Abstract
As phosphorus (P) exceeds 0.1 mg P L−1, the water is usually considered high enough to cause eutrophication. Thermally treated mussel shell (TMS), a calcium-rich (36.76%) biological material, was an environmentally friendly and low-cost adsorbent for P removal. To achieve very low concentrations of P, composite agents of PAC–TMS, Fe–TMS and La–TMS were prepared with the optimum weight ratio of 0.4, 0.2 and 0.2 (g g−1) [polyaluminium chloride (PAC), Fe(OH)3 or La2O3 to TMS] with the lower cost and high P adsorption efficiency, respectively, all of which had more than 80% of P removal rate after 12-h treatment of 5 mg L−1 P solution, much higher than that of TMS alone (50%). Isotherm experiment showed that these composite agents have maximum phosphate adsorption capacities of 91.74 mg P g−1 (PAC–TMS), 101.42 mg P g−1 (Fe–TMS) and 56.50 mg P g−1 (La–TMS), respectively. Additionally, the metal-modified TMS was almost independent of pH. Long-term efficient removal of P (< 0.1 mg L−1) was achieved during a 3-month removal trial of P-contaminated water while using these metal-modified TMS. Since metal-modified TMS both had calcium and other multivalent metal (Al, La or Fe), the modification pathway was mainly dependent on both enhance adsorption/precipitation by a simultaneous effect of the calcium and other multivalent metal.
Article Highlights
-
PAC–TMS, Fe–TMS and La–TMS with optimum ratio of 0.4, 0.2 and 0.2 for P removal
-
P adsorption capacities of 91.74(PAC–TMS), 101.42(Fe–TMS) and 56.50 mg g−1 (La–TMS)
-
Long-term efficient removal of P (< 0.1 mg L−1) via these metal-modified TMS
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Eutrophication is a global environmental problem that deteriorates water quality, damages ecosystems and threatens humans and animals health (Boeykens et al. 2017; Ruan et al. 2016; Deng et al. 2019). Phosphorous concentrations greater than 0.1 mg P L−1 are usually considered high enough to cause eutrophication (Kumar et al. 2019). In recent years, significant attention has been paid to phosphorus adsorption using biomass materials due to their low-cost and environmental-friendly properties, such as biochar from cellulosic material, biological shell material and so on (Dai et al. 2017; Micháleková-Richveisová et al. 2017; Paradelo et al. 2016; Roy 2017; Xiong et al. 2011; Zheng et al. 2019). These studies also showed that biomass adsorbents modified via physical pyrolysis and chemical treatment were commonly used in obtaining effective phosphorus adsorption to achieve very low concentrations of P. Biochar pyrolyzed from cellulosic materials has been modified by chemical compounds of aluminum (Novais et al. 2018), iron (Micháleková-Richveisová et al. 2017) and lanthanum modification (Qiu et al. 2017). There was little multivalent metal in cellulosic material, while biological shells are calcium-rich materials. Considering these, the direct modification of biological shell is more practical as low-cost adsorbent for achieving very low concentrations of P. Biological shell modification via pyrolysis was applied in most cases (Dai et al. 2017; Paradelo et al. 2016; Xiong et al. 2011), while chemical agents were seldom used (Arulvel et al. 2017). To improve the phosphorus removal performance of shells, more modification methods are demanded.
Waste mussel shells are abundant in shellfish aquaculture and the canning industries. Several studies have attempted to turn mussel shell into phosphorus absorbents (Paradelo et al. 2016; Romar-Gasalla et al. 2016; Xiong et al. 2011; Zapater-Pereyra et al. 2014). The raw powdered mussel shell was reported to have the maximum adsorption capacity 6.95 mg P g−1 and 18.23 mg P g−1 for phosphate, respectively (Xiong et al. 2011; Paradelo et al. 2016). A thermally treatment could increase the maximum adsorption capacity of mussel shell from 18.23 to 38.75 mg P g−1 for phosphate, and adsorption and precipitation both contributed to phosphate removal (Paradelo et al. 2016). However, the modification of thermally treated mussel shell is still needed to achieve very low concentrations of P. In this study, three metal-modified methods were attempted to improve the phosphorus removal performance of pyrolyzed mussel shell: polyaluminium chloride, iron and lanthanum oxide. In addition, lanthanum oxide has high phosphate affinity and often requires support materials such as activated carbon fiber (Zhang et al. 2011) and mesoporous silicate (Huang et al. 2015) to disperse the lanthanum. The pyrolyzed mussel shell powder could provide a much cheaper alternative support material for lanthanum.
