Abstract
The current work reports the synthesis of Fe-doped TiO2 nanoparticles by simple coprecipitation method and compares their photocatalytic efficiency to three different commercially available TiO2 (i.e., Sachtopore, Hombikat UV100 and Fluka) using methyl orange (MO) under UV light irradiation. X-ray diffraction (XRD), diffuse reflectance spectroscopy (DRS) and transmission electron microscopy (TEM) were used to characterize these synthesized Fe-doped TiO2 nanoparticles. In fact, the DRS results revealed that the bandgap of doped photocatalysts is smaller compared to undoped TiO2, leading to a slight shift in the absorption band towards the beginning of the visible light. In addition, XRD has shown that all samples were well crystallized and have only anatase phase. Accordingly, the photocatalytic investigation revealed that doped TiO2 exhibited improved photocatalytic efficiency of methyl orange (MO) compared to undoped TiO2 photocatalysts and depends on iron constituents, TiO2 types as well as the doses of synthesized nanomaterials.
Article Highlights
-
A simple coprecipitation method for the synthesis of Fe-doped TiO2 photocatalysts and comparison of their photocatalytic efficiency to undoped TiO2.
-
All Fe-doped TiO2 showed smaller bandgap compared to undoped TiO2 with only anatase phase.
-
Fe-doped TiO2 improves the photocatalytic degradation of dyes compared to undoped TiO2.
-
Photocatalytic efficiency of these doped TiO2 depends on their doses, the constituents of iron and TiO2 types.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
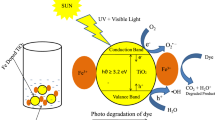
Air and water pollution are major problems facing modern societies. About half of the global production of synthetic textile dyes (700,000 tons per year) belong to azo products, which have chromophore (–N=N–) in their molecular structures (Yan-Xia et al. 2015). Various traditional physical and chemical techniques have been studied elsewhere (Yan-Xia et al. 2015; Auta et al. 2013; Taghizadeh et al. 2013; Ferroudj et al. 2013) to treat textile wastewater such as adsorption and chemical precipitation, but they are not favorable since these methods require further treatment to eliminate hazardous wastewater. However, advanced oxidation processes (AOPs) have proved to be an effective and promising treatment method for the removal of environmental contaminants (Bilińska et al. 2017; Meek et al. 2017). Thus, it is based on the excitation of a semiconductor mainly of titanium dioxide TiO2 using UV and/or visible light. Under the action of photons, this semiconductor (or photocatalyst) produces highly oxidizing free radicals. Consequently, it can oxidize a wide range of pollutants and ultimately mineralize them into CO2 and H2O moieties (Koupaie et al. 2013; Hejazi et al. 2020; Chatzisymeon et al. 2013; Pang et al. 2013).
Metal-oxide semiconductors are widely studied in several green applications such as photocatalytic environmental remediation, especially wastewater decontamination (Anjum et al. 2016; Srikanth et al. 2017; Zhang et al. 2017a; Gomathi Devi et al. 2014). Among the semiconductors studied, titanium dioxide (TiO2) has attracted considerable attention in the field of photochemistry (Sun et al. 2018), due to its low cost, its non-toxic nature, long-term stability and its strong photocatalytic activity (Weng et al. 2016). TiO2 is the most suitable semiconductor used in photocatalysis. However, its activity is limited to the UV region of the solar radiation. In fact, there are three naturally occurring TiO2 phases: anatase, rutile, and brookite. Therefore, the anatase phase is widely considered to have higher photocatalytic activity.
Doping is one of the most widely used approaches to shift the spectral response of wide bandgap semiconductors to visible light, where certain metal ions can be used as dopants such as copper (Zhang et al. 2017b), zinc (Naira et al. 2017) and nickel (Liu et al. 2017). Among the various dopants, iron has been extensively studied because the radius of Fe3+ (0.64 Å) is close to that of Ti4+ (0.68 Å) (Zahid et al. 2018). Therefore, Fe ions can be easily incorporated into the crystal lattice of TiO2 (Crişana et al. 2018). Doping with Fe3+ could introduce higher oxygen vacancies in the crystal lattice or on the surface of TiO2, which promote the photocatalytic activity by promoting the adsorption of water and the formation of hydroxyl groups on the surface of TiO2 (Han et al. 2009).
