Abstract
Nano-sized TiO2 particles containing anatase and rutile were applied to arsenic removal from water in natural groundwater conditions in the batch and column experiments. Arsenic concentrations were 200 µg L−1 and the pH range was 6–8.5 (similar to groundwater conditions). The results showed that anatase and rutile could adsorb 95.5% and 63.5% of arsenic in a solution after 60 min, respectively. In both adsorbents, arsenic adsorption was increased by increasing the adsorbent concentration. Increasing pH results increased adsorption in rutile more than anatase. The maximum adsorption capacity of 2.58 mg g−1 and 1.86 mg g−1 were calculated for anatase and rutile, respectively at the adsorbent concentration of 3 g L−1. Isotherm studies showed Freundlich model was more valid to the empirical adsorption data for both nanoparticles. The kinetics of the adsorption processes fitted well the pseudo-first-order adsorption model. To investigate the dynamic sorption, column study was carried out with fine and coarse silica sand porous media. According to the batch experiments, only anatase nanoparticles were injected into the column as an adsorbent at different doses. Breakthrough curves (BTC) showed the best efficiency of arsenic removal can be obtained by an adsorbent dose of 8 g L−1 in the fine sand column.
Article Highlights
-
TiO2nanoparticles (anatase and rutile) could be the effective adsorbent for reducing arsenic content from water.
-
The kinetic of the adsorption by rutile fitted well the pseudo-first-order adsorption model.
-
Anatase nanoparticle is more efficient than the rutile for the arsenic removal in groundwater conditions.
-
Anatase nanoparticle could be an instrumental agent for the in situ remediation in arsenic-contaminated aquifers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Natural water contaminations by heavy metals are important because of their toxicity for the human and environment (Al-Abdullah et al. 2018; Tang et al. 2016). Arsenic is a common toxic heavy metal contaminant and a high concentration of it has been reported in most parts of the world's groundwater. Millions of people in the world are dependent on groundwater for drinking water (Deedar and Aslam 2009; Erickson 2003; Jain and Loeppert 2000). Long-term drinking of water containing arsenic can cause changes in skin pigments and increase the risk of developing some cancers including liver, lungs, bladder and, kidney (Mujawar et al. 2018; Shakya and Ghosh 2018; Wang et al. 2002). According to the WHO (World Health Organization) guideline, the permitted value of arsenic in drinking water is 10 μg L−1 (WHO 2007).
There are many deletion techniques of arsenic from water that include various processes. Physical methods (Baskan and Pala 2010; Chowdhury and Yanful 2010; Sen et al. 2010; Xu et al. 2013; Yoon et al. 2008; Basha et al. 2011), chemical methods (Donia et al. 2011; Katsoyiannis et al. 2008; Kanel et al. 2005), and biological methods (Rahman and Hasegawa 2011; Ike et al. 2008) are applied. Among the techniques, adsorbents and adsorption processes have been considered in recent years because of their ease of use, low prices and, high efficiency, and finally, because it is more energy-saving than the others (Cui et al. 2015; Poorsadeghi et al. 2017). The efficiency of the adsorption process depends on the pH, temperature, type the dosage of the adsorbent; also, the availability of organic or inorganic materials in the medium (Malkoc and Nuhoglu 2005; Sheshmani et al. 2013). Metal oxide nanoparticles can serve as cheap and efficient adsorbents especially for inorganic ions (Tsydenov et al. 2014). Iron oxide (Aredes et al. 2012), zero-valent iron (Eljamal et al. 2011), aluminum oxide (Patra et al. 2012), Fe-Ti oxide (Chen et al. 2012), cupric oxide (Martinson and Reddy 2009), etc. are some metal oxide adsorbents which used to remove Arsenic from water. Remarkably, it is well-known that nanoscale’s TiO2 is a good adsorbent for arsenic removal from water due to some special properties, including its physical and chemical constancy, lower cost, and nontoxicity (Carp et al. 2004; Dutta et al. 2004; Ma and Tu 2011b; Pena et al. 2005). TiO2 exists as four different polymorphs in nature, include anatase, brookite, rutile, and TiO2 (B) which their crystal structures are tetragonal, orthorhombic, tetragonal, and monoclinic, respectively (Bang et al. 2005; Carp et al. 2004; Gupta and Tripathi 2011). The anatase and rutile forms are most often studied among all polymorphs (Zhang 2013).
In this study, arsenic reduction in aqueous solution by TiO2 nanoparticles with the conditions close to groundwater was investigated in batch and column studies. Batch experiments were conducted to assess the effect of time, nanoparticles dose, initial arsenic concentration and, pH on As concentration changes. Since groundwater temperature tends to remain relatively constant (Todd and Mays 2005), the effect of temperature changes on the removal of arsenic was not considered. There is a difficulty of simulating in the laboratory; therefore, other conditions (like the effect of gas pressure, etc.) were not considered. Langmuir, Freundlich, and Tempkin isotherms were performed to describe the equilibrium data separately. Until now, no report has been made on investigating the kinetics of arsenic adsorption by rutile; therefore kinetic studies were done to investigate the reaction rate and mechanism of the sorption process. Four kinetic models including pseudo-first-order, pseudo-second-order, Elovich, and intra-particle diffusion were studied for arsenic removal by both anatase and rutile. Column studies were conducted to investigate the dynamic sorption of arsenic by TiO2 nanoparticle. Two sizes of natural silica sand used as bed materials. The anatase nanoparticles were injected into the column and results were compared by drawing the breakthrough curves. Several studies investigated arsenic removal by TiO2 nanoparticles in batch and column experiments (Ali et al. 2014; Hafeznezami et al. 2017; Qiu et al. 2019). However, the column study with natural bed materials and nanoparticle injection is still not reported in the literature. This investigation aimed to evaluate the efficiency of applying TiO2 nanoparticles for arsenic removal from water in natural groundwater conditions. It will be useful for the in situ remediation in arsenic-contaminated aquifers.