Thus, pyrolyzed mussel shell powder was prepared and modified via three multivalent metal chemicals (Al, La or Fe), the phosphorus removal characteristics of which were examined via batch experiments, and the long-term performance of treating phosphorous-contaminated water was further investigated.
Materials and methods
Materials
Marine mussel shell was collected from a local mussel breeding plant in Zhoushan City in China. Polyaluminium chloride (PAC), lanthanum oxide (La2O3) and pure ferric chloride (FeCl3) were used as chemical modification agents for phosphorus removal in the study, respectively. The phosphate synthetic solution was prepared by dissolving potassium dihydrogen orthophosphate in deionized water. The real environmental water was collected from local contaminated river in Zhoushan city of China.
TMS preparation and modification
The mussel shell was washed to remove impurities and pulverized by grinder after drying in the oven. TMS was then obtained via pyrolyzing at 600 °C for 2 h in muffle furnace. Modification agents of PAC, La2O3 and Fe(OH)3 were mixed with TMS at different weight ratios to obtain composite phosphorus binding agents of PAC–TMS, La–TMS and Fe–TMS in the study. The weight ratio refers to the ratio of Fe(OH)3, La2O3 and PAC to TMS. La2O3 and TMS were physically mixed evenly with various weight ratios of 0.2, 0.4 and 0.8 to obtain aLa–TMS, bLa–TMS and cLa–TMS, respectively, and the same method was used to acquire aPAC–TMS, bPAC–TMS as well as cPAC–TMS. Fe–TMS was a mixture of TMS and the floc Fe(OH)3, and the floc was obtained via the reaction of FeCl3 (1 M) and NaOH (1 M) solution. Next, the floc was dried to a constant weight and then physically mixed evenly with of TMS at various weight ratios of 0.2, 0.4 and 0.8 to achieve the composite phosphorus binding agents of Fe–TMS. Phosphorus removal characteristics were all observed for different types of Fe–TMS, La–TMS and PAC–TMS. The La2O3 and PAC used in the form of a solid powder. The optimum mass ratio of the three composited phosphorus binding agents was ascertained via the phosphorus removal performance after 12-h treatment of 5 mg P L−1 synthetic solution under the same dosage (20 g L−1).
Batch experiments
Phosphorus (P) removal characteristics of Fe–TMS, La–TMS and PAC–TMS were further examined through the batch experiments of isotherm experiment, kinetics of P removal, the effect of adsorbents dosage, other ions and pH conditions. The batch experiments were conducted in a 250-mL conical flask inside a rotary mixer for 12 h (100 rpm and 25 °C) with initial P concentration of 50 mg L−1 and P binding agent dosage of 20 g L−1 (excluding isotherm experiments). The initial pH of batch experiments was all adjusted to 7.0 ± 0.2 except for the effect of pH experiment.
isotherm experiment: 100-mL solutions containing known concentrations of P (2–500 mg P L−1) were added to 0.1 g (1 g L−1) of composite agent in 250-mL conical flask in oscillator for 12 h.
kinetics of P removal: the sample was shaken for 0, 10, 20, 30, 60, 90, 150 and 210 min in a rotary mixer.
the effect of adsorbents dosage: the experiment was observed with various dosages of P binding agent (2, 5, 10, 20, 40, 60, 80 and 100 g L−1).
the effect of other ions: different concentrations of ammonia nitrogen, nitrate nitrogen and nitrite nitrogen (0, 2, 5, 10, 20 and 50 mg L−1) were added in P solution to examine the effect of other ions to optimum dosage of each mussel shell type in 500-mL conical flask with rotary mixer for 12 h.
the effect of pH: according to environmental water quality, different solution pH conditions of 6, 7 and 9 were further considered.