Generally, semiconductors are used in a variety of applications; for the removal of chemical pollutants (Ghaedi et al. 2013), photocatalysis (Black et al. 2018) and solar cell and gas sensors; have been widely reported (Liao et al. 2016) due to their special electronic and optical properties (Yanga et al. 2012). In recent years, methyl orange has been one of the most organic pollutants studied. However, the photocatalytic degradation of methyl orange (MO) in aqueous media is always chosen as a model reaction to study the photocatalytic efficiency of photocatalysts. In this study, three commercially available TiO2 were chosen to investigate their performances on photodegrading MO dye. Thus, several methods have been reported for the preparation of TiO2 semiconductors such as sol–gel, hydrothermal and coprecipitation. The coprecipitation method has certain advantages/disadvantages compared to conventional processes. In fact, the main advantage of the coprecipitation process is cost-effective; the large number of nanoparticles can be synthesized in a short time, high production yields and synthesis at room temperature (Cruz et al. 2018; Wu et al. 2016). However, this technique has several drawbacks such that the control of particle size distribution is limited (Nawaz et al. 2019). Grinding the calcined powder is sometimes required to achieve the desired particle size and contributes to reduced purity. The aim of this study is to prepare and assess the effects of the amount of Fe-doped TiO2 and the nature of TiO2 on the structural and optical properties of different TiO2 nanoparticles synthesized by the coprecipitation method.
Experimental
Materials
Commercially available TiO2 with different structures were obtained from different sources. TiO2 Hombikat UV-100 powder (Sachtleben Chemie, Germany; Purity > 99%) is composed of pure anatase and characterized by a specific surface area (SSA) larger than 250 m2/g (Chnirheb 2012). TiO2 Degussa P25 (Sigma-Aldrich, Germany; Purity > 99.5%) contains more than 80% anatase and 20% of rutile and a specific surface area (SSA) larger than 50 m2/g. TiO2 (Fluka; Purity > 99%) has a specific surface area of 10 m2/g and a crystal structure of 100% anatase. Ferrous sulfate (FeSO4, 99%), ferric chloride (FeCl3, 97%), HCl 37%, NaOH 97% were purchased from Sigma-Aldrich. All reagents were used without any further purification, and all stock and working solutions were prepared in deionized water.
Synthesis of Fe-Doped TiO2
Three different sources of TiO2 (Hombikat UV-100, Fluka, and Sachtopore) were assessed without any further treatment. Ferrous sulfate (FeSO4), ferric chloride (FeCl3) were used as precursors for iron. TiO2 semiconductor nanoparticles were prepared by coprecipitation method to obtain FeSO4 or FeCl3/TiO2 = 0.1% and 0.2% (wt) powders. The amount desired of iron and TiO2 were dissolved in 30 mL of HCl (0.25 M) solution. The solution was sonicated for 2 h to ensure the homogeneity. Furthermore, NaOH (0.1 M) was added into the solution as the co-precipitant to adjust the pH value. Then the solution was filtered and the precipitate was washed with deionized water and dried at 50 °C. The obtained photocatalysts were ground with a mortar and then calcined at 500 °C for 2 h with a constant heating rate of 5 °C/min.
Characterizations
The phase composition determined by X-Ray diffraction was carried out to identify the crystallinity of TiO2. Patterns of samples were obtained with a Bruker AXS D-8 diffractometer using Cu-Kα radiation in Bragg–Brentano geometry in the 2θ range from 10 to 90. The optical properties of the samples were analyzed by UV-1600 spectrophotometer. The transmittance spectra of the TiO2 suspensions were recorded on a UV–vis–NIR spectrophotometer Lambda 950 (Perkinelmer). The structural morphology of the prepared powders was analyzed by transmission electron microscopy (TEM) using Tecnai G2 12 TWIN, a 120 kV.
Photocatalytic Activity Measurement
The photocatalytic process was conducted using a pyrex beaker (10 ppm of methyl orange solution and 0.5 g/L of TiO2 photocatalyst) surrounded by a cylindrical reactor containing four vertical lamps. The instance between the light source and the solution was between 3 and 3.5 cm. Four 15 W lamps were produced as a source of UV irradiation. The same procedure was performed to study the photocatalytic performance of all photocatalysts. The reaction mixture was magnetically stirred in dark for 45 min to establish the adsorption/desorption equilibrium. Then, at regular time intervals, 2 mL of the solution was taken out, centrifuged and filtered through 0.2 µm Millipore filter. The filtrates obtained were then analyzed by UV–vis spectrophotometer.
