Abstract
Natural grasslands represent the second largest ecosystem in Turkey. However, the impact of varying nitrogen (N) fertilization rates on overall soil health indicators have not been reported in the country. A 2-year study was conducted in the Kahramnmaraş Plateau region in Turkey to evaluate the impacts of seven N application rates [i.e., 0 (N0), 50 (N50), 100 (N100), 150 (N150), 200 (N200), 250 (N250), and 350 (N350) kg N ha−1] on physical, chemical, and biological parameters of soil health. Nitrogen addition decreased bulk density by 8–12%, and increased aggregate stability by 3–5% and EC up to 110%. Application of ≥ 100 kg N ha−1 increased soil porosity up to 6.7%. Soil pH and C:N ratios were not affected by N addition. The lowest plant available water occurred with the N0 and N50 treatments, decreasing around 24% and 17% compared to N300. Soil organic carbon, total nitrogen, and C and N stocks increased with increasing N addition. Application of N300 rates increased C stocks between 4 and 34%, and N stocks between 15 and 22% compared to all other treatments. Compared to control, N250 increased microbial biomass carbon by 349% and nitrogen by 250%. Microbial respiration in the N250 and the N300 treatments was 97% and 129% greater than control. Addition of N fertilization for a first time in a grassland ecosystem with a previous history of long-term overgrazing, even at low rates, positively impacted several parameters of soil health, a positive impact that could ensure a greater sustainability of these fragil systems over the long-term.
Article Highlights
-
Microbial respiration (MR) in positively linked with the application of nitrogen as fertilizers in grasslands.
-
N fertilization, even at low rates, positively impacted several parameters of soil health in grassland ecosystems that have typically been exposed to intense overgrazing without addition of fertilizers in the past.
-
Balance N in soil also played imperative role in maintained of C stock in grasslands.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Timely production of quality fiber is essential to optimize animal production. However, the demand for quality fiber for animal feeding has largely exceeded the current fiber supply in Turkey and most countries around the world (Alçiçek et al. 2010). Different fertilization methods have been traditionally utilized across vast grassland areas in Turkey to ensure sufficient supply of quality fiber. Fertilization is one of the most efficient ways to improve productivity in grasslands that have been poorly managed but still conserve a minimum botanical composition to ensure future supply of feedstock biomass across the year (Çomaklı et al. 2005). Alongside with phosphorus, nitrogen (N) is the main crop yield-reducing nutrient (Kumar et al. 2019a; b; Adeyemi et al. 2020). However, N limitation in terrestrial ecosystems is ubiquitous, in part as a result of expensive nutrient reposition costs to crops (Battaglia et al. 2018), and constant short-term changes in the soil total N storage due to the dynamic nature of its cycle (Vitousek and Howarth, 1991; LeBauer and Treseder, 2008). Although there is a potential to increase biological N fixation (Diatta et al. 2020) and sustainable P nutrition with the use of phosphate-solubilizing bacteria in modern agroecosystems (Adnan et al. 2020), the use of N and P synthetic fertilizers in grasslands is the most common and widespread practice to increase plant productivity (Li et al. 2014; DeForest et al. 2004). While much effort has been devoted to measuring the aboveground impacts of increased anthropogenic N in crop productivity (Ronnenberg and Wesche 2011; Xu et al. 2015), much less has been done to elucidate the belowground impacts of these practices.
Soil available N is essential for soil microbial growth and overall regulation of the cycling processes of several nutrients in soil (Coleman and Crossley 1996). Likewise, changes in soil microbial biomass carbon (MBC) and N (MBN) or its activity rate may be an indication of changes in the rates of organic matter decomposition (Babur and Dindaroğlu 2020; Babur et al. 2021), humus formation (Magill and Aber 1998), nutrient cycling (Fisk and Fahey 2001), and interaction between soil microorganisms and plants (Bolat and Sensoy 2019).
Grassland soils represent a highly active organic carbon pool that plays an important global role in ecosystem sustainability (Yang et al. 2011). In these systems, N is the most limiting nutrient, mainly as a result of N replishnesment rates that are below the rate of plant N uptake and the reposition by natural sources such as precipitation, pollination, dust accumulation, and animal feces (Scurlock and Hall 1998; Bai et al. 2010; Fornara et al. 2013). The application of synthetic fertilizers can have a profound impact in the soil organic C pool and its functions in grassland ecosystems both in the short and long-terms (Wang et al. 2002). However, these impacts depend on the system under analysis (Vourlitis and Zorba 2007; Li et al. 2010; Bai et al. 2011). Previous research shows that application of N and P fertilizers decreased soil organic C in tundra soils (Mack et al. 2004), but increased it in grassland soils (Fornara et al. 2013). As a result, utilization of different soil fertility management will likely have a differential impact on soil productivity, soil carbon sequestration, and SMB and composition across different land use types (Li et al. 2014). Additionally, changes in some parameters could also be expected over time within a particular land use type. Research showed that while N fertilization in grassland soils may not impact or may even increase the SMB in the short-term (Johnson et al. 2005), overall decreases in SMB and respiration occured in the long-term (Söderström et al. 1983; Lovell et al. 1995; Allison et al. 2013). Increases in SMB (Roberge and Knowles 1967; Zhang et al. 2005) and microbial respiration (Salonius and Mahendrappa 1975) have also been reported in short-term incubation studies.
