Abstract
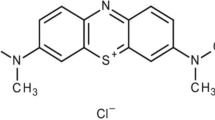
Many of the basic dyes are non- degradable and toxic pollutants in textile industrial wastewater. Methylene blue (MB) is a cationic dye that exists at a large scale in the textile wastewater which is discharged into the nearby environment without proper management, particularly in developing countries including Ethiopia. Therefore, this study aimed to evaluate the adsorption performance of MB from textile wastewater using locally prepared activated carbon produced from Parthenium hysterophorus stem. The plant samples were activated using H3PO4. The activated carbon was then characterized using proximate analysis, scanning electron microscope and Fourier transform infrared spectroscopy. Batch adsorption studies were conducted to determine the optimum condition for removal of MB from synthetic and real textile industrial wastewater under the effect of pH, contact time, adsorbent dose, and initial MB concentration. The adsorption isotherms were checked using Langmuir and Freundlich isotherms models. The result of the proximate analysis has shown that the activated carbon was composed of 5.7% moisture, 15.3% ash, 18.6% volatile matter and 60.4% fixed carbon. Using an aqueous solution, the maximum MB removal of 94% was achieved at the pH value 11, contact time 100 min, adsorbent dose 2 g in 100 mL and at the initial MB concentration 100 mg/L, whereas the maximum removal of MB from actual textile wastewater was found to be 91%. The Langmuir isotherm model was best fitted with experimental values at R2 was 0.99. Finally, it can be concluded that the activated carbon has a good adsorption capability and promising for MB removal from textile wastewater at an industrial scale.
Article Highlights
-
Wastewater pollution is the major concern of public and environmental health problem.
-
Water reclamation is the newly emerged approach to achieve sustainable development.
-
Methylene blue is a toxic and non-degradable chemical that discharged from the textile industry.
-
Adsorption is one of the promising treatment technology for Methylene blue remediation.
-
Activated carbon that produced from Parthenium hysterophorus is effective for Methylene blue removal from industrial wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Industrialization is water-intensive activities, particularly the textile industry is the fast-growing industry across the globe with a high-water consumption rate. Global freshwater bodies are polluted by different chemicals that discharged from the industrial sector (Ozturk and Cinperi 2018). The textile industry has complex unit operations which consist of bleaching, dyeing, printing, and stiffening of textile products. Each of these units is water-intensive and challenging for environmental sustainability. In addition, the textile industry is discharging a huge volume of high strength wastewater which is composed of non-degradable and resistant pollutants such as inorganic salts, high chemical oxygen demand (COD), heavy metals and dyes (Sen et al. 2019). Dyes are fundamentally organic compounds that bond chemically to the surface of the fabric to impart color which is termed as coloring agents (Sivarajasekar and Baskar 2014). The major pollutant in textile industrial wastewater is a dye which is highly visible even in small amounts (< 1 ppm) in the environment that can cause aesthetically unpleasant and toxicologically harmful (Rafatullah et al. 2010). In general, the dye is toxic and hazardous chemicals in the environment, particularly in the water bodies and biosphere. In addition, the dye can cause dysfunction of the kidneys, reproductive system, liver, brain, and nervous system of human beings (Adegoke and Solomon 2015). Approximately, 100,000 different types of dyestuffs are available on the global market but the annual production rate is about 7 × 105 t (Rafatullah et al. 2010; Ma et al. 2012). Methylene blue is a cationic dye with heterocyclic aromatic chemical compound (C16H18N3SC) which is commonly used for dyeing of cotton, wool and silk. About 40% of the synthetic dyes like methylene blue are toxic, mutagenic, and carcinogenic compounds that remained in industrial effluent and can cause severe public and environmental health problems (Hua et al. 2018).
The removal of dyes from textile industrial wastewater has been studied extensively using wastewater treatment technologies. Particularly, conventional wastewater treatment is composed of the preliminary, primary, secondary, and tertiary treatment stages. The removal of organic matter (BOD5) through the primary 30% and secondary treatment 85% were reported (Hunter et al. 2018). Recently, in addition to the conventional chemicals in the wastewater, over 100,000 micropollutants that belong to the chemical groups of the pesticides, personal care products, industrial chemicals, food additives, detergents, and antibiotic resistance genes were reported (Goswami et al. 2018). However, the removal of micropollutants through primary treatment was only < 20% and secondary treatment was about 60% (Guillossou et al. 2019). In general, conventional wastewater treatment plants are not effective for the removal of methylene blue from textile industrial wastewater. However, advanced wastewater treatments for dyes removal from textile industrial wastewater are very effective and efficient. The most applicable advanced wastewater treatments are chemical precipitation, nanofiltration, advanced oxidation process, ion exchange, reverse osmosis, membrane separation, electrocoagulation, and electrodialysis. However, these technologies are a very expensive and not sustainable mechanism for wastewater treatment in every sector, especially in developing countries (Fito et al. 2019). In addition, these methods required high energy, chemical, operational and capital inputs, and advanced technologies. Hence, such discrepancies in wastewater treatment methods encourage researchers to look for other effective and efficient treatment methods for dye removal from textile industrial wastewater.