Results and Discussion
Iron-TiO2 nanoparticles were synthesized with different types of TiO2 doped with iron. X-ray diffraction (XRD) analysis was performed to assess the structure and phase purity of the samples. Figure 1a shows XRD patterns of different type of Fe/TiO2 = 0.2% prepared by coprecipitation. It was observed that all the peaks are perfectly indexed to anatase TiO2 structure (JCPDS no. 21-1272; Fig. 1a). No other diffraction peaks of rutile or brookite were detected. Similar patterns were observed for doped and undoped photocatalysts. The absence of characteristic peaks of iron oxides phases may indicate the high metallic dispersion (Riaz et al. 2011, 2012; Sood et al. 2015) or due to the low concentration of Fe-doping (Riaz et al. 2011). We found that there is no appearance of any difference in crystallinity between doped and undoped TiO2. Furthermore, Fig. 1a shows the intensity of peaks that decreased by the introduction of dopant. In fact, the Scherrer equation (Eq. 1) was applied to calculate the average crystallite size of photocatalysts analyzed by XRD. In addition, the evaluation of the particle size obtained from the XRD patterns are listed in Table 1:
where D is the crystallite size, λ is the wavelength of the X-ray radiation (CuKα = 1.5406 Å), shape factor k is related to the crystallite shape, usually taken as 0.9. Moreover, the average grain sizes of the coprecipitated samples are listed in Table 1. It has been found that the amount of Fe has a small influence on the size and distribution of the photocatalysts. The results showed that the crystallite size of the sachtopore TiO2 and TiO2 UV100 increases with the increase in the doping concentration. For TiO2 fluka, the grain size increases by introducing an iron dopant unlike to TiO2 UV 100, which could be because the metals introduced were not incorporated into the TiO2 structure. On the other hand, in the case of TiO2 sachtopore, the size of the crystallite does not show any significant difference in particle size, by doping with iron.
Figure 1b shows the X-ray diffraction pattern of Fe/TiO2 = 0.1%. TiO2 nanoparticles were assigned to the anatase structure and no other phases as brookite or rutile were detected. The crystallite phases corresponding to the formation of mixed compounds (Fe2O3 or FexTiOy) are not observed due to the low concentration of iron ions. This could be assigned to fine dispersed iron ions in small amount in most of the TiO2 nanopowders. A slight shift in the position of the strongest peak relative to (101) was observed. This might be due to the introduction of iron, which revealed the substitution of Ti4 + by Fe3+ in the TiO2 network (Jiang et al. 2015). Fe3+ ions are incorporated into the crystal structure of TiO2 by substituting Ti4+ or by binding to interstitial sites (Rasoulnezhad et al. 2018). It has been observed that the intensity of the anatase decreased, which could be attributed to the deformation of the crystal lattice. In the case of sachtopore TiO2 and TiO2 UV100, the sizes of the crystallites increase with the increase in the concentration of dopants. By contrast, for TiO2 Fluka, the size of the crystallites of doped and undoped materials has not changed. Consequently, any modifications in the parameters of the crystal lattice and of the electronic structures were observed. Thus, Fe3+ ions were not incorporated into the crystal lattice of TiO2.
The morphologies of the as-prepared Fe/TiO2 = 0.2% were carried out by transmission electron microscopy (TEM). Figure 2 shows the morphologies of undoped and doped TiO2 nanoparticles with Fe. It can be observed that all synthesized titanium dioxide nanoparticles are spherical and have a morphology similar to that of undoped TiO2. TEM images illustrate planar surface with aggregates particles obtained for doped TiO2. Due to aggregation, the TiO2 particles are indistinguishable from each other and have irregular spheres.