Different fertilization rates can impact the size of the soil carbon pool by increasing the plant biomass productivity and enhancing floral diversity (Mack et al. 2004; Fornara and Tilman 2012; Qi, 2013), which can, in turn, increase the rates of microbial decomposition and SMB (Liu et al. 2010; Allison et al. 2013). However, changes in plant biomass can be species-related. For instance, Chen (2010) found that different N and N + P application rates significantly increased grass productivity while concomitantly decreasing that of legumes.
Different studies investigated the effects of N fertilization on chemical and microbiological parameters of soil quality in Europe and the USA, but none has been conducted in grassland areas in Turkey. In this country, grasslands represent the 2nd largest ecosystem after forests, occupying around 19% of the nation’s total area 2014. Most of this area is located in the country’s inner and southern temperate arid and semi-arid regions. Within this region, grasslands in the Kahramanmaraş Plateau are estimated to account for approximately 30% of the total grassland area in the country. In recent years, grassland ecosystems in this region were exposed to intense overgrazing which has resulted in floristic degradation and severe soil N deficiencies. A judicious N fertilization management could increase plant productivity and soil fertility in grassland areas while preserving the sustainability of these fragile ecosystems. However, information regarding the impact of different fertilization management in soil health, including soil organic carbon (SOC), nitrogen pools, and SMB in this region is not available at present.
In this study, we assessed the impact of different N rates on soil parameters that have a main role in grassland’s nutrient cycling and tend to quickly respond to changes in management, including SOC, total N, and microbial biomass dynamics. The objectives of this study were: (1) to investigate how different rates of N fertilization affect soil organic C and soil microbial variables such as microbial biomass C, microbial biomass N, microbial respiration (MR), the metabolic quotient (MR:MBC = qCO2), and microbial quotient (SOC/MBC); and (2) to determine the physico-chemical properties of soils that affect the soil microbial biomass following N addition.
Materials and Methods
Study Site
The study was conducted between November, 2018 and May, 2019 in a grassland area located in the Kuyumcular Village of South-Eastern Kahramnmaraş Plateau, Turkey (N 37° 30′ 02″–37° 26′ 26″ and E 36° 52′ 44″–36° 49′ 09″, 490 m above sea level). The study site has a 30 year mean annual precipitation of 750 mm, with 65% of the total annual precipitation occurring in the winter season, between December and March and a mean annual temperature of 15.8 °C (Institute of Kahramanmaraş Meteorology; https://www.yr.no/). August is the hottest month with an average temperature of 27.5 °C, while January is the coldest month with an average temperature of 3.9 °C. According to the Unified soil classification system (USCS), soil in the study site is classified as sandy loam (Holtz and Kovacs 1981). At the beginning of the study, vegetation was dominated by grasses such as Agrostis capillaris, Avena barbata, Bromus diandrus, Hordeum murinum ssp. glaucum, Lolium temulentum, Phlaris arundinacea, Phlaris paradoxa, Phleum pratense, and legumes such as Medicago polymorpha var. vulgaris, Melilotus officinalis, Trifolium lappaceum, Trifolium nigrescens ssp. petrisavrii. Although soil class in the study site is classified as a first-class soil, total net primary productivity and quality in the site is, on average, low, and the site never received nitrogen fertilization before the inception of the current study. To avoid animal grazing, the experimental area across the whole study was protected with fences.
Experimental Design
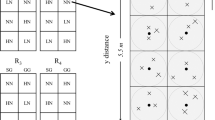
Seven treatments were utilized in this study, as follows: (1) N0 (unfertilized control), 0 kg N ha−1; (2) N50, 50 kg N ha−1; (3) N100, 100 kg N ha−1; (4) N150, 150 kg N ha−1; (5) N200, 200 kg N ha−1; (6) N250, 250 kg N ha−1; and (7) N300, 300 kg N ha−1. Granulated urea (46-0-0) was used as the fertilizer source in all treatments. Treatments were replicated three times, for a total of 21 experimental units (EU), in a completely randomized block design. Each EU was 3 m wide by 4 m in length (12 m2 per EU). All plots were separated by a 1 m wide unfertilized buffer in all sides. Phosphorus fertilizer was applied in the whole experimental area at a rate of 40 kg P ha−1 using triple superphosphate (TSP) fertilizer on November 15, 2018 (i.e., P was not a factor under analysis in this study). Nitrogen treatments were applied on March 10, 2019, following recommendations from a previous study in the same research area (Uslu 2005).
Sampling
In May 2019, four random samples of approximately 0.11 m2 each one (0.33 × 0.33 m) were at around 1 cm above the ground level in each EU. Plant samples were separated into three functional groups: grasses, legumes, and forbs. Following plant biomass harvest, two soil cores samples (8 cm diameter by 10 cm depth) were randomly collected at the center of each EU using a hand probe, and were then composited into one sample per EU and placed in paper bags. Later, composited soil samples were air-dried until constant mass weight at the laboratory for determination of physico-chemical soil properties. Additionally, two soil samples were taken for microbial analysis at each EU, composited, and field-stored in a cooler with dry ice until transportation to the laboratory where the the samples were stored at + 4 °C in a refrigerator until analysis. In both cases, soil samples were sieved to pass a 2-mm screen (Weidhuner et al. 2019) to remove plant roots, gravel, and coarse fragments.