Application of composite phosphorus binding agents for contaminated environmental water
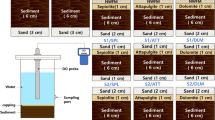
The real environmental water was collected from a local contaminated river Jingguan in Zhoushan of China. The phosphate (PO43−-P) concentration of real water was 0.2 mg L−1. To further highlight the phosphorus pollution, PO43−-P concentration in the real water was increased to 1.5 mg L−1 with the addition of monopotassium phosphate. To study the performance of binding phosphorus characteristics of optimum Fe–TMS, La–TMS and PAC–TMS during phosphorus-contaminated water treatment, seven white plastic rectangle devices (dimensions by 32 × 24 × 19 cm) with working volume of 10 L were developed with different optimum composite phosphorus binding agents and hydrodynamic condition simulated via aeration (Table 1). Each device was filled with 10 L of phosphorus contaminated water with the initial PO43−-P concentration of 1.5 mg L−1. Except control device W1, the rest devices W2–W7 were filled with the same dosage of composite phosphorus binding agents. The phosphorus was added in the devices on about 1-month and 2-month operation time for the further study of long-term performances, respectively. The distilled water was added into the devices W1–W7 to replenish the evaporation of water every day. The PO43−-P concentration variations in the devices W1–W7 were all examined every day.
Analytical method
The total carbon (TC), hydrogen (H) and calcium (Ca) contents were estimated between thermally treated mussel shell (TMS) and unmodified mussel shell (UMS). The content of Ca was measured by inductively coupled plasma mass spectrometry (PerkinElmer, USA) and the content of C and H was analyzed using an Element Analyzer (PerkinElmer, USA). Water quality indexes including Permanganate Index (CODMn), ammonium nitrogen (NH4+-N), nitrite nitrogen (NO2−-N), nitrate nitrogen (NO3−-N) and phosphate (PO43−-P) were measured according to standard method (SEPA 2002). pH was determined using a Delta320 precise pH meter.
All the data were statistically analyzed by OriginPro 8 (OriginLab, USA), with a p value less than 0.05 (p < 0.05) indicating statistically significant differences.
Results and discussion
Characteristics of TMS
The contents of TC and H in untreated mussel shell (UMS) were 14.42% and 0.71%, which were decreased to 12.99% (TC) and 0.08% (H), respectively, via the pyrolysis treatment in TMS. The decrease of TC in TMS was mainly attributed to the transformation of organic carbon in mussel shell to carbon oxide via the pyrolysis treatment. The content of Ca increased from 34.78 to 36.76% in TMS. The phosphorus removal efficiency and organics leaching levels of UMS and TMS were further determined following after 12-h treatment of 5 mg P L−1 synthetic solution at the same dosage (20 g L−1). The results showed that UMS and TMS both had approximately 50% P removal efficiency (0.125 mg P g−1). A similar pyrolyzed mussel shell achieved P removal efficiency of 77% and 89% after 24-h treatment of 15.5 mg P L−1 and 46.5 mg P L−1 synthetic solution, respectively (Paradelo et al. 2016). The P removal efficiency largely depends on the contact time, initial P concentration, pH and absorbents dosage (Xiong et al. 2011). In addition, the organics leaching level (CODMn) of UMS (2.82 mg L−1) was significantly higher than that of TMS (0.47 mg L−1). It was suggested that calcination at the high temperature of 600 °C was advantageous to remove organic matter, which avoided secondary organic pollution during water treatment.
A study has reported that crab shell (CaCO3-rich biomaterials) prepared at temperature 700–900 °C was lime-based biochar; while, CRB prepared at lower temperature of 300–600 °C was calcite-based biochar (Dai et al. 2017). Since CaCO3 is also the abundant component of mussel shell, TMS prepared at temperature 600 °C was seemed to be calcite-based biochar in this study. The calcite was almost insoluble in neutral or alkaline environment, and there was very limited Ca2+ dissolved in the solution. Thus, adsorption is vital for P removal via TMS in neutral or alkaline environment. It was reported that phosphate was adsorbed by calcite electrostatic and chemical interactions for pH between 7 and 8.2, and chemically adsorbed onto calcite for pH values greater than 8.2 (Karageorgiou et al. 2007). In addition, chemical precipitation between Ca2+ and phosphate can occur at low pH environment. A study has demonstrated P removal efficiency of mussel shell achieved the highest level at low pH of 1.5, which was related to the dissolution of Ca2+ ions from adsorbent (Xiong et al. 2011).