Optical measurements were carried out at room temperature by UV–vis spectroscopy. DRS spectra of TiO2 doped at 0.2% and of undoped TiO2 photocatalysts are presented in Fig. 3a. The samples were analyzed over a spectral range of 300–800 nm. The transmittance of titanium oxide nanoparticles has been shown to decrease. This decrease in the transmittance of TiO2 powders is due to the fundamental absorption of light. All the spectra have an abruptly increasing absorption edge. The optical bandgap Eg values of TiO2 was determined from their absorbance spectra. The Eg values for bandgap materials were calculated from the plot (αhυ)1/2 versus photon energy (hυ) and by extrapolation of the linear portion of the curves until they intercept the photon energy axis (Fig. 3a). Fe-doped TiO2 nanoparticles showed similar spectra compared to undoped TiO2 with a slight shift in the absorption threshold. Moreover, no significant change was between the undoped and Fe-doped TiO2 catalysts. On the other hand, for the different type of TiO2, the introduction of iron does not affect the value of the gap. A significant effect of iron precursors on the energy gap has not been observed. The bandgap values of iron-doped TiO2 powders vary from 3.22 to 2.97 eV. The DRS spectra of samples of TiO2 doped with iron have the same absorption threshold. The values of the energy gap thus obtained are in line with previous studies using the same method and different dopants (Khan et al. 2002; Irie et al. 2003). The absorption of Fe-doped TiO2 shift towards the beginning of the visible light zone due to the presence of iron. It has been observed that the amount of dopants (Fig. 3a) has a slight blue shift. The bandgap energies are reported in Table 1. In the case of TiO2 Fluka and TiO2 UV100, the energetic gap decreases with the increase in the amount of iron ions. This could be attributed to the introduction of conduction and valence bands. On the other hand, for the other types of TiO2, the iron content does not affect the value of the gap. Therefore, there is no significant effect of iron precursors on the energy gap for TiO2 sachtopore. The UV–vis diffuse reflectance spectra of Fe/TiO2 = 0.1% powders are shown in Fig. 3b. The spectra show a slight shift offset of the absorption edge by the introduction of iron ions. The absorption edge of TiO2 samples was shifted towards the beginning of the visible region compared to undoped TiO2 (Table 1). Furthermore, the absorption wavelength of the doped TiO2 was slightly increased but does not cause a significant change.
To assess the photoactivity of the samples, we conducted methyl orange (MO) degradation experiments. MO dye was chosen as a model pollutant due to its frequent use in the textile industry. MO initial concentration used in this work was 10 mg/L, the amount of catalysts was 0.5 g/L and the photocatalytic efficiency of the synthesized Fe-doped TiO2 samples was followed using Eq. (2).
where C/C0 is the normalized MO concentration, t is the reaction time, and k is the reaction rate constant (min−1). It has been observed that the degradation rate is faster in the case of TiO2 doped with Fe than in pure nanomaterials with TiO2. TiO2 sachtopore samples are more effective at degrading methyl orange than the other types of TiO2 used in current work. The photocatalytic efficiency of TiO2 depends on the nature of the catalyst used and the concentration of dopant. The best photo-performances are explained by the fact that the recombination time of the electron/hole pairs is longer (K et al. 2014), which favors surface-trapped electrons and surface-trapped holes of the photocatalysts (Irie et al. 2003). Some authors have suggested that Fe3+ increases the rate of recombination, while others reported the beneficial effects of Fe3+ in the separation of photogenerated electrons and holes, and on the shift of the absorption range towards the visible by reducing the bandgap. Thus leading to an increase in the photocatalytic activity (K et al. 2014; Moradi et al. 2018). TiO2 sachtopore nanoparticles exhibit higher photocatalytic activity and faster degradation rates than TiO2 fluka. Table 2 shows the values of the k rate constants and the half-life t1/2 using different photocatalysts. The degradation of methyl orange follows the pseudo first-order reaction model. The photoactivity is almost identical for all Fe-doped TiO2. The correlation coefficients calculated from the experimental data are relatively good.
The photocatalytic activities of as-synthesized Fe/TiO2 = 0.1% (the mass percentage of dopant in the titanium dioxide powder) for the degradation of methyl orange are shown in Fig. 4b. We found that compared with undoped TiO2, the prepared photocatalysts have a much higher photocatalytic efficiency than undoped TiO2. The presence of Fe3+ does not modify the position of the valence and conduction band of TiO2 (Adyani et al. 2018), but it creates new energy levels in the bandgap, between the valence and the conduction band (Kong et al. 2017). The higher photocatalytic activity of Fe-doped TiO2 in comparison to undoped TiO2 could be assigned to the substitution of Ti4+ ions with Fe3+, which acts as electron acceptors, promoting photogenerated charge separation leading to •OH radical formation on the surface of the oxide lattice (Mancuso et al. 2020; Khairy et al. 2014). The efficiencies of MO degradation for Fe-doped TiO2 = 0.2% is higher than Fe-doped TiO2 = 0.1% under UV irradiation for 2 h. This improvement in photocatalytic activity could be due to active surface sites and/or slower electron/hole recombination. According to Shen J H et al. work, the efficiencies of AO7 degradation over P25, bare, and Fe-doped TiO2 under UV irradiation for 6 h were 36%, 32% and 50%, respectively.