Among advanced treatment methods, adsorption has been found to be good treatment technology for dye removal (Schier et al. 2011; Gisi et al. 2016). Fundamentally, adsorption treatment technology is flexible, simple for design, relative ease of operation, cost-effective, high efficiency, recyclability, and environmentally friendly. Moreover, commercial activated carbon is universal adsorbent but very expensive to be used in many nations across the globe. Hence, searching for low cost, efficient, high carbon content and locally available precursor materials are still under investigation (Fito et al. 2019). However, non-conventional activated carbon has been produced from local materials such as cassava peel, cotton, orange peel, the bark of morinda tinctorial, the bark of the vitex negundo, bagasse fly ash, wheat straw, sawdust, avocado seed and crocus sativus leaves (Suneetha et al. 2015; Amalraj and Pius 2017; Dehghani et al. 2018; Niazi et al. 2018; Fito et al. 2019; Rizzo et al. 2019; Choong et al. 2020). However, these materials have still many limitations in terms of adsorption performance and the difficulty of adsorbent preparation. Hence, researchers are still looking for another potential precursor material like Parthenium hysterophorus for water and wastewater treatment.
Parthenium hysterophorus is a weed that is commonly grown in tropical and sub-tropical regions. This plant is aggressive and invasive with an allelopathic effect which can cause a serious threat to environmental ecology and biodiversity. However, the plant is very abundant in tropical regions including in Ethiopia without having economic value. However, the P. hysterophorus was identified as a good precursor for activated carbon development. In this regard, a few studies have been made to develop activated carbon from P. hysterophorus for water and wastewater purifications (Ajmal et al. 2006; Lata et al. 2008a; Chatterjee et al. 2012; Bapat and Jaspal 2016; Bedada et al. 2020). However, there are no extensive studies for MB removal from textile industrial wastewater using the activated carbon of P. hysterophorus. Therefore, this study aimed to evaluate the adsorption performance of MB from textile wastewater using locally prepared activated carbon produced from P. hysterophorus stem under the experimental design of a full factorial at pH (3 and 11), contact time (45 and 100 min), initial MB concentration (100 and 150 mg/L) and adsorbent doses (0.5 and 2 g per 100 mL). Finally, the full factorial adsorption performance was compared with the conventional approach which was studied simultaneously.
Materials and Methods
Adsorbent Preparation and Characterization
Adsorbent Preparation
Parthenium hysterophorus plant samples were collected from Addis Ababa Science and Technology University which is described by a high-altitude of 2300 m (8° 58′ N 38° 47′ E) above the sea level with the annual mean temperature of 15.9 °C, rainfall of 1089 mm and relative humidity of 60.7%. The P. hysterophorus samples were cut into small pieces of 5–10 mm and the different adsorbent preparation stages are indicated in Fig. 1. These pieces were washed properly with distilled water and dried later using the oven at 105 °C for 24 h. The dried P. hysterophorus mass was impregnated with concentrated H3PO4 in the ratio of 1:1 by weight which was kept at 130 ± 5 °C for 24 h. Afterward, pyrolysis was performed in the furnace at 500 °C for 2 h. The activated material was washed with distilled water several times to remove the acid attached to the surface of the activated carbon. Then, the activated carbon was dried at 110 °C in an oven for 24 h. Finally, it was grounded into the particle sizes of 250 µm which was kept in the airtight plastic bag until used for treatment (Bapat and Jaspal 2016).
Preparation stages of activated carbon from Parthenium hysterophorus weed ‘a’–‘d’. Where a indicates the raw Parthenium hysterophorus weed, b refers the phosphoric acid impregnated Parthenium hysterophorus weed, c is the pyrolyzed Parthenium hysterophorus adsorbent and d is ready-made activated carbon
Characterization of Activated Carbon
Proximate Analysis
The thermal drying method was used to determine the moisture content of activated carbon produced from P. hysterophorus. The adsorbent sample of 1.0 g was added into a crucible which was placed in an oven at a constant temperature of 110 °C for 2 h. Then, the sample was cooled in a desiccator to room temperature and weighed. Then, the difference between the initial and final mass of activated carbon was considered to be the moisture content of the material (Anisuzzaman et al. 2015; Fito et al. 2019). The moisture content in percentage (%) was computed using Eq. 1:
where MC is the moisture composition of activated carbon which is described in percentages, W1 is the weight of activated carbon before the application of thermal heating (g) and W2 is the weight of the adsorbent sample after thermal drying (g).
