraction. J Hazard Mater 40:191–201. https://doi.org/10.1016/0304-3894(94)00069-S
Guo S, Zha Y, Zhong H, Wang X, Xu D (2023) Numerical investigations of influence on thermal conductive heating in DNAPL-impacted soils by heterogeneity. J Contam Hydrol 258:104232. https://doi.org/10.1016/j.jconhyd.2023.104232
Harmon TC, Burks GA, Aycaguer AC, Jacckson K (2001) Thermally enhanced vapor extraction for removing PAHs from lampblack-contaminated soil. J Environ Eng 127:986–993. https://doi.org/10.1061/(ASCE)0733-9372(2001)127:11(986)
Hempfling R, Schulten HR (1990) Chemical characterization of the organic matter in forest soils by Curie point pyrolysis-GC/MS and pyrolysis-field ionization mass spectrometry. Org Geochem 15(2):131–145. https://doi.org/10.1016/0146-6380(90)90078-E
Horst J, Munholland J, Hegele P, Klemmer M, Gattenby J (2021) In situ thermal remediation for source areas: technology advances and a review of the market from 1988–2020. Groundwater Monit Rem 41:17–31. https://doi.org/10.1111/gwmr.12424
Kaleris V, Croisé J (1999) Estimation of cleanup time in layered soils by vapor extraction. J Contam Hydrol 36:105–129. https://doi.org/10.1016/S0169-7722(98)00138-7
Lighty JS, Pershing DW, Cundy VA, Linz DG (1988) Characterization of thermal desorption phenomena for the cleanup of contaminated soil. Nucl Chem Waste Manag 8(3):225–237. https://doi.org/10.1016/0191-815X(88)90030-7
Lighty JS, Silcox GD, Pershing DW, Cundy VA, Linz DG (1990) Fundamentals for the thermal remediation of contaminated soils. Particle and bed desorption models. Environ Sci Technol 24(5):750–757. https://doi.org/10.1021/es00075a022
Liu X, Murdoch LC, Falta RW, Tan T (2014) Experimental characterization of CVOC removal from fractured clay during boiling. Int J Heat Mass Transf 70:764–778. https://doi.org/10.1016/j.ijheatmasstransfer.2013.10.052
Liu Y, Huang F, Zhou D, Wang T, Wang G (2024) Study of factors affecting the remediation of contaminated soil through pneumatic fracturing and soil vapor extraction. J Hazard Mater 467:133776. https://doi.org/10.1016/j.jhazmat.2024.133776
Luo L, Chen Z, Cheng Y, Lv J, Cao D, Wen B (2019) Effects of dissolved organic carbon on desorption of aged phenanthrene from contaminated soils: a mechanistic study. Environ Pollut 254:113016. https://doi.org/10.1016/j.envpol.2019.113016
Merino J, Bucalá V (2007) Effect of temperature on the release of hexadecane from soil by thermal treatment. J Hazard Mater 143:455–461. https://doi.org/10.1016/j.jhazmat.2006.09.050
Mumford KG, Martin EJ, Kueper BH (2021) Removal of trichloroethene from thin clay lenses by electrical resistance heating: laboratory experiments and the effects of gas saturation. J Contam Hydrol 243:103892. https://doi.org/10.1016/j.jconhyd.2021.103892
Piña J, Merino J, Errazu AF, Bucalá V (2002) Thermal treatment of soils contaminated with gas oil: influence of soil composition and treatment temperature. J Hazard Mater B94:273–290. https://doi.org/10.1016/s0304-3894(02)00081-x
Poppendieck DG, Loehr RC, Webster MT (1999a) Predicting hydrocarbon removal from thermally enhanced soil vapor extraction systems 1. Laboratory studies. J Hazard Mater B 69:81–93. https://doi.org/10.1016/S0304-3894(99)00062-X
Poppendieck DG, Loehr RC, Webster MT (1999b) Predicting hydrocarbon removal from thermally enhanced soil vapor extraction systems 2. Field study. J Hazard Mater B 69:95–109. https://doi.org/10.1016/S0304-3894(99)00063-1
Roland U, Buchenhorst D, Holzer F, Kopinke FD (2008) Engineering aspects of radio-wave heating for soil remediation and compatibility with biodegradation. Environ Sci Technol 42:1232–1237. https://doi.org/10.1021/es0713680
Roland U, Holzer F, Trommler U, Hoyer Ch, Rabe C, Kraus M, Schneider J, Kopinke FD (2012) Applications of radio-frequency heating in environmental technology. Procedia Eng 42:161–164. https://doi.org/10.1016/j.proeng.2012.07.406