Physico-Chemical Properties
Soil moisture content (gravimetric method) was measured by oven-drying the soil samples for 24 h at 105 °C until constant mass weight (Gülçur 1974; Karaöz 1992). Soil pH and electrical conductivity (EC) was measured by glass electrode (1:2.5 w/v and 1:5 w/v soil/pure water suspension for pH and EC, respectively). SOC and total N (TN) concentrations were determined using the dichromate oxidation with K2Cr2O7 and the semi-micro Kjeldahl methods using air-dried soil samples, respectively (Kalembasa and Jenkinson 1973; Lu 1999). Soil bulk density was estimated using a stainless-steel core-sampling cylinder of known volume (Karaöz 1992). Then, SOC and TN values were multiplied by the bulk density in each EU to calculate the soil C and N stocks in units of Mg ha−1.
Biochemical Properties
Soil microbial biomass carbon (MBC; μg g−1) was analyzed from samples in the incubation beakers by using the chloroform-fumigation-extraction method (CFEM) (Vance et al. 1987). Soil field moisture content was adjusted to 50% of their water-holding capacity (WHC) before microbial analysis. Then, 30 g oven-dry weight was transferred separately into Petri-dishes within a desiccator. Sub-samples were fumigated with ethanol-free chloroform (CHCl3) for 24 h at 25 °C. Samples were extracted for MBC by adding 100–120 ml of 0.5 M K2SO4, shaking for 30 min, and then filtered through Whatman No. 2 paper. Three 30 g nonfumigated soil sub-samples were processed in the same manner. Finally, the SMBC was calculated as follows (Vance et al. 1987):
where KEC refers to the difference in extractable organic C between the fumigated and unfumigated samples and 2.64 is a factor to account for the biomass C released by fumigation extraction.
Soil microbial biomass nitrogen (MBN; μg g−1) was calculated following the method described by Brookes et al. (1985) and Anderson and Ingram (1996) as follows:
where FN is the difference between N extracted from fumigated and unfumigated samples, and 0.54 is the fraction of microbial biomass N released by fumigation extraction.
Soil microbial respiration (MR; mg CO2–C h−1 g−1) was determined over a 7-day period in the laboratory. Then, 30 g of the sieved field-moist soil samples was weighed into 250 ml Schott jars. Small beakers filled with 15 ml of 1 M NaOH were placed at the jars' soil surface to trap the evolved CO2. The jars were fastened airtight and incubated for 7 days at 25 °C. The moisture content of soil samples was periodically adjusted to 40–50% of the soil water-holding capacity (Babur 2019). At the end of the incubation period, the small beakers with the CO2 absorbed in the NaOH solution were removed and titrated with 0.1 M HCl after the addition of BaCl2. A NaOH solution without soil, incubated as above, was also titrated. Microbial respiration was calculated as the amount of CO2 evolution in the first 24 h incubation divided by the mass of dry soil. The metabolic quotient (qCO2) was calculated as the MR to MBC ratio (Anderson and Domsch 1990, 1993).
Data Analysis
Data were analyzed using SPSS 16.0 statistical software. Figures were plotted using Sigma Plot 10.0 software. A one-way ANOVA analysis was conducted to assess the impact of the seven treatments (six rates + unfertilized control) utilized in this study on selected parameters. When the analysis was significant, the control was removed from the analysis and a two-way MANOVA was conducted to test the overall effects of fertilization rates on measured parameters. The least significant difference (LSD) test was used to separate means at a P = 0.05 significance level.
Results
Soil Physical Properties
Highest bulk density was observed in the unfertilized control treatment (N0; 0.91 g cm−3). The addition of N fertilizer in the range between 50 and 300 kg N ha−1 decreased soil bulk density between 8 and 12%, with no differences among fertilizer rates.
Nitrogen rates at or greater than 100 kg N ha−1 increased soil porosity between 5.1 and 6.7% compared to the lowest soil porosity values observed in the unfertilized control (63.8%), with non-significant trends showing slightly higher values as more fertilizer was added. The minimum fertilization rate utilized in this study (i.e., 50 kg N ha−1) did not significantly affect the soil porosity when compared to the unfertilized control. All N fertilization rates increased aggregate stability between around 3 and 5% compared to minimum aggregate stability values of around 0.9 observed in the unfertilized control (Table 1).
Soil Chemical Properties
Soil pH was not impacted by different fertilization rates (Table 2). As a result, both before and after application of different N rates in this study, soil pH in the whole experimental area was classified as slightly alkaline. Increased application of N causes a significant increase in EC. A minimum EC of 654.0 ± 13.6 was observed in the unfertilized control. Application of N300 showed the highest EC (1374.0 ± 60.6), which was between 110 and 32% greater than EC for the N0 and N50 treatments, respectively, but no different than the EC of N100, N150, N200, and N250 (Table 2).