Materials and methods
Instruments and materials
The solutions were made by deionized water (EC < 0.8 μS cm−1). TiO2 nanoparticles at anatase and rutile crystalline phase were purchased from US Research Nano-materials Inc. (US Research Nano-materials, Inc., Houston, TX, USA). Anatase has a purity, diameter, and specific surface area of 99%, 18 nm, and 200–240 m2 g−1, respectively. Rutile has a purity, diameter, and specific surface area of 99%, 30 nm, and 35–60 m2 g−1, respectively. The pH adjustments were prepared by NaOH and HCl from CHEM-LAB (Chem-Lab NV, Zedelgem, Belgium); also, the standard stock solution of As (V) containing 1000 µg L−1 (CL01.0132). The quantitative determination of arsenic was done using graphite furnace atomic adsorption spectrophotometer (GF-AAS), Zeeman atomic adsorption spectrometer AA280Z with graphite tube. A pH meter (CyberScan 500) was used to adjust the pH of the arsenic stock solution. All the experiments were conducted in a laboratory at a temperature of 23 ± 2 ºC. To check the accuracy of each sampling test, three repetitions were performed; the data were obtained based on the average of their results. The Microsoft Excel (part of MS Office) was applied for data analysis and charts drawing.
Due to its high resistance to pressure and shock, a clear plexiglass cylinder was used for the preparation of horizontal flow fixed-bed columns. It was 35 cm long and an internal diameter of 2 cm equipped with an inlet and sampling valves. The bottoms of the columns were packed with polyethylene caps. To prevent from clogging nanoparticles, the outlet was not plugged in with cotton wool or glass wool. The column was filled with about 300 g of soil samples. The silica sand sieved in tow size ranges (0.35–0.5 and 1–2 mm) were used as bed materials to model the aquifers in a laboratory scale. Properties are shown in Table 1. 100 μm stainless mesh screens were used on both ends due to prevent to loss of the sands. The samples were washed sequentially according to the cleaning process used by Tian et al. (Tian et al. 2012). To simulate natural groundwater conditions, the column was placed horizontally; a peristaltic pump was fed arsenic solutions at 0.489 cm min−1 (constant Darcy's velocity), which was close to the typical groundwater velocity in aquifers (Kamrani et al. 2018). During the experiment, the column saturation was maintained. To inject the nanoparticles suspension, an injection vent was embedded close to the column inlet. At regular interval times, samples were collected in plastic bottles and analyzed for arsenic concentration. Schematic of the experimental setup used for column study is shown in Fig. 1.
Batch Experiment
Except for the experiments to specify the effect of pH on arsenic adsorption, other batch adsorption studies were performed at pH 7.0. The 50-ml solution samples of arsenic were prepared for all experiments in 250-ml Erlenmeyer flasks. After adding the adsorbent in determining doses, the suspensions were shaken at 180 oscillations per minute at 23 ± 2 °C and then centrifuged at 15,000g acceleration for 5 min. Samples of the supernatant were extracted and passed through a 0.22 μm polysulphone membrane filter for arsenic analysis. Arsenic percent, which was removed by the adsorbents, was calculated by Eq. (1):
where, C0 and Ce are the initial and final concentration (µg L−1) of As in the solution phase.
The experiments were implemented discontinuously, with changes in contact time, the concentration of nanoparticles, initial concentration of As and pH. The studies of isotherm for As adsorption by anatase and rutile were implemented. Except for the batch adsorption isotherms, a solution of 200 µg L−1 arsenic was used in other cases based on the fact that the natural contamination of arsenic of most groundwater is less than 200 µg L−1 (Ma and Tu 2011b).
To verify the time effect, the experiments were accomplished at a concentration of 200 µg L−1 of As in the presence of 3 g L−1 of TiO2 nanoparticles at pH = 7. To prevent the deposition of nanoparticles, the solutions were put into a shaker with 180 rpm. Samples were derived at 2, 6, 10, 15, 30, 45, and 60 min.
To evaluate the effect of TiO2 nanoparticles dose, 0.5, 1, 1.5, 2, 2.5,3, 3.5, and 4 g L−1 of nanoparticles were added to a solution of 200 µg L−1 of arsenic at pH = 7.
To assess the initial concentration of As, 3 g L−1of TiO2 nanoparticles were added to the solutions of 0.5, 1, 2, 3, 5, 7, and 10 mg L−1 of arsenic. The contact time was 6 h and pH = 7.
To evaluate the effect of pH changes, regarding the normal pH of groundwater, the pH variations from 6 to 8.5 was selected (Karmakar et al. 2017; Mandal and Mayadevi 2008). 0.01 Normal HCl and NaOH solutions are used to adjust pH. 3 g L−1 adsorbent was added to 200 µg L−1 arsenic solution. Suspension samples, shake for 60 min at 180 rpm.
The Study of Adsorption Isotherms
An isotherm is an important tool that describes the relationship between adsorbent concentration and adsorption capability. There are many isotherm models to explain the distribution of adsorbent between the liquid and solid phases. In this research, the adsorption capacity of anatase and rutile nanoparticles was calculated, using Freundlich, Langmuir, and Tempkin isotherms models.