Sabour MR, Jalali SHS, Dezvareh G (2017) Comprehensive model for remediation of sandy soils contaminated with volatile organic compounds using thermal enhancement of soil vapor extraction method. Water Air Soil Pollut 228:239. https://doi.org/10.1007/s11270-017-3414-5
Schulten HR (1987) Pyrolysis and soft ionization mass spectrometry of aquatic/terrestrial humic substances and soils. J Anal Appl Pyrol 12(2):149–218. https://doi.org/10.1016/0165-2370(87)85063-5
Shi JX, Yang Y, Lu XJ, Xi BD, Li J, Xiao C (2021) Effect of water-level fluctuation on the removal of benzene from soil by SVE. Chemosphere 274:129796. https://doi.org/10.1016/j.chemosphere.2021.129796
Sun H, Qin X, Yang X, Zhao Y (2020) Study on the heat transfer in different aquifer media with different groundwater velocities during thermal conductive heating. Environ Sci Pollut Res 27:36316–36329. https://doi.org/10.1007/s11356-020-09131-2
Sun C, Xu C, Zhao Y, Zhang W, Li X, Zhang Q, Wu B, Ma F, Gu Q (2023) A numerical simulation of thermally-enhanced soil vapor extraction with a validation of an actual contaminated site. Chemosphere 338:139413. https://doi.org/10.1016/j.chemosphere.2023.139413
Sun X, Zhao L, Huang M, Hai J, Liang X, Chen D, Liu J (2024) In-situ thermal conductive heating (TCH) for soil remediation: a review. J Environ Manage 351:119602. https://doi.org/10.1016/j.jenvman.2023.119602
Tse KKC, Lo SL (2002) Desorption kinetics of PCP-contaminated soil: effect of temperature. Water Res 36:284–290. https://doi.org/10.1016/S0043-1354(01)00191-9
USEPA (1991) Soil vapor extraction technology: reference handbook (EPA/540/2-91/003). In: Office of research and development, USEPA, Cincinnati, OH
Ussiri DAN, Johnson CE (2003) Characterization of organic matter in a northern hardwood forest soil by 13C NMR spectroscopy and chemical methods. Geoderma 111:123–149. https://doi.org/10.1016/S0016-7061(02)00257-4
Wang W, Chen C, Xu W, Li C, Li Y (2021) Experimental research on heat transfer characteristics and temperature fise law on in situ thermal remediation of soil. J Therm Anal Calorim 147:3365–3378. https://doi.org/10.1007/s10973-021-1064-1
Webb SW, Phelan JM (1997) Effect of soil layering on NAPL removal behavior in soil-heated vapor extraction. J Contam Hydrol 27:285–308. https://doi.org/10.1016/S0169-7722(96)00094-0
Wu Y, Feng Y, Fan L, Wang Q, Song X, Yu Z (2022) Effects of moisture content and dry bulk density on the thermal conductivity of compacted backfill soil. J Zhejiang Univ Sci A 23:610–620. https://doi.org/10.1631/jzus.A2100673
Xu X, Hu N, Wang Q, Fan L, Song X (2022) A numerical study of optimizing the well spacing and heating power for in situ thermal remediation of organic-contaminated soil. Case Stud Therm Eng 33:101941. https://doi.org/10.1016/j.csite.2022.101941
Yu Y, Liu L, Yang CY, Kang WH, Yan ZY, Zhu YM, Wang JG, Zhang HJ (2019) Removal kinetics of petroleum hydrocarbons from low-permeable soil by sand mixing and thermal enhancement of soil vapor extraction. Chemosphere 236:124319. https://doi.org/10.1016/j.chemosphere.2019.07.050
Zheng Q, Yang C, Feng S, Wu S, Zhang X (2023) Influence mechanism of thermally enhanced phase change on heat transfer and soil vapour extraction. J Contam Hydrol 257:104202. https://doi.org/10.1016/j.conhyd.2023.104202
Funding
This work was funded by National Natural Science Foundation of China (Grant no. 21207010).
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, Y., Kang, W., Song, J. et al. The Mechanism for Remediation of Low-Permeable Soil Contaminated with Petroleum Hydrocarbons by Thermally-Enhanced Soil Vapor Extraction Combined with Sand Mixing. Int J Environ Res 18, 86 (2024). https://doi.org/10.1007/s41742-024-00639-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41742-024-00639-w