Screening of the optimum ratio of TMS and modifying agent for phosphorus removal
The P removal efficiency largely depends on the contact time, initial P concentration, pH and absorbents dosage. TMS had only 50% P removal efficiency after 12-h treatment of 5 mg P L−1 synthetic solution at the same dosage (20 g L−1). It was seemed that adsorbent TMS was not efficient to achieve very low concentration of P after 12-h treatment in the neutral environment, which was necessary to be further modified by other agents. The P removal efficiency of individual modifying agents of La2O3, PAC and Fe(OH)3 was examined after 12-h treatment of 5 mg P L−1 synthetic solution at the same dosage (20 g L−1). Results showed that La2O3 alone had P removal rate of 92.5%, and the water color was brown and not acceptable both for the PAC and Fe(OH)3 alone for P removal. Thus, the optimum ratio of TMS and modifying agent is essential for efficient phosphorus removal. The weight ratios of the modified agents to TMS of 0.2, 0.4 and 0.8 were further examined at 12 h of 5 mg P L−1 synthetic solution with the same dosage of 20 g L−1. The P removal performance is shown in Fig. 1.
PAC–TMS with the ratio of 0.2 (marked as aPAC–TMS), 0.4 (marked as bPAC–TMS) and 0.8 (marked as cPAC–TMS) had P removal rates of 86.4% (0.216 mg P g−1), 95.4% (0.238 mg P g−1) and 95.8% (0.239 mg P g−1), respectively. These results demonstrated that the P removal rate significantly increased to the highest level when the proportion of PAC and TMS increased from 0.2 to 0.4. Since PAC material is cheap, the optimum ratio of 0.4 was recommended. Phosphate exhibits different ionization forms in different pH condition. The main forms of phosphate were H2PO4−, HPO42− and PO43− at the varying pH conditions of 2.1–7.2, 7.2–12.3 and above 12.3, respectively (Pan et al. 2009). This experiment was conducted in neutral environment (pH = 7.0). TMS as CaCO3-rich materials, the CaCO3 solubility was around 15 mg L−1 in neutral water, which theoretically could only bind 2.79 mg P L−1 with Ca2+ ions according to Eq. 1 (Rietra et al. 2001; Yin et al. 2017). However, abundant Al3+ ions could release from PAC in neutral environment, which could efficiently bind with PO43− to form the AlPO4 in neutral environment (Eq. 2) (Toor and Kim 2019), which was the pathway to modify the TMS.
Composite P binding agents aLa–TMS, bLa–TMS and cLa–TMS with ratios of La2O3 to TMS of 0.2, 0.4 and 0.8, respectively, achieved P removal rates of 87.2% (0.218 mg P g−1), 92.4%(0.231 mg P g−1) and 94.2% (0.236 mg P g−1), respectively. La2O3 had a P removal rate of only 92.5% (0.231 mg P g−1), which was similar to that of bLa–TMS. The results showed that the P removal rate significantly increased by 5.2% and 1.8% when the proportions of La2O3 and TMS were increased from 0.2 to 0.4 and from 0.4 to 0.8, respectively. Considering the high cost of La2O3, aLa–TMS with a ratio of 0.2 was the optimal composite P binding agent. Lanthanum has a good affinity towards phosphorus and has an especially strong anti-interference ability in complex systems containing phosphorus (Chen et al. 2012; Lin et al. 2018). These contribute to an efficient removal of phosphorus by La–TMS. Qiu et al. (2017) also reported that LaPO4·xH2O is demonstrated to be the dominant pathway for specific adsorption by La2O3.
In terms of the composite P binding agents Fe–TMS, P removal rates of 76.6% (0.191 mg P g−1) and 81.8% (0.204 mg P g−1) were achieved for aFe–TMS and bFe–TMS with ratios of 0.2 and 0.4, respectively. As the ratio of Fe–TMS increased to more than 0.8, the color of the water was brown, which was not acceptable for applications in natural waters. In consideration of the water color and P removal rates, composite P binding agent aFe–TMS with a proportion of 0.2 was recommended. Ferric hydroxide is an efficient method for P removal mainly by ion exchange adsorption in neutral water environment (Ren et al. 2015), but it easily caused high treatment cost. The combination of TMS and ferric hydroxide in this study could largely decrease the cost. As Fe3+ ions dissolved in acid solution, precipitation between Fe3+/Ca2+ and phosphate (FePO4) could simultaneously occur. A similar study has reported that a synergistic effect for P removal was examined at Fe/Ca molar ratio of 7:3–4:1 (Qiu et al. 2015). In addition, the batch results demonstrated there was still residual P concentration in solution (> 0.1 mg L−1) after 12-h treatment of TMS-based composite phosphorus binding agents, which might be mainly attributed to the insufficient contact time to achieve very low concentration of P.