On the other hand, TiO2 doped with Fe inhibits the recombination of the photogenerated electrons and holes. Fe ion with an appropriate concentration can trap photogenerated electrons. The half-life is the time required for a quantity to reduce to half its initial value. It is clearly observed that the nanoparticles of TiO2 doped Fe present a good photocatalytic effect. The corresponding first order degradation rate constant for all doped samples is much higher than that of undoped samples for the photodegradation of MO under the same experimental conditions. The photocatalytic activities of the photocatalysts could be influenced by the different physicochemical properties, the size of the crystallites or the specific surface.
Conclusion
Fe-doped TiO2 nanoparticles were prepared by a simple coprecipitation method. The XRD patterns did not show the presence of iron oxide phases for all samples. Optical study showed a slight decrease in the bandgap energies, leading to an increase in the wavelength of the absorption edge. The photocatalytic activity of the Fe-doped TiO2 was assessed under UV light irradiation. The photodegradation of methyl orange follows a pseudo first-order kinetic model. The photocatalytic capacity indicated that the as-prepared nanoparticles exhibited improved photocatalytic activity compared with undoped TiO2 towards the removal of methyl orange. All prepared samples are more photoactive under UV irradiation than undoped TiO2. The photocatalytic efficiency of Fe-doped TiO2 coprecipetated nanoparticles depend on the nature of TiO2, the quantity of photocatalysts as well as iron components. In fact, the highest degradation rate of MO was 25% in 2 h for Fe-doped TiO2 (sachtopor) instead of undoped TiO2 (5%).
References
Adyani SM, Ghorbani MA (2018) Comparative study of physicochemical and photocatalytic properties of visible light responsive Fe, Gd and P single and tri-doped TiO2 nanomaterials. J Rare Earth 36(1):72–85
Anjum M, Miandad R, Waqas M, Gehany F, Barakat MA (2016) Remediation of wastewater using various nano-materials. Arab J Chem. https://doi.org/10.1016/j.arabjc.2016.10.004
Auta M, Hameed BH (2013) Coalesced chitosan activated carbon composite for batch and fixed-bed adsorption of cationic and anionic dye. Coll Surf B 105:199–206
Bilińska L, Gmurek M, Ledakowicz S (2017) Textile wastewater treatment by AOPs for brine reuse. Process Saf Environ Prot 109:420–428
Black AP, Suzuki H, Higashi M, Frontera C, Ritter C, De C, Sundaresan A, Abe R, Fuertes A (2018) New rare earth hafnium oxynitride perovskites with photocatalytic activity in water oxidation and reduction. Chem Comm 54:1525–1528
Chatzisymeon E, Petrou C, Mantzavinos D (2013) Photocatalytic treatment of textile dye house effluents with simulated and natural solar light. Global NEST J 15:21–28
Chnirheb A, Harir M, Kanawati B, Fekete A, El Azzouzi M, Hertkorn N, Schmitt-Kopplin Ph (2012) Efficiency and degradation products elucidation of the photodegradation of mefenpyrdiethyl in water interface using TiO2 P-25 and Hombikat UV100. J Environ Sci 24:1686–1693
Crişana M, Mardareb D, Ianculescuc A, Drăgana N, Niţoid I, Crişana D, Voicescua M, Todana L, Oanceae P, Adomniţeib C, Dobromirf M, Gabrovskag M, Vasile B (2018) Iron doped TiO2 films and their photoactivity in nitrobenzene removal from water. Appl Surf Sci 455:201–215
Cruz IF, Freire C, Araújo JP, Pereira C, Pereira AM (2018) Multifunctional ferrite nanoparticles: from current trends toward the future, pp 63–116. Chapter 3: Portugal