Soil Water-Related Parameters
A consistent trend showed increases in field capacity, permanent wilting point, and plant available water with higher N application rates (Table 3). The lowest field capacity and permanent wilting point were observed in the N0, N50, and N100 treatments, with reductions between 4 and 7% compared to maximum values observed in the N300 treatments for both parameters. The lowest plant available water was observed in the N0 and N50 treatments, with decreases between 24 and 17% when compared to the maximum plant available water content in the N300 treatment (Table 3).
Soil Organic Carbon and Total Nitrogen
Soil organic carbon (SOC) and total nitrogen (TN) concentrations gradually increased with increases in N application rates (Table 4). Maximum SOC (6.37 ± 0.24%) and TN (0.48 ± 0.007%) were measured in the N300 treatment in both cases. These values represent an increase of 35% and 30% compared with the lowest SOC values in the N0 and N50 treatments, respectively, and increases between 16 and 27% compared to minimum TN in N0, N50, N100, and N150 treatments (Table 4). Moreover, SOC and TN in the N300 were not different from values in the N250 treatment. Although C:N ratio slightly increased with increasingly higher N rates, this trend was not statistically significant, resulting in all N fertilization treatments having a similar C:N ranging between 12.6 and 13.5 (Table 4).
In the range of soil C stock observed in our study (39.0–52.3 Mg ha−1), increases in N application rates gradual and significantly increased soil C stocks (Fig. 1a). Application of 300 kg N ha−1 resulted in the highest C stock (52.3 ± 1.99 Mg ha−1), a value that was 4% (vs. N250) to 34% (vs. N0) greater when compared to the other N treatments. Moreover, the N250 treatment increased the soil C stock by 29% compared to N0, but it was not different than N50, N100, N150, and N200 rates.
Maximum N stock values measured in the N300 treatment were 22%, 16%, and 15% higher than N stocks in N0, N50, and N100, respectively, but similar to N stocks in N150, N200, and N250 (Fig. 1b). In both cases, unfertilized control N0 had the lowest C (39.0 ± 1.71 Mg ha−1) and N stocks (3.13 ± 0.08 Mg ha−1) (Fig. 1a, b).
Soil Biochemical Properties and Microbial Respiration
Compared to the lowest MBC and MBN values obtained with N0, the N250 treatment increased MBC by 349%, from 211.3 to 950.8 µg g−1 (Fig. 2a) and MBN by 250%, from 21.6 to 75.6 µg g−1 (Fig. 2b). Although there were no differences in MBN between the N250 and N300 treatments, MBC was 29% greater in the N250 compared to the N300 rate. In this study, the minimum N application rate (N50) increased MBC by 53% and MBN by 25% compared to N0.
All N application treatments increased soil microbial respiration (MR) compared to unfertilized control N0 (1.723 μg CO2–C g−1 h−1), with greatest increased of 97% and 129% when compared to the N250 (3.389 μg CO2–C g−1 h−1) and the N300 treatments (3.945 μg CO2–C g−1 h−1), respectively (Fig. 3). Results of PCA showed that biomass C and N are more closely associated with changes in MR. Effect of N200, N250, and N300 was dominant for the differences in biomass C, N, and MR (Fig. 4). Pearson correlation showed that biomass C and N, and stock C and N were significant and positively correlated with MR (Fig. 5).
Discussion
A strong positive correlation between total N in soil and particle size distribution was observed in the current study due to soil particles' ability to retain soil nutrients. Seneviratne et al. (2009) reported that soil total N is correlated with particle size distribution. Jiao et al. (2014) argued that particle size distribution is affected by available soil nutrients and other factors, including soil organic matter. Conversely, Percival et al. (2000) reported no correlation between particle size distribution and nutrient availability. Zhang et al. (2016) reported the correlation of available soil N with silt as N is easily decomposable and can be adsorbed by silt more efficiently than by sand and clay.
Particle density, bulk density, and soil porosity are essential indicators of soil compaction and can be profoundly affected by changes in management. Our results showed that N application gradually decreased soil bulk density and increased soil porosity compared to the unfertilized control N0 (Table 1), which is in agreement with reports from Rasool et al. (2008). Thus, a reduction in soil compaction in grassland exosystems could be potentially achieved if N fertilization is adopted as a long-term management practice. Soil compaction is characterized by an increase in the penetration resistance that is influenced by soil structural characteristics and functions (Abu-Hamdeh 2003).
We found no difference in the soil aggregate stability, an important indicator of soil structural development and C dynamics in soil, when N was applied in rates between 50 and 300 kg N ha−1. Similarly, Hati et al. (2008) reported no changes in soil aggregate stability with N rates. Xin et al. (2016) also reported that mineral fertilizers including NPK and NP did not increase aggregate stability following 23 years of fertilizer application. The NH4+ form of N tends to decrease aggregate stability by dispersing organic binding agents inside aggregates and soil colloids (Fonte et al. 2009). However, Hati et al. (2006) reported that long-term (31 years) dual application of compost and mineral fertilizer significantly increased aggregate stability compared to unfertilized control, likely due to the beneficial binding effect of compost in soil aggregates and soil colloids.