Composite P binding agents bPAC–TMS, aLa–TMS and aFe–TMS were all low-cost adsorbent for P removal, because TMS was still the major component, accounting for 60%, 80% and 80%, respectively. P retention on TMS was mainly attributed to adsorption of phosphate on the surface of adsorbent in neutral environment, which was also the major pathway for P removal via bPAC–TMS, aLa–TMS and aFe–TMS in neutral environment. In addition, phosphate can be partially bound by the released Ca2+, Fe3+, Al3+ and La3+ cations on the surface of adsorbent. Thus, composite P binding agents bPAC–TMS, aLa–TMS and aFe–TMS all had two metal cations, which could both bind phosphate resulting in phosphate adsorption/precipitation. Thus, PAC, Fe(OH)3 and La2O3 all modified TMS for phosphate removal in this study.
Phosphorus removal characteristics of TMS-based composite phosphorus binding agents
Efficient composite P binding agents bPAC–TMS, aLa–TMS and aFe–TMS were obtained based on TMS. The phosphorus removal characteristics of the three composite P binding agents was further examined via the various tests of adsorption isotherms, adsorption kinetics of P removal, effect of dosage, and the effect of other ions and different solution pH conditions.
Adsorption isotherms
The results of the adsorption isotherm experiments are shown in Fig. 2 and Table 2. At a dosage of 20 g L−1, the adsorption capacities of bPAC–TMS, aLa–TMS and aFe–TMS were correlated with the concentration of P in the solution. The adsorption capacity of phosphorus increased with an increase in the phosphorus concentration. The linear equations for Langmuir isotherm adsorption and Freundlich isotherm adsorption were used to fit the data. The governing equations of these models are written as follows:
where qe and qmax are the amount of adsorption and maximum adsorption, ce is the concentration, and KL and KF are the Langmuir and Freundlich adsorption equilibrium constants.
The results showed that the Langmuir model had a higher correlation coefficient than the Freundlich model (Fig. 2 and Table 2), which indicated that a monolayer homogeneous adsorption process occurred during P adsorption via the composite agents. The maximum phosphate adsorption capacity of bPAC–TMS, aLa–TMS and aFe–TMS were 91.74 mg g−1, 56.50 mg g−1 and 101.42 mg g−1. The raw powdered mussel shell was reported to have the maximum adsorption capacity of 6.95 mg P g−1 and 18.23 mg P g−1 for phosphate, respectively (Xiong et al. 2011; Paradelo et al. 2016), and a higher value of 38.75 mg P g−1 by TMS (Paradelo et al. 2016), which were all much lower than that of bPAC–TMS, aLa–TMS and aFe–TMS. This result suggested that the composite agents exhibited a high ability to remove phosphate.
Kinetics study
Figure 3a shows the results of adsorption kinetics. The P adsorption capacity and removal rates of the three kinds of P binding agents were enhanced with an increase in the contact time, which increased rapidly in the first 10 min and then slowly increased between 10 and 90 min. Adsorption equilibrium was obtained within 90 min. Linear equations of a pseudo-first-order reaction kinetic model and a pseudo-second-order reaction kinetic model were used to fit the kinetic data. The governing equations of these models are written as follows:
where qe and qt are the amounts of adsorption at time t, and k1 and k2 are the pseudo-first-order and pseudo-second-order adsorption rate constants.
In terms of the fitting coefficient of determination (R2) in Table 2, the adsorption kinetics of the three kinds of phosphorus binding agents was more satisfactory described by the pseudo-second-order reaction kinetic model (R2 > 0.97), which indicated that the adsorption of P on biochars was conducted through the inner sphere complex (Wang et al. 2011).