Freundlich isotherm can be applied for non-ideal sorption on heterogeneous surfaces, as well as, multilayer sorption. The calculation was done by the Freundlich equation (Danish et al. 2013):
where, qe represents the amount of adsorbent uptake per amount of sorbent at the equilibrium (mg g−1), Ce corresponds to the equilibrium concentration (mg L−1). Kf is a parameter that indicates the relative adsorption capacity of the adsorbent (mg g−1) and 1/n is the adsorption intensity (Arsénio et al. 2017). When the equation is linearized, both parameters Kf and 1/n are found by linear regression Eq. (3):
The adsorption process is allowed to understand by the slope, 1/n. Degree of nonlinearity between adsorption and solution concentration is expressed by the value of n. (Dehvari et al. 2017; Desta 2013).
Commonly the value of n is above 1 and it may be because of site distribution on the adsorbent surface or other factors that decrease the adsorbent- adsorbate interaction with increasing surface density (Reed and Matsumoto 1993). Also, n values between 1 and 10 correspond to favorable adsorption (Naiya et al. 2008).
The Langmuir isotherm shows homogeneous and monolayer adsorption on the adsorbent surface that contains the limited number of adsorption sites which have uniform situations without adsorbent displacement, in the plane surface. (Dehvari et al. 2017; Moftakhar et al. 2016). The Langmuir isotherm described, using the equation:
where, qm is maximum adsorption capacity (mg g−1) and b is Langmuir constant depends on the adsorption energy. Plotting (Ce/qe) versus (Ce) shows a line in a first-order equation that qm and b are achieved by its slope and y-intercept (Chen et al. 2011; Hashemzadeh et al. 2019).
To survey As removal capacity on both TiO2 nanoparticles, adsorption isotherms were conducted by adding increasing amounts of As in 3 g L−1 TiO2 at pH = 7. Initial concentrations of arsenic were 0.5, 1, 2, 3, 5, 7, and 10 mg L−1. Anatase and rutile were added to the solution and shaken for 6 h with 180 rpm and pH = 7.
Tempkin isotherm assumes that the heat adsorption of adsorbent–adsorbate interactions decreases linearly with surface coverage rather than logarithmically. (Hashemzadeh et al. 2019; Khelifi et al. 2018). This model is explained by Eq. (5):
The linearized form of it is:
where, R is the universal gas constant (0.0083 kJ mol−1 K−1), T is the absolute temperature (K), KT and b are the equilibrium binding constant (L mg−1), and the Tempkin isotherm constant (KJ mol−1), respectively. Plotting (qe) versus (ln Ce) allows for estimating KT and b from the slope and y-intercept of the straight line (Moftakhar et al. 2016).
Kinetic Studies
The kinetic equations survey the adsorbed ions transfer per unit time that shows the reaction rate and mechanism with the potential controlling steps such as chemical reactions, mass transport, and pore diffusions (Hashemzadeh et al. 2019). There are two kinetic models according to the nature of adsorption: (I) sorption reaction models and (II) sorption diffusion models (Sarici-Ozdemir 2012). In this research, the pseudo-first-order, pseudo-second-order, and Elovich kinetic models from the first group and intra-particle diffusion model from the second were chosen to investigate the kinetics of As adsorption.
Pseudo-first-order kinetics model supposes that the adsorption is commensurate to the difference between equilibrium adsorption capacity (qe) and the adsorption at time t (qt), in mg g−1. This model is mathematically described by Eq. (7) (Khelifi et al. 2018; Moftakhar et al. 2016):
Lagergren model is the linearized form of Eq. (7) and represented as the Eq. (8):
where qt is the capacity of adsorbate at time t (mg g−1), qe is the maximum adsorption capacity (mg g−1), k1 is the constant of pseudo-first-order rate (min−1). By scattering log (qe−qt) versus t in Eq. (8) a straight line is obtained which k1 and qe could be determined from its slope and y-intercept, respectively. The constant k1 is a criterion that shows how fast the reaction proceeds.
The pseudo-second-order model is a nonlinear form of the equation that explains the relation between the mass of adsorbate per unit mass of adsorbent versus time (Moftakhar et al. 2016). It's expressed by the Eq. (9):
There are several linear forms of Eq. (9). The Eq. (10) is the one that is represented by Ho and McKey (Ho and McKay 1999):
where, k2 is the pseudo-second-order rate constant (g mg−1 min−1). By plotting t/qt versus t the kinetic parameters, k2 and qe are estimated using the y-intercept and slope of the line obtained. The constant k2 is a criterion that shows how fast the reaction proceeds.
When the chemical reaction or diffusion is dominated in the adsorption process, the Elovich equation should be used. This model describes the kinetics of adsorption of homogeneous surfaces (Eq. 11):
where, b is the initial adsorption rate (mg g−1 min−1), and a is the desorption constant (mg g−1) that depends on chemical and structural characteristics of the sorbent and the coefficient of solute diffusion. By plotting qt versus log t, a and b are estimated, using the y-intercept and slope of the straight line obtained (Moftakhar et al. 2016).