Plant available water gradually increased with N application rates between 100 and 300 kg N ha−1. Increases in plant available water could result from an increase in N use efficiency through a reduction in the losses from denitrification, immobilization, leaching, and volatilization (Raun and Johnson 1995), although information about the role of N addition in the promotion of water use efficiency is scarse.
Nitrogen fertilization did not affect soil pH in our study. Conversely, all N rates gradually increased EC in this study, with maximum EC resulting from the application of the N300 treatment. However, final EC values from all the treatments under analysis were classified as non-saline soils (i.e., EC ≤ 2000 µS cm−1), which do not limit plant growth due to salinity (USSL Staff 1954). Supra-optimum levels of N addition, on the other hand, may cause over accumulation of NO3− and, thus, increases in soil salinity and impairment of waterbodies (Kirchmann et al. 2002). Utilization of grasses as catch crops can utilize the excess amount of NO3−, thus reducing its lateral runoff and vertical leaching in the soil profile. However, this approach needs further development to explore the varying potential that various grasses and other catch crops can have to reduce the risk of N loss from agroecosystems.
Soil organic C and total N contents gradually increased with increased N addition, although numerical differences were only significant when 250 and 300 kg N−1 were added, with increases ranging between 24 and 35% compared to the unfertilized control (Table 4). In both cases, N300 rate caused the highest increases in soil organic C and total N contents. These findings are similar to other findings highlighting the importance that N fertilization has to increase soil C and N stocks (Cai and Qin 2006; Tong et al. 2009). The variation in the impact of N addition on soil organic C and total N contents could be related to their application rates, suggesting that grassland soils have acted as a C sink. Tong et al. (2009) also reported an increase in soil organic C and total N contents after 17 years of cropping at eight experimental sites. They reported paddy soils as a C sink affected by fertilizer, management practices, and climate (Tong et al. 2009). However, the increases in soil organic C, total N, and C and N stocks with increasing N application rates in our study were higher to those reported by Tong et al. (2009) in China. Increasing SOC and TN with the addition of greater N rates in our study was of similar magnitude in each case, resulting in no changes in the soil C:N ratios across different N rates. Gal et al. (2007) reported strong positive correlations between soil organic C and total N that determined the stability of soil C:N ratios in the plough soil layer. The C:N ratios in our study ranged between 12.6 and 13.5 across all the different N fertilization rates, thus suggesting that short-term addition of various rates of N fertilizer would not have an immediate effect in the C:N ratios of grassland soils.
The addition of increasingly higher rates of N fertilizer increased soil microbial respiration because of enhanced growth and respiration of both root and microbial population (Lovell and Hatch 1998). Similarly, cumulative soil respiration increased with the application of 50, 100, and 200 kg N ha−1 over a 2-year period in a study conducted in N-deficient soils in a temperate grassland in Inner Mongolia, China (Peng et al. 2011). Conversely, Burton et al. (2004) reported no response in root and microbial respiration with increased N in a forest in Michigan due to limited nutrients resources other than NO3–N. Wang et al. (2019) reported seasonal variations in soil respiration as a result of different N application rates in a long-term study in a loam soil in China. They concluded that the addition of N in N-deficient ecosystems could play an important role in soil C losses through increasing soil respiration (Peng et al. 2011). In our study, N addition positively impacted both microbial biomass C and C stocks and this might have had immediate positive effects on soil respiration rates, although the increase in soil respiration in our study was lower compared to values reported by Peng et al. (2011). Xu and Wan (2008) also reported increases in CO2 emissions following the application of different urea rates. In our study, CO2 emissions from microbial respiration were maximized with the N250 and N300 rates, similar to results from Aber et al. (1989).
Conclusion
In this study, nitrogen fertilization improved most physical, chemical, and biological parameters of soil quality in a grassland environment in Turkey that had not receive any anthropogenic N fertlization before. Although maximum values for most parameters resulted from application of 250 and 300 kg N ha−1, significant increases in soil porosity, aggregate stability, plant available water, biomass C and N ocurred with application of either 50 or 100 kg N ha−1, which were the two lowest N rates utilized in this study. Soil C stocks and soil microbial respiration, on the other hand, were only affected by the addition of 250 or 300 N ha−1. In conclusion, N fertilization, even at low rates, can positively impact several soil health parameters over the short term when applied to grassland ecosystems that have typically been exposed to intense overgrazing and have no history of fertility management.