Ferroudj N, Nzimoto J, Davidson A, Talbot D, Briot E, Dupuis V, Abramson S (2013) Maghemite nanoparticles and maghemite/silica nanocomposite microspheres as magnetic Fenton catalysts for the removal of water pollutants. Appl Catal B: Environ 136:9–18
Ghaedi M, Karimi F, Barazesh B, Sahraei R, Daneshfar A (2013) Removal of reactive orange 12 from aqueous solutions by adsorption on tin sulfide nanoparticle loaded on activated carbon. J Ind Eng Chem 19:756–763
Gomathi Devi L, Kavitha R (2014) Review on modified N-TiO2 for green energy applications under UV/visible light: selected results and reaction mechanisms. RSC Adv 4:28265–28299
Han F, Kambala VSR, Srinivasan M, Rajarathnam D, Naidu R (2009) Tailored titanium dioxide photocatalysts for the degradation of organic dyes in wastewater treatment: a review. Appl Catal A- Gen 359:25–40
Hejazi R, Mahjoub AR, Cheshm Khavarb AH, Khazaeea Z (2020) Fabrication of novel type visible-light-driven TiO2@MIL-100 (Fe) microspheres with high photocatalytic performance for removal of organic pollutants. J Photochem Photobio A: Chem 400:112644
Irie H, Watanabe Y, Hashimoto K (2003) Carbon-doped anatase TiO2 powders as a visible-light sensitive photocatalyst. Chem Lett 32:772–773
Jiang X, Wang F, Li G, Qi L, Liu P, Ding Y, Zhang S, Yang M (2015) Low temperature synthesis and mechanism of finely dispersed nanorod rutile titanium dioxide. RSC Adv 5:62160–62166
Khairy M, Zakaria W (2014) Effect of metal-doping of TiO2 nanoparticles on their photocatalytic activities toward removal of organic dyes. Egyp J Pet 23:419–426
Khan SUM, Al-Shahry M, Ingler WB (2002) Efficient photochemical water splitting by a chemically modified n-TiO2. Sci 297:2243–2245
Kong L, Wang C, Wan F, Zheng H, Zhang X (2017) Synergistic effect of surface self-doping and Fe species-grafting for enhanced photocatalytic activity of TiO2 under visible-light. Appl Surf Sci 396:26–35
Koupaie EH, Moghaddam MRA, Hashemi SH (2013) Successful treatment of high azo dye concentration wastewater using combined anaerobic/aerobic granular activated carbon-sequencing batch biofilm reactor (GAC-SBBR): simultaneous adsorption and biodegradation processes. Water SciTechnol 67:1816–1821
Liao Y, Wang X, Ma Y, Li J, Wen T, Jia L, Zhong Z, Wang L, Zhang D (2016) New mechanistic insight of low temperature crystallization of anodic TiO2 nanotube array in water. Cryst Grow Desig 16(4):1786–1791
Liu Y, Chen X (2017) Black titanium dioxide for photocatalysis. Semiconduct Semimet 97:393–428
Mancuso A, Sacco O, Sannino D, Pragliola S, Vaiano V (2020) Enhanced visible-light-driven photodegradation of Acid Orange 7 azo dye in aqueous solution using Fe-N co-doped TiO2. Arab J Chem. https://doi.org/10.1016/j.arabjc.2020.05.019
Meek ME (2017) AOPs in hazard characterization for human health. Curr Opin Toxicol 3:80–86
Moradi V, Jun MBG, Blackburn A, Herring RA (2018) Significant improvement in visible light photocatalytic activity of Fe doped TiO2 using an acid treatment process. Appl Surf Sci 427(B):791–799
Naira RG, Mazumdarb S, Modak B, Bapatb R, Ayyub P, Bhattacharyya K (2017) The role of surface O-vacancies in the photocatalytic oxidation of Methylene Blue by Zn-doped TiO2: a mechanistic approach. J Photochem Photobiol A 345(1):36–53
Nawaz M, Sliman Y, Ercan I, Lima-Tenório MK, Tenório-Neto ET, Kaewsaneha C, Elaissari A (2019) Magnetic and pH-responsive magnetic nanocarriers, Thailand pp 41–85 Chapter 2.