Another step that controls the reaction rate in the adsorption process is intra-particle diffusion in the solid phase. The Eq. (12) which is known as Weber and Morris's equation describes this adsorption kinetics model, mathematically (Ho 2006).
where kint is the constant that describes the intra-particle diffusion rate (mg g−1 min−0.5) and C is a constant that depends on the border layer thickness (mg g−1). Based on the equation if the adsorption rate is influenced by the diffusion process, plotting qt versus t0.5 will be a straight line and, if the intra-particle diffusion is the rate-determining step, the line passes through the origin (0,0) (Cui et al. 2015; Moftakhar et al. 2016).
Column Studies
According to the batch experiments results, rutile nanoparticles could not reduce the arsenic concentration, below the drinking standard. Therefore, the column experiments were conducted with just anatase as TiO2 nanoparticles. The column was wet-packed by adding the sands vertically into each column during the upward flow direction. Care was taken to achieve uniform distribution in sediment fabric and to avoid the entrance of air bubbles into the column. Also, care was taken to stop leakage. The packed soil columns were assumed to be homogeneous. The tracer test was performed using 5 mg L−1 NaCl solution to obtain hydrodynamic dispersion of the column. The longitudinal dispersion coefficient of the column was determined by drawing the breakthrough curve (BTC) of the tracer test. Initially, 300 ml of deionized water as a background solution was fed with the velocity of 0.0008 m s−1 into the column in steady-state flow condition using a peristaltic pump. Then 50 ml of NaCl solution was flushed to the column. The electrical conductivity of effluents were measured using an Ec-meter to detect the amount of NaCl passed through the column. Finally, 400 ml of deionized water was supplied into the column until no NaCl was detected in the effluent. The BTCs were drawn by the concentration of tracer in the effluent that was measured in regular time.
To simulate the arsenic-contaminated aquifer, the solution of 200 µg L−1 arsenic was fed with the velocity of 0.489 cm min−1 into the column in steady-state flow condition using a peristaltic pump. The Arsenic concentration was measured in the effluent solution. when it reached the initial concentration (200 µg L−1), the column became ready for TiO2 injection. The point was reached after feeding approximately 10 times the pore volume (10 PVs) of the initial solution. In this case, the removal of arsenic by column material is not considered.
Due to simulation of the effect of adding TiO2 nanoparticles to arsenic-contaminated aquifers, 2 times the pore volume (2 PVs) of TiO2 suspensions with concentrations of 3, 5, and 8 g L−1 were injected to the column. The injection speed was 0.25 ml s−1. The column outlet samples were collected at regular interval time and centrifuged at 20,000g acceleration for 5 min. Supernatant was extracted and passed through a 0.22 μm polysulphone membrane filter for arsenic analysis. From the normalized effluent of arsenic concentration (C/C0) for each column experiment, BTCs were plotted. The tests were continued until the adsorbent materials were exhausted (C/C0 = 1). The duration of the experiments was approximately 140––330 min. The column experiments were conducted at the normal pH = 7.5.
Results and Discussion
The Effect of Contact Time
Figure 2 illustrates the effect of contact time on arsenic adsorption using TiO2 adsorbents. The data show that in both adsorbents, arsenic adsorption has been increased by increasing the contact time; but two phases are recognizable. In the first phase of adsorption, which takes about 30 min, approximately 90% and 60% of the arsenic is removed from the solution by anatase and rutile, respectively. After that, the adsorption efficiency is reduced, and As adsorption is almost constant, which indicates the second phase of adsorption. This can be explained in two ways: (I) in the first phase, all adsorption sites on the adsorbent surface are empty and are available for surface adsorption. (II) at the beginning of the adsorption process, higher amounts of arsenic increase the chance of collision between arsenic ions and adsorbent levels (Jegadeesan et al. 2010). While in the second phase, with the adsorption sites reduction, access of arsenic ions to residual sites is difficult, and therefore, the adsorption is almost constant. Also, the repulsion force between arsenic adsorbed onto the solid surface and the arsenic ions in the solution gradually reduce arsenic adsorption (Kocabaş-Ataklı and Yürüm 2013). The curves also show that anatase has a greater ability to arsenic removal than rutile. That is because of its specific surface area. As mentioned before, the specific surface area of anatase is 200–240 m2 g−1 while it is 35–60 m2 g−1 for rutile. A higher specific surface produces more adsorption sites and makes anatase more absorbable than rutile. Also, anatase has some differences with rutile in the crystal structure, which causes more ability to arsenic adsorption. Each octahedron in anatase is in contact with 10 surrounding octahedrons that share four common edges while those of rutile are 8 and, linked by two common edges.(Fujishima et al. 2000; Ma and Tu 2011a; Hiemstra and Van Riemsdijk 1996). Such a finding was similar to that made in previous researches by Kocabas et. Al (2013), and Gupta et. al. (2013).
The Effect of Adsorbent Dosage
Figure 3 shows the arsenic adsorption with doses of 0.5–4 g L−1 of nanoparticles. As shown, the adsorption percentage was increased by an enhancement in TiO2 nanoparticle concentration. It happens because there is not enough active site to adsorb all arsenic ions at low concentrations of the adsorbent. In other words, almost all active adsorption sites were occupied by arsenic ions when there was still a lot of As ions exist in the solution (Özer et al. 2004). Likewise, increasing adsorbent dosage causes accessibility of more binding sites for the complex of As ions. But from 3 to 4 g L−1 of anatase dosage, arsenic adsorption was not remarkably changed by increasing the dose of the adsorbent. It is because the maximum adsorption of As (close to 100%) has happened and there were not some As ions in the solution. But for rutile, arsenic removal increased with increasing in adsorbent dosage. Because of its lower ability to uptake all the arsenic ions. This is compatible with results by Ma et al. (2011), Cui et al. (2015), and some others.