Effect of dosage on P removal
The optimal dosage experiment for the three kinds of phosphorus binding agents is shown in Fig. 3b. In the case of a fixed initial phosphate concentration of 50 mg L−1, the P removal rate of bPAC–TMS was significantly increased to 98.8% as the dosage added increased to 5 g L−1, which was considered as the optimal dosage of bPAC–TMS. The removal rate of aLa–TMS rapidly increased within the dosage range of 0–5 gL−1, and highly efficient removal of P (98.8%) was achieved as the dosage approached 10 g L−1, indicative of the optimal dosage of aLa–TMS. Meanwhile, the optimal amount of aFe–TMS was 5 g L−1 with the effective P removal of 97.3%. Thus, the optimal dosages of bPAC–TMS, aLa–TMS, and aFe–TMS were 5 g L−1, 10 g L−1 and 5 g L−1, respectively. As the maximum phosphate adsorption capacity of bPAC–TMS, aLa–TMS and aFe–TMS was 91.74 mg g−1, 56.50 mg g−1 and 101.42 mg g−1, the dosage of 10 g L−1 and 5 g L−1 for 50 mg L−1 was enough. However, there was still residual P concentration detected in solution. It might be speculated that the contact time of 12 h was not enough to achieving the very low concentration of P.
Effect of inorganic nitrogen occurrence and pH on P removal
Since the occurrences of inorganic nitrogen (ammonia nitrogen, nitrate nitrogen and nitrite nitrogen) are very common in natural waters, the effect of varying concentrations of inorganic nitrogen (0–50 mg L−1) on P removal performance was observed using composite P binding agents of bPAC–TMS, aLa–TMS and aFe–TMS. Results demonstrated that efficient P removal (> 90%) is obtained from bPAC–TMS, aLa–TMS and aFe–TMS with the coexistence of NH4+-N, NO3−-N and NO2−-N in the range of 0–50 mg L−1.
According to pH values in environmental water, the P removal by bPAC–TMS, aLa–TMS and aFe–TMS was investigated at varying pH values of 6, 7 and 9 (Fig. 4). Phosphate exhibits different ionization forms in different pH condition. The P removal rate of aLa–TMS was maintained at high levels of almost 100%, which was not affected by the pH dynamics. Efficient P removal (92.2–99.1%) using bPAC–TMS was also achieved in a pH range of 4–13. Additionally, aFe–TMS produced highly efficient P removal of (97.8–99.6%) in the pH range of 6–7 and was reduced to 83% at a pH value of 9, respectively. The modification agents of PAC and Fe(OH)3 mainly enhanced the precipitation of TMS, and the chemical precipitation also depended on pH. However, La had good affinity of P over wide range of pH. In this respect, aLa–TMS was a good choice. However, three composite agents all achieved more than 80% of P removal efficiency at pH range of 6–9. Therefore, the modification agents all extended TMS to use in wider range of pH circumstances. The phosphorus removal using ferric, calcium salts or PAC was all dependent of pH change. However, the application of these composite P binding agents in this study could reduce the consumption of reagents for pH adjustment.
Application of composite phosphorus binding agents for contaminated environmental water
Figure 5 shows the long-term P removal performances of composite phosphorus binding agents for phosphorus contaminated water. The initial P concentration of systems W1–W7 was 1.52 mg L−1. The results showed that P concentrations rapidly declined in the initial 3 days via addition of P binding agents, with removal rates of 0.66 (W2), 0.42 (W3), 0.56 (W4), 0.68 (W5), 0.69 (W6) and 0.74 mg (Ld)−1 (W7). To achieve very low concentration of P (< 0.1 mg L−1), more than 1 day was required. Results further suggested that the contact time of 12 h was one of the factors that caused residual P concentration during batch experiments in this study. In contrast, no significant decrease of P was observed in device W1 without agent addition. After the secondary addition of phosphorus (on day 33), the rapid decrease in P concentrations were also detected in device W2–W7, with removal rates of 0.60 (W2), 0.68 (W3), 0.62 (W4), 0.73 (W5), 0.58 (W6) and 0.74 mg (Ld)−1 (W7) in the initial 3 days.