For volatile matter determination, 1 g of activated carbon was placed in a preheated crucible and incinerated in the muffle furnace at 800 °C for 8 min with no contact of air under the standardized conditions. The adsorbent sample was then cooled in the desiccator and weighed. Finally, the volatile matter of activated carbon was calculated using Eq. 2:
where VM (%) is the volatile matter of the activated carbon in percentage, W1 (g) is the weight of the activated carbon before heating the sample and W2 (g) is the weight of activated carbon after heating the ash content of activated carbon was determined by the thermal drying method. Some empty crucibles were preheated and weighed. Activated carbon of 1 g was added in these preheated crucibles and placed in the furnace which was ignited at 500 °C for 4. Then, the adsorbent samples were cooled at room temperature in the desiccator. Finally, the adsorbent samples were weighed and the ash content was calculated using Eq. 3:
where AC is the ash content of the activated carbon in percentage, W1 (g) is the weight of the activated carbon before the heating process and W2 (g) is the weight of the ash after ignition process (Anisuzzaman et al. 2015; Fito et al. 2019). Finally, the fixed carbon (FC) of activated carbon was calculated from the summation of all the percentages of moisture content, ash content, and volatile matter content which was subtracted from 100%. Equation 4 was applied to calculate the fixed carbon of the activated carbon (Nwabanne and Igbokwe 2012):
Scanning Electron Microscope (SEM)
The surface morphology of the prepared activated carbon was determined using scanning electron microscopy at different resolutions. The sample preparation and scanning were performed as per standard operating procedures of the machine Scanning electron microscope (SEM, JEOL JSM-7600F FEG-SEM, JEOL, USA) (Hegazy et al. 2014; Nure et al. 2017).
Fourier Transform Infrared (FTIR)
The activated carbon sample was mixed with KBr in the ratio of 2:200. The mixture sample was crushed in a mortar to obtain a homogeneous powder mixture which was introduced into a molder to get a very fine plate. The plate analysis was then carried out by the FTIR spectrophotometer (FTIR, ThermoNicolet 5700, Waltham, MA USA). Finally, the adsorbent spectra were scanned over a wavelength of 4000–400 cm−1 (Hegazy et al. 2014; Nure et al. 2017).
Synthetic and Actual Wastewater Preparation
In preparation of the stock solution, 1 g of MB (C16H18N3SCl) was weighed and added into 1 L of Erlenmeyer flask. Following this, distilled water was poured into the flask to the marked level of 1000 mL and complete dissolution was performed using a stirring glass rod. Then, different working solutions were prepared using the concept of the dilution process. However, the real textile industrial wastewater was collected from Ayka Addis textile factory which is located in the south-west direction of Addis Ababa about 19 km. The wastewater samples were collected using a pre-clean polyethylene plastic bottle with diluted nitric acid and distilled water. Then, the samples were transported to the laboratory and preserved in a refrigerator at 4 °C to minimize the change in the physicochemical properties of the wastewater. Finally, absorption of the MB solution was carried out using a UV–Visible spectrophotometer (Agilent technology, Cary 100 UV–Visible spectrophotometer, Santa Clara, USA) at the wavelength (λmax) of 668 nm.
Optimization of MB Adsorption
Under the investigation of full factorial experimental design, four factors with two levels were used and marked as 24. This factorial approach has resulted in 16 adsorption runs, as indicated in Table 1. Literature values were used to fix each level of adsorption factors. The main benefit of the factorial experimental approach is to evaluate interactions among the adsorption factors and their impacts on the treatment performances. The combination of each factor with a lower and higher level of the run was done randomly. Different concentrations of MB solution of 100 mL were poured into 250 mL conical flasks and mixed with different amounts of adsorbent at constant room temperature. These solutions were agitated using orbital shaker at 125 rpm. Then, the separation of adsorbent from the adsorption treatment solution was performed using the centrifugal process at 3500 r/min for 30 min which was later filtered via Whatman filter paper 42 (Nure et al. 2017; Fito et al. 2019). Finally, the concentration of MB was determined using a UV–Visible spectrophotometer at a wavelength of 668 nm.
The percentage removal of MB from textile industrial wastewater was determined using Eq. 5:
where R% is the removal percentage of MB from textile industrial wastewater, Ci (mg/L) is the initial MB concentration and Cf (mg/L) is the final MB concentration recorded after adsorption treatment.
The Effect of Factors on Adsorption
In the conventional approach, one factor can be varied during the adsorption treatment keeping other factors constant at the optimum point (pH value 11, contact time 100 min, adsorbent dose 2 g in 100 mL and at the initial MB concentration 100 mg/L). Based on this information, the effect of pH at 3, 5, 8 and 11; the adsorbent dose of 0.5, 1, 1.5 and 2 g in 100 mL; contact time of 45, 60, 80,100 and 120 min; and initial MB concentration of 100,120, 140 and 150 mg/L were checked at constant room temperature.