Effect of Initial Arsenic Concentration
Figure 4 displays the effect of arsenic concentration, using both anatase and rutile nanoparticles. As shown, increasing the arsenic initial concentration can lead to a reduction of adsorption percentage by using nanoparticles. This is due to the fact that at low concentrations of arsenic, all As ions in solution can get in touch with accessible sites on the surface of the TiO2 nanoparticles. While at higher concentrations of arsenic, there are not enough active sites on the surface of nanoparticles for all arsenic ions in the solution (Poorsadeghi et al. 2017; Shojaei and Shojaei 2017).
The Effect of pH
The effect of pH changes on the arsenic adsorption by titanium nanoparticles in the groundwater pH range (6–8.5) is illustrated in Fig. 5. It shows that arsenic adsorption has been increased using both anatase and rutile by increasing the pH. The cause of it sounds to be the removal of the hydroxyl ions from the coordinate layer at the TiO2 surface which creates a positive position on the surface of the nanoparticles and it can adsorb more arsenic anions from an alkaline solution (Kocabaş-Ataklı and Yürüm 2013). At pH of 6, the adsorption was more than 90% for anatase and with increasing pH, its adsorption capability increases slightly. While arsenic adsorption by rutile is improved more with increasing pH and it just could adsorb more than 75% of arsenic ions in pH = 8.5. It is because the amount of pHzpc (pH at the potential of zero point charge) in anatase solution is higher than the rutile solution. Therefore, the rutile particle surface would be more negatively charged than anatase with increasing pH (Ma and Tu 2011a). Ma et al. (2011a), Bang et al. (2005), Pena et al. (2005), and Tsydenov et al. (2014) found similar results in their studies.
Studies of Adsorption Isotherms
Figure 6 illustrates the isotherm plots and the coefficients obtained for the isotherm model. Parameters are listed in Table 2. The As adsorption on both anatase and rutile nanoparticles are more corresponds to the Freundlich isotherm, based on the correlation coefficients. It shows the multilayered and heterogeneous adsorption of arsenic on the surface of the TiO2 nanoparticles; because of the non-uniform or multi-layered distribution of active sites on the anatase and rutile surface (Gupta et al. 2013). The values of n = 1.74 for anatase and n = 1.66 for rutile represent that the As adsorption process by these nanoparticles is desirable and represents an almost strong bond between the adsorbents and the arsenic ions. Also, it shows the physical sorption of arsenic on the surface of the nanoparticles and represents that anatase has a greater arsenic adsorption capacity than rutile. These results were in line with the results in similar researches by Ma et al. (2011a), Dutta et al. (2013), Pena et al. (2013), Danish et al. (2013), and Kocabas et al. (2013). While Bang (2005), Gupta (2013), Cui (2014) found the Langmuir isotherm was fitted the best. The maximum adsorption capacities of this adsorbent have been estimated to be 2.58 and 1.86 for anatase and rutile nanoparticles, respectively.
Kinetic Studies
The experimental data indicate that the pseudo-first-order equation appeared to be the better-fitting model for the removal of arsenic by both TiO2 nanoparticles because of their regression coefficients (R2 = 0.999, 0.995. Also, calculated values of qe in the pseudo-first-order model are in line with the experimented values of qe that were 15.9 for anatase and 10.6 for rutile. In other researches, Cui et.al (2015), Pena et.al (2005), and Danish et.al (2013) found that the pseudo-second-order model is more appropriate for arsenic removal using anatase nanoparticles but for rutile, no similar research has been reported. Nevertheless, high values of R2 in the Elovich model can be assigned to the heterogeneous surface of both adsorbents. Kocabas et al. (2013) found similar results in the Elovich model. The parameters of the four mentioned kinetic models are arranged in Table 3. Figure 7 illustrates the pseudo-first-order, pseudo-second-order, and Elovich Linear kinetic models of arsenic adsorption.
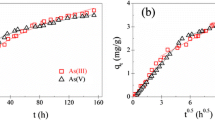
The regression coefficients of both anatase and rutile in the intra-particle diffusion model are R2 = 0.872 and 0.934, respectively, which are not appropriate in compression with the other regression coefficients (Fig. 8a). It seems that this model is not able to interpret the adsorption process, and the intra-particle diffusion is not the rate-determining step. But as seen in Fig. 8b, due to the line segmentation, values of R2 have been increased. The adsorption process consists of two different phases similar to what was achieved in the contact time part: (I) adsorption of arsenic on the TiO2 surface which, is done quickly. (II) The intra-particle diffusion of arsenic ions into adsorption sites of TiO2 nanoparticles which, is done gradually. The first phase has been associated with film diffusion and surface adsorption. While the second one represents the intra-particle diffusion into the porous structure after saturating the solid surface (Suteu et al. 2016). The increased values of R22 can confirm the intra-particle diffusion process at the end of the reaction. Also, the higher value of R22 for rutile displayed that the intra-particle diffusion in rutile is more efficient than anatase in the second phase. Overall the higher values of kint 1 in comparison with kint 2 represent the surface adsorption is more significant than intra-particle diffusion and is the rate-determining processes of adsorption.
Column Studies
The BTC of the tracer in the sand columns is illustrated in Fig. 9. The symmetrical shape of the BTCs for both samples shows that the hydraulic conductivity is high enough in both columns and, the tracer was not adsorbed by the porous media.