The third addition of P was conducted after 2-month operation (on day 64). Device W2–W7 with different composite phosphorus binding agents (bPAC–TMS, aLa–TMS and aFe–TMS) addition still achieved efficient P removal, having removal rates of 0.59 (W2), 0.69 (W3), 0.38 (W4), 0.69 (W5), 0.48 (W6) and 0.7 mg (Ld)−1 (W7) in the initial 3 days. In addition, no P release was detected after efficient removal of P. The results indicated that the water disturbance via aeration might have advantageous to improve P removal, especially for P binding agents of aLa–TMS and aFe–TMS. Meanwhile, the P concentration in device W1 increased to approximately 4.0 mg L−1 after 2 months of operation. P eutrophication is a growing concern globally. Phosphorous concentrations greater than 0.1 mg P L−1 are usually considered high enough to cause eutrophication (Kumar et al. 2019). Less than 0.1 mg L−1 of P concentrations in devices W2–W7 was all detected in approximately a week after each P addition. Thus, the three agents of bPAC–TMS, aLa–TMS and aFe–TMS adapted well to low P concentration of contaminated water and had long-term efficient removal of P. In addition, no P desorption was detected.
Conclusion
Calcium-rich biological material TMS was an environmentally friendly and low-cost adsorbent for P removal. A calcium-rich material of TMS was effectively metal modified via PAC, Fe(OH)3 and La2O3 to remove P. Composite agents of PAC–TMS, Fe–TMS and La–TMS prepared at different optimum weight ratios had the P removal rate more than 80% of after 12 h treatment of 5 mg L−1 P solution, which was much higher than that of TMS alone (50%). These composite agents had maximum phosphate adsorption capacities of 91.74 mg P g−1 (PAC–TMS), 101.42 mg P g−1 (Fe–TMS) and 56.50 mg P g−1 (La–TMS), respectively. The metal-modified TMS all extended the use of TMS in a wider range of pH conditions and achieved very low concentration of P in natural water. Composite P binding agents of PAC–TMS, La–TMS and Fe–TMS both had two metal cations, which could both bind phosphate via simultaneous adsorption/precipitation.
References
Arulvel S, Elayaperumal A, Jagatheeshwaran MS (2017) Electroless nickel—phosphorus coating on crab shell particles and its characterization. J Solid State Chem 248:87–95
Boeykens SP, Piol MN, Samudio Legal L, Saralegui AB, Vázquez C (2017) Eutrophication decrease: phosphate adsorption processes in presence of nitrates. J Environ Manag 203:888–895
Chen N, Feng C, Zhang Z, Liu R, Gao Y, Li M, Sugiura N (2012) Preparation and characterization of lanthanum (III) loaded granular ceramic for phosphorus adsorption from aqueous solution. J Taiwan Inst Chem Eng 43(5):783–789
Dai L, Tan F, Li H, Zhu N, He M, Zhu Q, Hu G, Wang L, Zhao J (2017) Calcium-rich biochar from the pyrolysis of crab shell for phosphorus removal. J Environ Manag 198:70–74
Deng Y, Ruan Y, Ma B, Timmons MB, Lu H, Xu X, Zhao H, Yin X (2019) Multi-omics analysis reveals niche and fitness differences in typical denitrification microbial aggregations. Environ Int 132:105085
Huang W, Yu X, Tang J, Zhu Y, Zhang Y, Li D (2015) Enhanced adsorption of phosphate by flower-like mesoporous silica spheres loaded with lanthanum. Microporous Mesoporous Mater 217:225–232
Karageorgiou K, Paschalis M, Anastassakis GN (2007) Removal of phosphate species from solution by adsorption onto calcite used as natural adsorbent. J Hazard Mater 139(3):447–452
Kumar SP, Korving L, van Loosdrecht M, Witkamp GJ (2019) Adsorption as a technology to achieve ultra-low concentrations of phosphate: research gaps and economic analysis. Water Res X 4:100029
Lin L, Li Z, Song X, Jiao Y, Zhou C (2018) Preparation of chitosan/lanthanum hydroxide composite aerogel beads for higher phosphorus adsorption. Mater Lett 218:201–204
Micháleková-Richveisová B, Frišták V, Pipíška M, Ďuriška L, Moreno-Jimenez E, Soja G (2017) Iron-impregnated biochars as effective phosphate sorption materials. Environ Sci Pollut Res 24(1):463–475