Adsorption Isotherms
Adsorption isotherm was investigated using initial MB concentrations of 100, 120,140, and 150 mg/L at the fixed adsorbent dose 2 g, the contact time of 100 min and pH 11. The two most common adsorption isotherm models, the Langmuir and Freundlich were used. Langmuir isotherm assumes that all binding sites have equal affinity and formation of adsorbate monolayer on the surface of the adsorbent. The linearized form of the Langmuir isotherm model was indicated by Eq. 6:
where qmax (mg/g) is the maximum adsorption capacity of the MB adsorbed per unit mass of activated carbon, KL (L/mg) is the Langmuir constant which related to equilibrium adsorption constant. Ce (mg/L) and qe (mg/g) are the equilibrium values of MB concentration and adsorption capacity, respectively. In addition, the dimensionless constant parameter called separation constant (RL) of Langmuir adsorption was measured using Eq. 7:
where Co (mg/L) is the initial MB concentration.
Principally, the Freundlich isotherm is associated with heterogeneous surfaces of the adsorbent (activated carbon). The linearized form of Freundlich isothermal equation used in this study was described using Eq. 8:
where Kf ((mg/g)(L/mg)1/n) and ‘n’ (unitless) are the Freundlich constants which represent adsorption capacity and adsorption intensity, respectively. Basically, ‘n’ is used to describe the favorability of the adsorption process (Pongener et al. 2018; Islam et al. 2019).
Results and Discussion
Characterization of Activated Carbon
Proximate Analysis
Proximate analysis of activated carbon was performed and the percentage results were described in terms of the moisture content 5.70, volatile matter 18.60, ash content 15.30, and fixed carbon 60.40, as indicated in Table 2. Fixed carbon and ash content are the most important parameters of the proximate analysis to judge the quality of the adsorbent materials. Normally, a good precursor material should have a high percentage of the fixed carbon with a very small component of ash content. In the proximate analysis, fixed carbon refers to the non-volatile carbon, whereas the ash content is undesirable inorganic oxides which can be observed during preparations of activated carbon. The fixed carbon of many locally prepared activated carbons was very small. For instance, the fixed carbon of the bagasse fly ash 42.20% (Nure et al. 2017), original algae biomass 7.73%, residual biomass of Spirulina platensis algae 9.92%, biochar 59.88% (Nautiyal et al. 2016), Catha edulis 53.00% (Fito et al. 2019), apple pulp 6.56% and the apple peel 7.40% (Hesas et al. 2013). Basically, the result of the proximate analysis does not only depend on the precursor material but it is highly influenced by carbonization processes because of the variable degrees of dehydration, decomposition and elimination reactions are expected at different temperatures (Nautiyal et al. 2016). The fixed carbon composition of activated carbon in this study was 60.40% which is higher than many local prepared activated carbons but still lower than conventional activated carbon. In line with the commercial activated carbon, the average fixed carbon percentage of 77.45% was reported (Nautiyal et al. 2016). In general, the P. hysterophorus carbonaceous material can be taken as a good precursor for activated carbon development that can be contributed to combating water pollution to ensure water sustainability.
Fourier Transform Infrared Spectroscopy (FTIR)
The surface functional groups of P. hysterophorus activated carbon was determined through the identification of FTIR spectra. Six clear peaks were observed which are indicated in Fig. 2. These dominant peaks are at 3346 cm−1, 2656 cm−1, 2341 cm−1, 1855 cm−1, 1546 cm−1 and 1070 cm−1. The FTIR spectrum reveals the complex nature of the adsorbent as evidenced by the presence of many peaks. The peak obtained at 3346 cm−1 indicates the existence of free and intermolecular bonded hydroxyl groups. It is also related to the valence vibration of the hydrogen-bonded O–H groups. The shoulder band at 2656 cm−1 originated from the C–H stretching in the (–CH2) and (–CH3) groups. The relatively wide band at 2341 cm−1 corresponds to the symmetric (NH3+) stretching frequency or the carbon–oxygen bonds in the ketene groups. A very small peak near 1855 cm−1 is assigned to the C=O stretching vibrations of ketones, aldehydes, lactones, or carboxyl groups. The weak intensity of this peak indicates that this sample contains a small number of carboxyl groups. The band at 1546 cm−1 indicates the presence of (C–H, –C=C–) and aromatic C=C) groups. Finally, 1070 cm−1 represents C–O single bond and also indicates CH bending vibration of C–H2 and C–H3 (Meski et al. 2011).
Scanning Electron Microscope (SEM) Analysis
The SEM analysis of the activated carbon was done before and after the adsorption of MB from textile industrial wastewater. The image of surface morphology is indicated in Fig. 3a, b. The surface morphology of activated carbon was assessed. The micrograph clearly shows the presence of highly porous nature and differences in pore sizes. In this study, the surface of the activated was magnified 4000 times which was clearly shown that the different pore sizes and their distributions. The creation of pores and pore-volume on the activated carbon might be associated with the contribution to chemical activation by phosphoric acid. The heterogeneous and non-uniform shape of the pores with several cracks was observed on the surface of the adsorbent but these values were diminished after the adsorption treatment, since the surface interacted with MB which was well bound to the inner walls of the pores in the carbon matrix.