References
Aber JD, Nadelhoffer K, Steudler P et al (1989) Nitrogen saturation in northern forest ecosystems. Bioscience 39:378–386
Abu-Hamdeh NH (2003) Effect of compaction and deep tillage on soil hydraulic and aeration properties and wheat yield. Commun Soil Sci Plant Anal 34:2277–2290
Adeyemi O, Keshavarz-Afshar R, Jahanzad E, Battaglia ML, Luo Y, Sadeghpour A (2020) Effect of wheat cover crop and split nitrogen application on corn yield and nitrogen use efficiency. Agronomy 10:1081
Adnan M, Fahad S, Zamin M, Shah S, Mian IA, Danish S, Zafar-ul-Hye M, Battaglia ML, Naz RMM, Saeed B, Saud S, Ahmad I, Yue Z, Brtnicky M, Holatko J, Datta R (2020) Coupling phosphate-solubilizing bacteria with phosphorus supplements improve maize phosphorus acquisition and growth under lime induced salinity stress. Plants 9:900
Alçiçek A, Kılıç A, Ayhan V, Özdoğan M (2010) “Türkiye’de kaba yem üretimi ve sorunları”, Türkiye Ziraat Mühendisliği VII. Teknik Kongresi. TMMOB Ziraat Mühendisleri Odası (ZMO) 11–15 Ocak 2010, Cilt:2, S.1071–1080, Ankara
Allison SD, Lu Y, Weihe C, Goulden ML, Martiny AC et al (2013) Microbial abundance and composition influence litter decomposition response to environmental change. Ecology 94(3):714–725
Anderson TH, Domsch KH (1990) Application of ecophysiological quotients (qCO2 and qD) on microbial biomass from soils of different cropping histories. Soil Biol Biochem 22:251–255
Anderson TH, Domsch KH (1993) The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions, such as pH, on the microbial biomass of forest soils. Soil Biol Biochem 25:393–395
Anderson JM, Ingram JSI (1996) Tropical soil biology and fertility a handbook of methods, 2nd edn. Cab International, Wallingford
Babur E (2019) Effects of parent material on soil microbial biomass carbon and basal respiration within young afforested areas. Scan J For Res 34(2):94–101
Babur E, Dindaroğlu T (2020) Seasonal changes of soil organic carbon and microbial biomass carbon in different forest ecosystems. In: Environmental factors affecting human health, Ivan Uher, IntechOpen. https://doi.org/10.5772/intechopen.90656
Babur E, Dindaroğlu T, Solaiman Z, Battaglia ML (2021) Microbial respiration, microbial biomass and activity are highly sensitive to forest tree species and seasonal patterns in the Eastern Mediterranean Karst Ecosystems. Sci Total Environ 775:145868
Bai YF, Wu JG, Clark CM, Naeem S, Pan QM et al (2010) Tradeoffs and thresholds in the effects of nitrogen addition on biodiversity and ecosystem functioning: evidence from inner Mongolia Grasslands. Glob Chang Biol 16:358–372
Bai JB, Xu XL, Song MH, He YT, Jiang J et al (2011) Effects of temperature and added nitrogen on carbon mineralization in alpine soils on the Tibetan Plateau. Ecol Environ Sci 20(5):855–859
Battaglia ML, Groover G, Thomason WE (2018) Harvesting and nutrient replacement costs associated with corn stover removal in Virginia. Virginia Cooperative Extension Publication. CSES-229NP
Bolat İ, Şensoy H (2019) Microbial biomass soil content and activity under black alder and sessile oak in the Western Black Sea Region of Turkey. Int J Environ Res 13(5):781–791
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Burton AJ, Pregitzer KS, Crawford JN, Zogg GP, Zak DR (2004) Simulated chronic NO3 deposition reduces soil respiration in northern hardwood forests. Glob Change Biol 10:1080–1091
Cai ZC, Qin SW (2006) Dynamics of crop yields and soil organic carbon in a longterm fertilization experiment in the Huang-Huai-Hai Plain of China. Geoderma 136:708–715
Chen LY (2010) Effect of N, P addition on N:P stioehiometry of different functional groups in Potentilla fruticosa community in a sub-alpine meadow. Thesis, Lanzhou University, Lanzhou, Gansu, China
Coleman DC, Crossley DA (1996) Fundamentals of soil ecology. Academic Press, San Diego
Çomaklı B, Güven M, Koç A, Menteşe Ö, Bakoğlu A, Bilgili A (2005) Azot, Fosfor ve Kükürtle Gübrelemenin Ardahan Meralarının Verim ve Tür Kompozisyonuna Etkisi, Türkiye VI. Tarla Bitkileri Kongresi, Antalya, Türkiye 2:757–761
DeForest JL, Zak DR, Pregitzer KS, Burton AJ (2004) Atmospheric nitrate deposition, microbial community composition, and enzyme activity in Northern hardwood forests. Soil Sci Soc Am J 68(1):132
Diatta AA, Thomason WE, Abaye O, Thompson TL, Battaglia ML, Vaughan LJ, Lo M, Leme JFDC (2020) Assessment of nitrogen fixation by mungbean genotypes in different soil textures using 15N natural abundance method. J Soil Sci Plant Nutr. https://doi.org/10.1007/s42729-020-00290-2
Fisk MC, Fahey TJ (2001) Microbial biomass and nitrogen cycling responses to fertilization and litter removal in young Northern hardwood forests. Biogeochemistry 53(2):201