According to the batch experiment results, only anatase nanoparticle was injected into the column as an adsorbent because of its more efficient. Figure 10 shows the BTC with the injection of 3, 5 and 8 g L−1 of anatase nanoparticles. In all experiments, initially, the relative arsenic concentration gradually decreased. It then remains almost constant for a while and finally, it has been increased to the initial concentration (200 µg L−1). The graphs show that the maximum adsorption in fine sand is greater than the coarse sand. This can be explained by the enhancement of residence time of anatase with the decrease in grain size. It means that because of the higher mobility of anatase in the coarse sand, some of them are removed from the column without reaching their maximum adsorption capacity. Checking the column exhausting times also confirms this. At different anatase concentrations, the coarse sand column exhausted about 20, 40, and 55 min earlier than the fine sand, respectively, indicating faster removal of the nanoparticles from the column. The maximum adsorption of arsenic at different doses of anatase is shown in Table 4.
The maximum adsorption of arsenic in fine sand is also more delayed than coarse sand in 3 and 5 g L−1 of anatase nanoparticles injection. It may occur because of the reduction in the effective porosity of porous media, increases dead-end pores due to a decrease in grain size. While after injecting 8 g L−1, no significant delay was observed in maximum adsorption of both columns (Fig. 10c). It may occur because the high dose injection of the adsorbent at the beginning of the experiment reduces the effective porosity of coarse sand by blocking the pores. As a result, the time to reach maximum adsorption is almost similar to that of fine sand. But over time, the dead-end pores gradually open and the nanoparticles get out of the column sooner than the fine sand.
The maximum adsorption in both columns has been increased by increasing the dose of adsorbent injected. Also, the efficiency of columns in arsenic removal has been increased. The best efficiency of arsenic removal was obtained for an anatase dose of 8 g L−1 in the fine sand column, so that the permitted value of arsenic in drinking water (< 10 μg L−1) was measured in the effluent. Figure 11 shows the BTC of tow grain size sand columns with injecting different doses of anatase nanoparticles.
Conclusions
In this research, the ability of TiO2 nanoparticles (anatase and rutile) as adsorbents was investigated to remove arsenic from water in laboratory conditions close to natural groundwater conditions. According to the results, arsenic removal by both adsorbents generally has been increased during the time, so that, two fast and slow phases are recognized before and after 30 min. The maximum arsenic was adsorbed with 3 g L−1 of TiO2 nanoparticles and 200 µg L−1 of arsenic at pH 7 for 60 min. In the groundwater pH range, arsenic sorption has been increased by increasing pH; whereas the increment for rutile is more than anatase. Additionally, the equilibrium data illustrated most correspondence with the Freundlich isotherm model. The results represented the As sorption is multi-layer and anatase and rutile have heterogeneous surfaces. The experimental data demonstrated that the pseudo-first-order equation appeared to be better-fitting model for removal of As by both anatase and rutile nanoparticles. Maximum adsorption capacities were assessed to be 2.58 and 1.86 for anatase and rutile nanoparticles, respectively. According to the results of the batch experiments, anatase nanoparticle is more efficient than the rutile for arsenic removal in groundwater conditions. Column studies with anatase injection into polluted columns showed that the maximum adsorption of arsenic in fine sand is greater than the coarse sand. Also, the maximum adsorption of arsenic in fine sand is more delayed. Arsenic concentration decreased with increasing adsorption doses; the permitted value of arsenic in drinking water (< 10 μg L−1) was obtained for an adsorbent dose of 8 g L−1 in the fine sand column.
References
Al-Abdullah J, Al Lafi AG, Alnama T, Wa AM, Amin Y, Alkfri MN (2018) Adsorption mechanism of lead on wood/nano-manganese oxide composite. Iran J Chem Chem Eng 37:131–144
Ali I, Al-Othman ZA, Alwarthan A, Asim M, Khan TA (2014) Removal of arsenic species from water by batch and column operations on bagasse fly ash. Environ Sci Pollut Res 21:3218–3229
Aredes S, Klein B, Pawlik M (2012) The removal of arsenic from water using natural iron oxide minerals. J Clean Prod 29:208–213
Arsénio dS, Abreu AS, Moura I, Machado AV (2017) Polymeric materials for metal sorption from hydric resources. In: Water purification. Elsevier, pp 289–322
Bang S, Patel M, Lippincott L, Meng X (2005) Removal of arsenic from groundwater by granular titanium dioxide adsorbent. Chemosphere 60:389–397
Basha CA, Somasundaram M, Kannadasan T, Lee CW (2011) Heavy metals removal from copper smelting effluent using electrochemical filter press cells. Chem Eng J 171:563–571