Optimization for MB Removal
In this optimization experiments, MB removal performances from aqueous solution using full factorial experimental design and the corresponding laboratory results are shown in Table 3. The maximum MB removal was found to be 93.8% at the adsorbent dosage 2 g/100 mL, pH 11, initial MB dye concentration 100 mg/L, and contact time of 100 min at constant room temperature. The second maximum removal of 91.2% was recorded at optimum adsorbent dose 2 g/100 mL, pH 11, and contact of time 100 min. However, the minimum MB removal of 69.2% was observed at the adsorbent dosage of 0.5 g/100 mL, pH 3, initial MB concentration 100 mg/L, and contact time of 45 min. This was supported by the test done on the sample of actual textile wastewater taken from Ayka Addis textile industry which has an initial MB concentration of 86.5 mg/L. In line with this actual wastewater, the maximum removal of 89% was obtained. In factorial adsorption treatment, it is impossible to differentiate the contribution of each factor very clearly for the removal of the adsorbates. However, it has the main advantages of showing the interaction effect and also to reduce the total number of experiments to obtain the maximum adsorption performances. In a similar finding, 62.7–98.3% removal of rhodamine-B dyes from aqueous solution onto formaldehyde and phosphoric acid activated carbon of P. hysterophorus was reported (Lata et al. 2008b).
Batch Adsorption Studies
The Effects of pH
The effect of pH on removal of MB was studied by varying the pH values from 3 to 11 at initial MB concentration of 100 mg/L, the adsorbent dosage of 2 g, and contact time of 100 min. These different pH values resulted in variation of MB removal which is shown in Fig. 4. The lowest MB removal of 68% was recorded at the pH value of 3 but gradually increased with increasing pH values to the alkaline range. Finally, the maximum MB removal efficiency of 96% was observed at pH 11. Principally, as pH values increased, the adsorbent surface develops several negative sites to bind with many more cations adsorbates, whereas decreasing of pH can force the adsorbent surface to develop positive sites to bind with anionic adsorbates. This indicates that basic pH is favorable for the MB removal from textile industrial wastewater. Basically, the surface chemistry and interactions between the adsorbate and adsorbent are determined by the pH of the solution which in turn influences the adsorption removal efficiency. In basic media, the concentration of OH− ions in the solution is increased. This creates the conducive surface chemistry for removal of MB which has a cationic surface. This can cause the surface of the activated carbon of P. hysterophorus to become deprotonated and the negative charge on the surface was developed. Then, the removal mechanisms have two possibilities which can be either the electrostatic attractions between the activated carbon and the MB adsorbate or covalent bond between these two parties. In general, the alkaline pH enhancing the MB removal efficiency due to both the aqueous chemistry and surface binding sites which resulted in increasing the rate of dye adsorption as well.
The Effects of Contact Time
The impact of MB removal was studied under the variation of contact time 45–120 min at the pH 11, initial MB concentration 100 mg/L, the adsorbent dosage of 2 g/100 mL. MB removal performances under the given conditions are shown in Fig. 5. The adsorption efficiency was increasing sharply until the time reached 100 min at the performance of 93.5%. Then, the adsorption performance nearly became constant. This result is almost the same as the maximum removal value observed under the full factorial approach. The adsorbate diffusion in aqueous solution towards the adsorbent is ultimately determined the time consumed and the adsorption efficiency. This value indicated that 100 min is sufficient for adsorbate to interact with the surface of the adsorbent to achieve equilibrium. In general, it is possible to conclude that the time necessary to reach the equilibrium condition for the removal of the MB molecules onto activated carbon was established to be 100 min.
The Effect of Initial MB Concentration
Initial MB concentration was one of the most important adsorption factors which have an impact on the rate of MB uptake on the surface of activated carbon. The effects of initial MB concentration which ranged from 100 to 250 mg/L at the constant adsorbent dose of 2 g/100 mL, pH 11, and contact time 100 min were studied, and the experimental results are illustrated in Fig. 6. As initial MB concentrations increased from 50 to 120 mg/L, adsorption performances were also increased gradually to a maximum value of 93.3%. Then, the removal efficiency was nearly becoming a constant until the MB concentration was raised to 150 mg/L. Finally, the MB removal was decreased with increasing the initial MB concentration. This decreasing situation of removal might be attributed to the saturation point of the active sites. In general, at lower adsorbate concentrations, the adsorbate removal and adsorption rate are found to be very high but after the saturation point of the adsorption, the amount adsorbates are much higher than the active sites of the adsorbent which would result in low adsorption performances.