Novais SV, Zenero MDO, Barreto MSC, Montes CR, Cerri CEP (2018) Phosphorus removal from eutrophic water using modified biochar. Sci Total Environ 633:825–835
Pan B, Wu J, Pan B, Lv L, Zhang W, Xiao L, Wang X, Tao X, Zheng S (2009) Development of polymer-based nanosized hydrated ferric oxides (HFOs) for enhanced phosphate removal from waste effluents. Water Res 43:4421–4429
Paradelo R, Conde-Cid M, Cutillas-Barreiro L, Arias-Estévez M, Nóvoa-Muñoz JC, Álvarez-Rodríguez E, Fernández-Sanjurjo MJ, Núñez-Delgado A (2016) Phosphorus removal from wastewater using mussel shell: investigation on retention mechanisms. Ecol Eng 97:558–566
Qiu L, Zheng P, Zhang M, Yu X, Abbas G (2015) Phosphorus removal using ferric–calcium complex as precipitant: parameters optimization and phosphorus-recycling potential. Chem Eng J 268:230–235
Qiu H, Liang C, Yu J, Zhang Q, Song M, Chen F (2017) Preferable phosphate sequestration by nano-La(III) (hydr)oxides modified wheat straw with excellent properties in regeneration. Chem Eng J 315:345–354
Ren J, Li N, Li L (2015) Granulation and ferric oxides loading enable biochar derived from cotton stalk to remove phosphate from water. Bioresour Technol 178(1):119–125
Rietra PJ, Hiemstra T, Riemsdijk WH (2001) Interaction between calcium and phosphate adsorption on goethite. Environ Sci Technol 35(16):3369–3374
Romar-Gasalla A, Rivas-Pérez IM, Paradelo-Núñez R, Nóvoa-Muñoz JC, Arias-Estévez M, Fernández-Sanjurjo MJ, Álvarez-Rodríguez E, Núñez-Delgado A (2016) Phosphorus retention on forest and vineyard soil samples, mussel shell, pine-sawdust, and on pyritic, granitic and waste materials. Geoderma 280:8–13
Roy ED (2017) Phosphorus recovery and recycling with ecological engineering: a review. Ecol Eng 98:213–227
Ruan YJ, Deng YL, Guo XS, Timmons MB, Lu HF, Han ZY, Ye ZY, Shi MM, Zhu SM (2016) Simultaneous ammonia and nitrate removal in an airlift reactor using poly(butylene succinate) as carbon source and biofilm carrier. Bioresour Technol 216:1004–1013
SEPA (2002) Standard Methods for the Examination of Water and wastewater, 4th edn. China Environmental Science Press, Beijing, pp 200–281
Toor UA, Kim D-J (2019) Effect of pH on phosphorus (P) dissolution and recovery from polyaluminum chlorides (PAC) sludge. J Environ Manag 239:142–149
Wang Z, Nie E, Li J, Yang M, Zhao Y, Luo X, Zheng Z (2011) Equilibrium and kinetics of adsorption of phosphate onto iron-doped activated carbon. Environ Sci Pollut Res 19(7):2908–2917
Xiong J, Qin Y, Islam E, Yue M, Wang W (2011) Phosphate removal from solution using powdered freshwater mussel shells. Desalination 276(1–3):317–321
Yin H, Yan X, Gu X (2017) Evaluation of thermally-modified calcium-rich attapulgite as a low-cost substrate for rapid phosphorus removal in constructed wetlands. Water Res 115(1):329–338
Zapater-Pereyra M, Malloci E, van Bruggen JJA, Lens PNL (2014) Use of marine and engineered materials for the removal of phosphorus from secondary effluent. Ecol Eng 73:635–642
Zhang L, Wan L, Chang N, Liu J, Duan C, Zhou Q, Li X, Wang X (2011) Removal of phosphate from water by activated carbon fiber loaded with lanthanum oxide. J Hazard Mater 190(1):848–855
Zheng Y, Wang B, Wester AE, Chen J, He F, Chen H, Gao B (2019) Reclaiming phosphorus from secondary treated municipal wastewater with engineered biochar. Chem Eng J 362:460–468
Acknowledgements
The work was supported by the Fundamental Research Funds for Zhejiang Universities (2019J00047) and the Program of Xinmiao Talents in Zhejiang Province (2017R411014).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Yin, H., Liu, L., Lv, M. et al. Metal-Modified Mussel Shell for Efficient Binding of Phosphorus in Eutrophic Waters. Int J Environ Res 14, 135–143 (2020). https://doi.org/10.1007/s41742-020-00250-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41742-020-00250-9