Baskan MB, Pala A (2010) A statistical experiment design approach for arsenic removal by coagulation process using aluminum sulfate. Desalination 254:42–48
Carp O, Huisman CL, Reller A (2004) Photoinduced reactivity of titanium dioxide. Prog Solid State Chem 32:33–177
Chen L, He B-Y, He S, Wang T-J, Su C-L, Jin Y (2012) Fe-Ti oxide nano-adsorbent synthesized by co-precipitation for fluoride removal from drinking water and its adsorption mechanism. Powder Technol 227:3–8
Chen R, Zhi C, Yang H, Bando Y, Zhang Z, Sugiur N, Golberg D (2011) Arsenic (V) adsorption on Fe3O4 nanoparticle-coated boron nitride nanotubes. J Colloid Interface Sci 359:261–268
Chowdhury SR, Yanful EK (2010) Arsenic and chromium removal by mixed magnetite–maghemite nanoparticles and the effect of phosphate on removal. J Environ Manage 91:2238–2247
Cui J, Du J, Yu S, Jing C, Chan T (2015) Groundwater arsenic removal using granular TiO 2: integrated laboratory and field study. Environ Sci Pollut Res 22:8224–8234
Danish MI, Qazi IA, Zeb A, Habib A, Ali Awan M, Khan Z (2013) Arsenic removal from aqueous solution using pure and metal-doped titania nanoparticles coated on glass beads: adsorption and column studies. J Nanomater 2013:1–17
Deedar N, Aslam I (2009) Evaluation of the adsorption potential of titanium dioxide nanoparticles for arsenic removal. J Environ Sci 21:402–408
Dehvari M, Ehrampoush MH, Ghaneian MT, Jamshidi B, Tabatabaee M (2017) Adsorption kinetics and equilibrium studies of reactive red 198 dye by cuttlefish bone powder. Iran J Chem Chem Eng 36:143–151
Desta MB (2013) Batch sorption experiments: Langmuir and Freundlich isotherm studies for the adsorption of textile metal ions onto teff straw (Eragrostis tef) agricultural waste. J Thermodyn 2013:1–7
Donia AM, Atia AA, Mabrouk DH (2011) Fast kinetic and efficient removal of As (V) from aqueous solution using anion exchange resins. J Hazard Mater 191:1–7
Dutta PK, Ray AK, Sharma VK, Millero FJ (2004) Adsorption of arsenate and arsenite on titanium dioxide suspensions. J Colloid Interface Sci 278:270–275
Eljamal O, Sasaki K, Tsuruyama S, Hirajima T (2011) Kinetic model of arsenic sorption onto zero-valent iron (ZVI). Water Quality Exposure Health 2:125–132
Erickson BE (2003) Field kits fail to provide accurate measures of arsenic in groundwater. Int J Environ Sci Technol 37:35A-38A. https://doi.org/10.1021/es0323289
Fujishima A, Rao TN, Tryk DA (2000) Titanium dioxide photocatalysis. J Photochem Photobiol 1:1–21
Gupta K et al (2013) Effect of anatase/rutile TiO2 phase composition on arsenic adsorption. J Dispersion Sci Technol 34:1043–1052
Gupta SM, Tripathi M (2011) A review of TiO 2 nanoparticles. Chin Sci Bull 56:1639–1657
Hafeznezami S et al (2017) Remediation of groundwater contaminated with arsenic through enhanced natural attenuation: batch and column studies. Water Res 122:545–556
Hashemzadeh M, Nilchi A, Hassani AH, Saberi R (2019) Synthesis of novel surface-modified hematite nanoparticles for the removal of cobalt-60 radiocations from aqueous solution. Int J Environ Sci Technol 16:775–792. https://doi.org/10.1007/s13762-018-1656-4
Hiemstra T, van Riemsdijk WH (1996) A surface structural approach to ion adsorption: the charge distribution (CD) model. J Colloid Interface Sci 179:488–508. https://doi.org/10.1006/jcis.1996.0242
Ho Y-S (2006) Isotherms for the sorption of lead onto peat: comparison of linear and non-linear methods. Polish journal of environmental studies 15
Ho Y-S, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Ike M, Miyazaki T, Yamamoto N, Sei K, Soda S (2008) Removal of arsenic from groundwater by arsenite-oxidizing bacteria. Water Sci Technol 58:1095–1100
Jain A, Loeppert RH (2000) Effect of competing anions on the adsorption of arsenate and arsenite by ferrihydrite. J Environ Qual 29:1422–1430
Jegadeesan G, Al-Abed SR, Sundaram V, Choi H, Scheckel KG, Dionysiou DD (2010) Arsenic sorption on TiO2 nanoparticles: size and crystallinity effects. Water Res 44:965–973
Kamrani S, Rezaei M, Kord M, Baalousha M (2018) Transport and retention of carbon dots (CDs) in saturated and unsaturated porous media: role of ionic strength, pH, and collector grain size. Water Res 133:338–347
Kanel SR, Manning B, Charlet L, Choi H (2005) Removal of arsenic (III) from groundwater by nanoscale zero-valent iron. Int J Environ Sci Technol 39:1291–1298
Karmakar S, Bhattacharjee S, De S (2017) Experimental and modeling of fluoride removal using aluminum fumarate (AlFu) metal organic framework incorporated cellulose acetate phthalate mixed matrix membrane. J Environ Chem Eng 5:6087–6097
Katsoyiannis IA, Ruettimann T, Hug SJ (2008) pH dependence of Fenton reagent generation and As (III) oxidation and removal by corrosion of zero valent iron in aerated water. Int J Environ Sci Technol 42:7424–7430
Khelifi O, Nacef M, Affoune AM (2018) Nickel (II) adsorption from aqueous solutions by physico-chemically modified sewage sludge. Iran J Chem Chem Eng 37:73–87
Kocabaş-Ataklı ZÖ, Yürüm Y (2013) Synthesis and characterization of anatase nanoadsorbent and application in removal of lead, copper and arsenic from water. Chem Eng J 225:625–635