The Effects of Adsorbent Dose
In this section, MB removal efficiency was studied under the change of adsorbent dose from 0.5 to 2 g/1000 mL at constant initial concentration 100 mg/L, contact time 100 min, and pH value 11. The results of the findings are displaced in Fig. 7. Adsorption performances were gradually increased from 90 to 95% which was nearly the same result of the maximum MB removal obtained under the full factorial method. This was observed due to the fact that increasing the adsorbent dose was provided with sufficient adsorbent sites. However, the interactions of the adsorbent sites and adsorbates might be limited after the saturation point. Thus, a large amount of the adsorbent can reduce the adsorbent capacity which was likely due to the particle aggregations and resulted in decreasing the total surface area of the adsorbent (Nethaji et al. 2013).
Adsorption Isotherm
MB adsorption was studied at different initial MB concentrations of 100, 120,140, and 150 mg/L at the fixed adsorbent dose of 2 g/100 mL, contact time 100 min, and pH 11. Langmuir and Freundlich’s isotherms were employed to judge whether the adsorbent surface was homogenous and heterogeneous. The linearized Eq. (9) of Langmuir isotherm was used and the results of the sketch are indicated in Fig. 8.
From the plot of Ce vs Ce/qe, the value of qmax was calculated from the slope (1/qmax) of the linearized equation of the Langmuir. Thus, the qmax value of 11.37 was found. Similarly, the Langmuir constant (KL) was calculated from the intercept of the graph (1/(qmax KL) and its result of 0.15 was recorded. The calculated RL value of 0.0625 was obtained which was closer to zero. There are four options for separation factors such as the favorable adsorption at 0 < RL < 1, unfavorable adsorption at RL > 1, linear adsorption at RL is equal to 1 and irreversible adsorption at RL is equal to 0. Since the RL value of this study was between 0 and 1 (0 < RL < 1) which indicated that the adsorption process was favorable. Furthermore, the coefficient of the determination (R2) of the graph was 0.99 which was fairly close to 1. Therefore, this isothermal model was best fitted with the experimental data.
The linearized Freundlich isotherm Eq. (10) was used and the sketched graph is displayed in Fig. 9:
where qe is the amount of MB adsorbed per gram of the adsorbent at equilibrium (mg/g), Ce is the equilibrium concentration of adsorbate (mg/L), Kf is Freundlich isotherm constant (mg/g), n is the adsorption intensity.
The plot of log Ce vs log qe was sketched and the slope of 0.49 and intercept of 0.35 was obtained. The value of Kf was calculated from the intercept of the equation and the result of 2.54 was found. Similarly, the n value was calculated from the slope of the linearized equation which resulted in n value of 2.05. The R2 value of the linearized form of Freundlich isotherm was 0.98. Based on this analysis, the experimental data was also best fitted with Freundlich isothermal model. In general, adsorption of MB onto activated carbon was favorable and the R2 value from the graph was close to 1 which presented that Langmuir model is best describes the adsorption mechanism which indicated that the adsorption process is a homogeneous and single layer.
Conclusions
Parthenium hysterophorus plant stem was used to develop activated carbon at the laboratory level. The plant samples were activated with phosphoric acid and high temperature. This activated carbon was characterized and encouraging results were found. This indicated that the material can be taken as a good precursor for adsorbent development. Moreover, the activated carbon was employed for the removal of MB from textile industrial wastewater and the maximum removal of 94% was found at pH value 11, contact time 100 min, adsorbent dose 2 g in 100 mL, and at the initial MB concentration 100 mg/L. Under similar treatment conditions, 91% maximum MB removal from actual textile wastewater was recorded. Nearly, similar percentages of maximum MB removal were also recorded under different conventional adsorption treatment approaches. The mechanism of the adsorption was checked and the Langmuir adsorption isotherm was best fitted with the experimental data at the R2 value of 0.99, indicating that the adsorption process is a homogeneous and single layer. Likewise, the RL value was found to be between 0 and 1 which confirmed that the adsorption process was favorable. Hence, this activated carbon can be considered as a locally available, environmentally friendly, and effective alternative adsorbent for MB removal from textile industrial wastewater. In general, it can be concluded that the activated carbon produced from P. hysterophorus is a promising adsorbent but still it is highly recommendable to work on the detailed investigation of the precursor material before its application at industrial level for water and wastewater treatment.
Availability of DATA and Materials
All data are fully available without restriction.