Fonte SJ, Yeboah E, Ofori P, Quansah GW, Vanlauwe B, Six J (2009) Fertilizer and residue quality effects on organic matter stabilization in soil aggregates. Soil Sci Soc Am J 73:961–966
Fornara DA, Tilman D (2012) Soil carbon sequestration in prairie grasslands increased by chronic nitrogen addition. Ecology 93:2030–2036
Fornara DA, Banin L, Crawley MJ (2013) Multi-nutrient vs. nitrogen-only effects on carbon sequestration in grassland soils. Glob Chang Biol 19:3848–3857
Gal A, Tony JV, Erika M, Eileen JK, William WM (2007) Soil carbon and nitrogen accumulation with long-term no-till versus moldboard plowing overestimated with till-zone sampling depth. Soil Till Res 96:42–51
Gülçur F (1974) Soil Physical and Chemical Analysis Methods (In Turkish). İstanbul University Publication No. 1970, Forest Faculty Publication No. 201, İstanbul, Turkey: Kutulmuş Printing
Hati KM, Swarup A, Singh D, Misra AK, Ghosh PK (2006) Long-term continuous cropping, fertilisation, and manuring effects on physical properties and organic carbon content of a sandy loam soil. Aust J Soil Res 44:487–495
Hati KM, Swarup A, Mishra B, Manna MC, Waniari RH, Mandal KG, Misra AK (2008) Impact of long-term application of fertilizer, manure and lime under intensive cropping on physical properties and organic carbon content of an Alfisol. Geoderma 148:173–179
Holtz RD, Kovacs WD (1981) An introduction to geotechnical engineering (chapter 3). Prentice Hall
Jiao S, Zhang M, Wang Y et al (2014) Variation of soil nutrients and particle size under different vegetation types in the Yellow River Delta. Acta Ecol Sin 34:148–153
Johnson D, Leake JR, Read DJ (2005) Liming and nitrogen fertilization affects phosphatase activities, microbial biomass and mycorrhizal colonisation in upland grassland. Plant Soil 271:157–164
Kalembasa SJ, Jenkinson DS (1973) A comparative study of titrimetric and gravimetric methods for the determination of organic carbon in soil. J Sci Food Agric 24:1085–1090
Karaöz MÖ (1992) Leaf and litter analysis methods (In Turkish). J Fac For Istanbul U Ser B 42(1–2):57–71
Kirchmann H, Johnston AE, Bergstrom LF (2002) Possibilities for reducing nitrate leaching from agricultural land. Ambio 31:404–408
Kumar P, Lai L, Battaglia ML, Kumar S, Owens V, Fike J, Galbraith J, Hong CO, Faris R, Crawford R, Crawford J, Hansen J, Mayton H, Viands D (2019a) Impacts of nitrogen fertilization rate and landscape position on select soil properties in switchgrass field at four sites in the USA. CATENA 180:183–193
Kumar S, Lai L, Kumar P, Feliciano YMV, Battaglia ML, Hong CO, Owens VN, Fike J, Farris R, Galbraith J (2019b) Impacts of nitrogen rate and landscape position on soils and switchgrass root growth parameters. Agron J 111(3):1046–1059
LeBauer DS, Treseder KK (2008) Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89(2):371
Li YC, Song CC, Hou CC, Song YY (2010) Effects of nitrogen input on meadow marsh soil N2O emission and organic carbon mineralization. Chin J Ecol 29(11):2091–2096
Li JH, Yang YJ, Li BW, Li WJ, Wang G et al (2014) Effects of nitrogen and phosphorus fertilization on soil carbon fractions in alpine meadows on the Qinghai-Tibetan Plateau. PLoS ONE 9(7):e103266. https://doi.org/10.1371/journal.pone.0103266
Liu ZF, Fu BJ, Zheng XX, Liu GH (2010) Plant biomass, soil water content and soil N:P ratio regulating soil microbial functional diversity in a temperate steppe: a Regional Scale Study. Soil Biol Biochem 42(3):445–450
Lovell RD, Hatch DJ (1998) Stimulation of microbial activity following spring applications of nitrogen. Biol Fertil Soils 26:28–30
Lovell RD, Jarvis SC, Bardgett RD (1995) Soil microbial biomass and activity in long term grassland: effects of management changes. Soil Biol Biochem 27(7):969–975
Lu RK (1999) Soil and agro-chemical analytical methods. China Agricultural Science and Technology Press, Beijing, China 107:147–150
Mack MC, Schuur EAG, Bret Harte MS, Shaver GR, Chapin FS III (2004) Ecosystem carbon storage in arctic tundra reduced by long term nutrient fertilization. Nature 431:440–443
Magill AH, Aber JD (1998) Long-term effects of experimental nitrogen additions on foliar litter decay and humus formation in forest ecosystems. Plant Soil 203(2):301
Peng Q, Dong Y, Qi Y et al (2011) Effects of nitrogen fertilization on soil respiration in temperate grassland in Inner Mongolia, China. Environ Earth Sci 62:1163–1171
Percival HJ, Parfitt RL, Scott NA (2000) Factors controlling soil carbon levels in New Zealand grassland is clay content important? Soil Sci Soc Am J 64:1623–1630
Qi R (2013) Response of plant community to nitrogen and phosphorous additions in sub-alpine meadows of the Qinghai Tibetan Plateau. Thesis, Lanzhou University, Lanzhou, Gansu, China