Ma L, Tu S (2011a) Removal of arsenic from aqueous solution by two types of nano TiO 2 crystals. Environ Chem Lett 9:465–472
Ma L, Tu S (2011b) Arsenic removal from water using a modified rutile ore and the preliminary mechanisms. Desalination Water Treatment 32:445–452
Malkoc E, Nuhoglu Y (2005) Investigations of nickel (II) removal from aqueous solutions using tea factory waste. J Hazard Mater 127:120–128
Mandal S, Mayadevi S (2008) Adsorption of fluoride ions by Zn–Al layered double hydroxides. Appl Clay Sci 40:54–62
Martinson CA, Reddy K (2009) Adsorption of arsenic (III) and arsenic (V) by cupric oxide nanoparticles. J Colloid Interface Sci 336:406–411
Moftakhar MK, Yaftian MR, Ghorbanloo M (2016) Adsorption efficiency, thermodynamics and kinetics of Schiff base-modified nanoparticles for removal of heavy metals. Int J Environ Sci Technol 13:1707–1722. https://doi.org/10.1007/s13762-016-0969-4
Mujawar MN, Sahu JN, Abdullah EC (2018) Synthesis of novel magnetic biochar using microwave heating for removal of arsenic from waste water. Iran J Chem Chem Eng 37:111–115
Naiya T, Bhattacharya A, Das S (2008) Removal of Cd (II) from aqueous solutions using clarified sludge. J Colloid Interface Sci 325:48–56
Özer A, Özer D, Özer A (2004) The adsorption of copper (II) ions on to dehydrated wheat bran (DWB): determination of the equilibrium and thermodynamic parameters. Process Biochem 39:2183–2191
Patra AK, Dutta A, Bhaumik A (2012) Self-assembled mesoporous γ-Al2O3 spherical nanoparticles and their efficiency for the removal of arsenic from water. J Hazard Mater 201:170–177
Pena ME, Korfiatis GP, Patel M, Lippincott L, Meng X (2005) Adsorption of As (V) and As (III) by nanocrystalline titanium dioxide. Water Res 39:2327–2337
Poorsadeghi S, Kassaee M, Fakhri H, Mirabedini M (2017) Removal of arsenic from water using aluminum nanoparticles synthesized through arc discharge method. Iran J Chem Chem Eng 36:91–99
Qiu S, Yan L, Jing C (2019) Simultaneous removal of arsenic and antimony from mining wastewater using granular TiO2: Batch and field column studies. J Environ Sci 75:269–276
Rahman MA, Hasegawa H (2011) Aquatic arsenic: phytoremediation using floating macrophytes. Chemosphere 83:633–646
Reed BE, Matsumoto MR (1993) Modeling cadmium adsorption by activated carbon using the Langmuir and Freundlich isotherm expressions. Sep Sci Technol 28:2179–2195
Sarici-Ozdemir C (2012) Adsorption and desorotion kinetcs methylene blue onto activated carbon. Physicochem Prob Min Process 48:441–545
Sen M, Manna A, Pal P (2010) Removal of arsenic from contaminated groundwater by membrane-integrated hybrid treatment system. J Membr Sci 354:108–113
Shakya A, Ghosh P (2018) Arsenic, iron and nitrate removal from groundwater by mixed bacterial culture and fate of arsenic-laden biosolids. Int J Environ Sci Technol 16:5901–5916
Sheshmani S, Arab FM, Amini R (2013) Iron (iii) hydroxide/graphene oxide nano composite and investigation of lead adsorption. J Appl Res Chem 6:17–23 ((in Persian))
Shojaei S, Shojaei S (2017) Experimental design and modeling of removal of acid green 25 dye by nanoscale zero-valent iron. Euro-Mediterranean J Environ Integration 2:2–15
Suteu D, Zaharia C, Badeanu M (2016) Kinetic modeling of dye sorption from aqueous solutions onto apple seed powder. Cellulose Chem and Technol 50:1085–1091
Tang X et al (2016) Chemical coagulation process for the removal of heavy metals from water: a review. Desalination Water Treat 57:1733–1748
Tian Y, Gao B, Wu L, Muñoz-Carpena R, Huang Q (2012) Effect of solution chemistry on multi-walled carbon nanotube deposition and mobilization in clean porous media. J Hazard Mater 231:79–87
Todd D, Mays L (2005) Groundwater hydrology third edition vol 3. Jhon Wiley and Sous,
Tsydenov DE, Shutilov AA, Zenkovets GA, Vorontsov AV (2014) Hydrous TiO2 materials and their application for sorption of inorganic ions. Chem Eng J 251:131–137
Wang JP, Qi L, Moore MR, Ng JC (2002) A review of animal models for the study of arsenic carcinogenesis. Toxicol Lett 133:17–31
WHO (2007) Arsenic in drinking water. Fact sheet No. 210 vol 12.
Xu P, Capito M, Cath TY (2013) Selective removal of arsenic and monovalent ions from brackish water reverse osmosis concentrate. J Hazard Mater 260:885–891
Yoon S-H, Lee JH, Oh S, Yang JE (2008) Photochemical oxidation of As (III) by vacuum-UV lamp irradiation. Water Res 42:3455–3463
Zhang B (2013) Removal of TiO2 nanoparticles by conventional water treatment processes. Clemson University
Acknowledgements
This research was part of a Ph.D. thesis conducted at Kharazmi University. The authors gratefully acknowledge the National Water & Wastewater Engineering Company of Iran for their technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors state that they have no conflict of interest associated with this work.
Rights and permissions
About this article
Cite this article
Nazari, A., Nakhaei, M. & Yari, A.R. Arsenic Adsorption by TiO2 Nanoparticles Under Conditions Similar to Groundwater: Batch and Column Studies. Int J Environ Res 15, 79–91 (2021). https://doi.org/10.1007/s41742-020-00298-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41742-020-00298-7