References
Adegoke KA, Solomon O (2015) Dye sequestration using agricultural wastes as adsorbents. Water Resour Ind 12:8–24. https://doi.org/10.1016/j.wri.2015.09.002
Ajmal M, Rao RAK, Ahmad R, Khan MA (2006) Adsorption studies on Parthenium hysterophorous weed: removal and recovery of Cd(II) from wastewater. J Hazard Mater 135:242–248. https://doi.org/10.1016/j.jhazmat.2005.11.054
Amalraj A, Pius A (2017) Removal of fluoride from drinking water using aluminum hydroxide coated activated carbon prepared from bark of Morinda tinctoria. Appl Water Sci 7:2653–2665. https://doi.org/10.1007/s13201-016-0479-z
Anisuzzaman SM, Joseph CG, Taufiq-Yap YH et al (2015) Modification of commercial activated carbon for the removal of 2,4-dichlorophenol from simulated wastewater. J King Saud Univ Sci 27:318–330. https://doi.org/10.1016/j.jksus.2015.01.002
Bapat SA, Jaspal DK (2016) Parthenium hysterophorus: novel adsorbent for the removal of heavy metals and dyes. Glob J Environ Sci Manag 2:135–144. https://doi.org/10.7508/gjesm.2016.02.004
Bedada D, Angassa K, Tiruneh A et al (2020) Chromium removal from tannery wastewater through activated carbon produced from Parthenium hysterophorus weed. Energy Ecol Environ 5:184–195. https://doi.org/10.1007/s40974-020-00160-8
Chatterjee S, Kumar A, Basu S, Dutta S (2012) Application of response surface methodology for methylene blue dye removal from aqueous solution using low cost adsorbent. Chem Eng J 181–182:289–299. https://doi.org/10.1016/j.cej.2011.11.081
Choong CE, Wong KT, Jang SB et al (2020) Fluoride removal by palm shell waste based powdered activated carbon vs. functionalized carbon with magnesium silicate: implications for their application in water treatment. Chemosphere 239:124765. https://doi.org/10.1016/j.chemosphere.2019.124765
De Gisi S, Lofrano G, Grassi M, Notarnicola M (2016) Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: a review. Sustain Technol 9:10–40. https://doi.org/10.1016/j.susmat.2016.06.002
Dehghani MH, Farhang M, Afsharnia M, Mckay G (2018) Adsorptive removal of fluoride from water by activated carbon derived from CaCl2-modified Crocus sativus leaves: equilibrium adsorption isotherms, optimization, and influence of anions. Chem Eng Commun. https://doi.org/10.1080/00986445.2018.1423969
Fito J, Said H, Feleke S, Worku A (2019) Fluoride removal from aqueous solution onto activated carbon of Catha edulis through the adsorption treatment technology. Environ Syst Res 8:1–10. https://doi.org/10.1186/s40068-019-0153-1
Goswami L, Vinoth Kumar R, Borah SN et al (2018) Membrane bioreactor and integrated membrane bioreactor systems for micropollutant removal from wastewater: a review. J Water Process Eng 26:314–328. https://doi.org/10.1016/j.jwpe.2018.10.024
Guillossou R, Le Roux J, Mailler R et al (2019) Organic micropollutants in a large wastewater treatment plant: what are the benefits of an advanced treatment by activated carbon adsorption in comparison to conventional treatment? Chemosphere 218:1050–1060. https://doi.org/10.1016/j.chemosphere.2018.11.182
Hegazy AK, Abdel-Ghani NT, El-Chaghaby GA (2014) Adsorption of phenol onto activated carbon from seaweed: determination of the optimal experimental parameters using factorial design. Appl Water Sci 4:273–281. https://doi.org/10.1016/j.jtice.2011.04.003
Hesas RH, Arami-Niya A, Wan Daud WMA, Sahu JN (2013) Preparation and characterization of activated carbon from apple waste by microwave-assisted phosphoric acid activation: application in methylene blue adsorption. BioResources 8:2950–2966
Hua Y, Xiao J, Zhang Q et al (2018) Facile synthesis of surface-functionalized magnetic nanocomposites for effectively selective adsorption of cationic dyes. Nanoscale Res Lett 13:99
Hunter RG, Day JW, Wiegman AR, Lane RR (2018) Municipal wastewater treatment costs with an emphasis on assimilation wetlands in the Louisiana coastal zone. Ecol Eng. https://doi.org/10.1016/j.ecoleng.2018.09.020
Islam MN, Khan MN, Mallik AK, Rahman MM (2019) Preparation of bio-inspired trimethoxysilyl group terminated poly(1-vinylimidazole)-modified-chitosan composite for adsorption of chromium (VI) ions. J Hazard Mater 379:120792. https://doi.org/10.1016/j.jhazmat.2019.120792
Lata H, Garg VK, Guptaa RK (2008a) Adsorptive removal of basic dye by chemically activated Parthenium biomass: equilibrium and kinetic modeling. Desalination 219:250–261. https://doi.org/10.1016/j.desal.2007.05.018
Lata H, Mor S, Garg VK, Gupta RK (2008b) Removal of a dye from simulated wastewater by adsorption using treated parthenium biomass. J Hazard Mater 153:213–220. https://doi.org/10.1016/j.jhazmat.2007.08.039