Ozawa K, Emori M, Yamamoto S, Yukawa R, Yamamoto S, Hobara R, Fujikawa K, Sakama H, Matsuda I (2014) Electron-hole recombination time at TiO2 single-crystal surfaces: influence of surface band bending. J Phys Chem Lett 5(11):1953–1957
Pang YL, Abdullah AZ (2013) Fe3+ doped TiO2 nanotubes for combined adsorption–sonocatalytic degradation of real textile wastewater. Appl Catal B 129:473–481
Rasoulnezhad H, Hosseinzadeh G, Yekrang J (2018) Preparation and characterization of nanostructured S and Fe co-doped TiO2 thin film by ultrasonic-assisted spray pyrolysis method. J Nanostruct 8(3):251–258
Riaz N, Chong F, Dutta BK, Man ZB, Khan MS, Nurlaela E (2011) Effect of calcination temperature on Orange II photocatalytic degradation using Cu: Ni/TiO2 under visible light. Third National Postgraduate Conference (NPC), IEEE: University Teknologi PETRONAS (UTP), Tronoh, Malaysia 1−5.
Riaz N, Chong FK, Dutta BK, Man ZB, Khan MS, Ramli RM (2012) Azo dye degradation using Cu/TiO2 under visible light: effect of metal loading. 2nd International Conference on Process Engineering and Advanced Materials (ICPEAM), IEEE: Kulala Lumpur 6.
Shen JH, Chuang HY, Jiang ZW, Liu XZ, Horng JL (2020) Novel quantification of formation trend and reaction efficiency of hydroxyl radicals for investigating photocatalytic mechanism of Fe-doped TiO2 during UV and visible light-induced degradation of acid orange 7. Chemosphere 251:126380
Sood S, Umar A, Mehta SK, Kumar Kansal SK (2015) Highly effective Fe-doped TiO2 nanoparticles photocatalysts for visiblelight driven photocatalytic degradation of toxic organic compounds. J Colloid Interface Sci 450:213–223
Srikanth B, Gouthama R, Badri Narayana R, Ramprasatha A, GopinathK P, Sankaranarayanan AR (2017) Recent advancements in supporting materials for immobilised photocatalytic applications in waste water treatment. J Environ Manag 200:60–78
Sun L, Guan J, Xu Q, Yang X, Wang J, Hu X (2018) Synthesis and applications of molecularly imprinted polymers modified TiO2 nanomaterials: a review. Polymers 10(11):1248
Taghizadeh F, Ghaedi M, Kamali K, Sharifpour E, Sahraie R, Purkait MK (2013) Comparison of nickel and/or zinc selenide nanoparticle loaded on activated carbon as efficient adsorbents for kinetic and equilibrium study of removal of Arsenazo (III) dye. Powder Techn 245:217–226
Weng XL, Zeng QS, Zhang YL, Dong F, Wu ZB (2016) Facile approach for the syntheses of ultrafine TiO2 nanocrystallites with defects and C heterojunction for photocatalytic water splitting. ACS Sustain Chem Eng 4:4314–4320
Wu W, Jiang C, Roy VAL (2016) Designed synthesis and surface engineering strategies of magnetic iron oxide nanoparticles for biomedical application. Nanoscale 8:19421–19474
Yanga G, Yanb Z, Xiao T (2012) Preparation and characterization of SnO2/ZnO/TiO2 composite semiconductor with enhanced photocatalytic activity. Appl Surf Sci 258:8704–8712
Yan-Xia H, Chuan-Lu Y, Mei-Shan W, Xiao-Guang M, Li-Zhi W (2015) Enhancing the visible-light absorption of TiO2 with the use of key N Co, and Na dopant concentrations. Sol Energ Mat Sol 132:94–100
Zahid R, Manzoor M, Rafq A, Ikram M, Nafees M, Butt AR, Hussain SG, Ali S (2018) Infuence of iron doping on structural, optical and magnetic properties of TiO2 nanoparticles. Elect Mater Lett 14:587–593
Zhang DQ, Eng CY, Stuckey DC, Zhou Y (2017a) Effects of ZnO nanoparticle exposure on wastewater treatment and soluble microbial products (SMPs) in an anoxic-aerobic membrane bioreactor. Chemosphere 171:446–459
Zhang W, Yin JR, Tang XQ, Zhang P, Ding YH (2017b) Density functional theory studies on the structural and physical properties of Cu-doped anatase TiO2 (101) surface. Physica E 85:259–263
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Ellouzi, I., El hajjaji, S., Harir, M. et al. Coprecipitation Synthesis of Fe-Doped TiO2 from Various Commercial TiO2 for Photocatalytic Reaction. Int J Environ Res 14, 605–613 (2020). https://doi.org/10.1007/s41742-020-00280-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41742-020-00280-3