Rasool R, Kukal SS, Hira GS (2008) Soil organic carbon and physical properties as affected by long-term application of FYM and inorganic fertilizers in maize–wheat system. Soil Till Res 101:31–36
Raun W, Johnson GV (1995) Soil-plant buffering of inorganic nitrogen in continuous winter wheat. Agron J 87(5):827–834
Roberge MR, Knowles R (1967) The ureolytic microflora in a black spruce (Picea mariana Mill.) humus. Soil Sci Soc Am Proc 31:76–79
Ronnenberg K, Wesche K (2011) Effects of fertilization and irrigation on productivity, plant nutrient contents and soil nutrients in southern Mongolia. Plant Soil 340(1–2):239–251
Salonius PO, Mahendrappa MK (1975) Microbial respiration and exchangeable ammonium in podzol organic horizon materials treated with urea. Can J For Res 5:731–734
Scurlock JMO, Hall DO (1998) The global carbon sink: a grassland perspective. Glob Chang Biol 4(2):229–233
Seneviratne G, Henakaarchchi MPNK, Weerasekara MLMAW et al (2009) Soil organic carbon and nitrogen pools as influenced by polyphenols in different particle size fractions under tropical conditions. J Natl Sci Found Sri Lanka 37:67–70
Söderström B, Bååth E, Lundgren B (1983) Decreases in soil microbial activity and biomasses owing to nitrogen amendments. Can J Microbiol 29:1500–1506
Tong C, Xiao H, Tang G, Wang H, Huang T, Xia H, Keith SJ, Li Y, Liu S, Wu J (2009) Long-term fertilizer effects on organic carbon and total nitrogen and coupling relationships of C and N in paddy soils in subtropical China. Soil Till Res 106(1):8–14
Uslu ÖS (2005) Research on the botanical composition and effects of different fertilizer applications on the yield and botanical composition of Yeniyapan range in Araplar village, Kahramanmaraş. Ph.D. Thesis. University of Çukurova, Institute of Natural and Applied Sciences, Department of Field Crops, School of Natural and Applied Sciences, Adana, Turkey
USSL Staff (1954) Diagnosis and improvement of saline and alkali soils. USDA Handbook No 60 Washington DC, USA
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Vitousek PM, Howarth RW (1991) Nitrogen limitation on land and in the sea: How can it occur? Biogeochemistry 13:87–115
Vourlitis GL, Zorba G (2007) Nitrogen and carbon mineralization of semi-arid shrubland soil exposed to long-term atmospheric nitrogen deposition. Biol Fert Soils 43:611–615
Wang GX, Qian J, Cheng GD, Lai YM (2002) Soil organic carbon pool of grassland soils on the Qinghai-Tibetan Plateau and its global implication. Sci Total Environ 291:207–217
Wang R, Hu Y, Wang Y, Ali S, Liu Q, Guo S (2019) Nitrogen application increases soil respiration but decreases temperature sensitivity: combined effects of crop and soil properties in a semiarid agroecosystem. Geoderma 1(353):320–330
Wedin DA, Tilman D (1996) Influence of nitrogen loading and species composition on the carbon balance of grasslands. Science 274:1720–1723
Weidhuner A, Keshavarz Afshar R, Luo Y, Battaglia M, Sadeghpour A (2019) Sample grinding size affects nitrogen and carbon estimate of a wheat cover crop. Agron J 111:3398–3402. https://doi.org/10.2134/agronj2019.03.0164
Xin X, Zhang J, Zhu A, Zhang C (2016) Effects of long-term (23 years) mineral fertilizer and compost application on physical properties of fluvo-aquic soil in the North China Plain. Soil Till Res 1(156):166–172
Xu W, Wan S (2008) Water- and plant-mediated responses of soil respiration to topography, fire, and nitrogen fertilization in a semiarid grassland in northern China. Soil Biol Biochem 40:679–687
Xu P, Leng Y, Zeng GM, Huang DL, Lai C, Zhao MH, Wei Z, Li NJ, Huang C, Zhang C, Li FN, Cheng M (2015) Cadmium induced oxalic acid secretion and its role in metal uptake and detoxification mechanisms in Phanerochaete chrysosporium. Appl Microbiol Biotechnol 99(1):435–443
Yang H, Shaojie MU, Chengming SUN, Jianlong LI, Weimin JU (2011) Summary of research on estimation of organic carbon storage in grassland ecosystem. Chin J Grassl 33:107–114
Zhang YD, Sun ZH, Shen YX (2005) Effect of fertilization on soil microorganism of deteriorated grassland in dry-Hot Valley Region of Jinsha River. J Soil Water Conserv 19(2):88
Zhang J, Li P, Jia C, Li Z, Tang H, Yang Y (2016) Distribution of soil nitrogen and its relationship with particle size along the Dan river valley, China. Environ Earth Sci 75(5):406
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Babur, E., Uslu, Ö.S., Battaglia, M.L. et al. Nitrogen Fertilizer Effects on Microbial Respiration, Microbial Biomass, and Carbon Sequestration in a Mediterranean Grassland Ecosystem. Int J Environ Res 15, 655–665 (2021). https://doi.org/10.1007/s41742-021-00336-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41742-021-00336-y