Ma J, Jia Y, Jing Y et al (2012) Kinetics and thermodynamics of methylene blue adsorption by cobalt-hectorite composite. Dye Pigment 93:1441–1446. https://doi.org/10.1016/j.dyepig.2011.08.010
Meski S, Ziani S, Khireddine H et al (2011) Factorial design analysis for sorption of zinc on hydroxyapatite. J Hazard Mater 186:1007–1017. https://doi.org/10.1016/j.jhazmat.2010.11.087
Nautiyal P, Subramanian KA, Dastidar MG (2016) Adsorptive removal of dye using biochar derived from residual algae after in situ transesterification: alternate use of waste of biodiesel industry. J Environ Manage 182:187–197. https://doi.org/10.1016/j.jenvman.2016.07.063
Nethaji S, Sivasamy A, Mandal AB (2013) Adsorption isotherms, kinetics and mechanism for the adsorption of cationic and anionic dyes onto carbonaceous particles prepared from Juglans regia shell biomass. Int J Environ Sci Technol 10:231–242. https://doi.org/10.1007/s13762-012-0112-0
Niazi L, Lashanizadegan A, Sharififard H (2018) Chestnut oak shells activated carbon: preparation, characterization and application for Cr(VI) removal from dilute aqueous solutions. J Clean Prod 185:554–561. https://doi.org/10.1016/j.jclepro.2018.03.026
Nure JF, Shibeshi NT, Asfaw SL et al (2017) COD and colour removal from molasses spent wash using activated carbon produced from bagasse fly ash of Matahara sugar factory, Oromiya region, Ethiopia. Water SA 43:470–479. https://doi.org/10.4314/wsa.v43i3.12
Nwabanne J, Igbokwe P (2012) Application of response surface methodology for preparation of activated carbon from palmyra palm nut. N Y Sci J 5:18–25
Ozturk E, Cinperi NC (2018) Water efficiency and wastewater reduction in an integrated woolen textile mill. J Clean Prod 201:686–696. https://doi.org/10.1016/j.jclepro.2018.08.021
Pongener C, Bhomick PC, Supong A et al (2018) Adsorption of fluoride onto activated carbon synthesized from Manihot esculenta biomass—equilibrium, kinetic and thermodynamic studies. J Environ Chem Eng 6:2382–2389. https://doi.org/10.1016/j.jece.2018.02.045
Rafatullah M, Sulaiman O, Hashim R, Ahmad A (2010) Adsorption of methylene blue on low-cost adsorbents: a review. J Hazard Mater 177:70–80. https://doi.org/10.1016/j.jhazmat.2009.12.047
Rizzo L, Malato S, Antakyali D et al (2019) Consolidated vs new advanced treatment methods for the removal of contaminants of emerging concern from urban wastewater. Sci Total Environ 655:986–1008. https://doi.org/10.1016/j.scitotenv.2018.11.265
Schier L, Lima D, Dennison M et al (2011) Adsorption modeling of Cr, Cd and Cu on activated carbon of different origins by using fractional factorial design. Chem Eng J 166:881–889. https://doi.org/10.1016/j.cej.2010.11.062
Sen SK, Patra P, Das CR et al (2019) Pilot-scale evaluation of bio-decolorization and biodegradation of reactive textile wastewater: an impact on its use in irrigation of wheat crop. Water Resour Ind 21:100106. https://doi.org/10.1016/j.wri.2019.100106
Sivarajasekar N, Baskar R (2014) Adsorption of basic red 9 on activated waste Gossypium hirsutum seeds: process modeling, analysis and optimization using statistical design. J Ind Eng Chem 20:2699–2709. https://doi.org/10.1016/j.jiec.2013.10.058
Suneetha M, Sundar BS, Ravindhranath K (2015) Removal of fluoride from polluted waters using active carbon derived from barks of Vitex negundo plant. J Anal Sci Technol 6:1–19. https://doi.org/10.1186/s40543-014-0042-1
Acknowledgements
We would like to thank Ethiopian Road Authority (ERA) and Addis Ababa Science and Technology University (AASTU) for the research fund and facilities which was done collaboratively for national capacity building.
Funding
This research work was supported by the Ethiopian Road Authority
Author information
Authors and Affiliations
Contributions
JF contributed to experimental design, the inception of the idea, experimental supervision, manuscript writing and editing, and revision, whereas SA and KA mainly contributed to manuscript editing and revision, and in addition, experiment work and data collection were carried out by SA.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interests.
Rights and permissions
About this article
Cite this article
Fito, J., Abrham, S. & Angassa, K. Adsorption of Methylene Blue from Textile Industrial Wastewater onto Activated Carbon of Parthenium hysterophorus. Int J Environ Res 14, 501–511 (2020). https://doi.org/10.1007/s41742-020-00273-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41742-020-00273-2