Abstract
The objective of this study was to analyze the influence of periphyton biofilm on nutrient releases from sediments in ponds and lakes. The physico-chemical parameters in the overlying water, the TP content, and the P forms in the sediments and periphyton biofilm were measured. The results showed that (1) periphyton biofilm could change the physico-chemical indexes in the overlying water; furthermore, the periphyton biofilm influenced the release of N and P from sediments, and its extent of influence was closely related to the nutrient levels of the sediments. (2) The periphyton biofilms effectively reduced the concentrations of N and P in sediments with different nutritional levels (P < 0.05). The average daily TN removal rates were 52% and 25% in the pond and lake microcosms, respectively, and the average daily TP removal rates were 1.06% and 20.9% in the pond and lake microcosms, respectively. This result suggested that periphyton biofilms acted as biological buffers for nutrient cycling between sediments and overlying water; these buffering effects were mainly through the absorption, filtration, and precipitation of nutrients. (3) The TP content and P forms of the periphyton biofilm were different between the pond and lake microcosms, which indicated that the P forms of the periphyton biofilm were potentially influenced by the nutritional levels of the sediments.
Article Highlights
-
There are different in the influence of periphyton biofilm on nutrient releases from sediments in ponds and lakes.
-
Periphyton biofilm could change the physico-chemical indexes in the overlying water; furthermore, the periphyton biofilm influenced the release of N and P from sediments, and its extent of influence was closely related to the nutrient levels of the sediments.
-
The TP content and P forms of the periphyton biofilm were different between the pond and lake microcosms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Compared with heavy metal pollution (Das Sharma 2019), lake eutrophication also has become a serious environmental problem (Najar et al. 2017), especially for shallow lakes in China, and eutrophication has resulted in serious blue–green algal blooms in recent years (Wang et al. 2006; Yang et al. 2013). As major nutrients in aquatic ecology, nitrogen (N), and phosphorus (P) have been recognized as the most critical nutrients that limit lake productivity. Excessive inputs of N and P have resulted in the eutrophication of freshwater bodies, which has subsequently led to the deterioration of these aquatic environments (Zhang et al. 2015). In the last decade, exogenous nutrient substances have been controlled; however, source control is not always possible, especially because nutrients may also come from internal sources, such as lake sediments (Dittrich et al. 2011). Some related studies have reported that P and N releases from sediments may have a significant impact on water quality and may result in continuing eutrophication (Xie et al. 2003).
The release of P from sediments is a highly complex process and involves a number of physical, chemical, and biological processes (Christophoridis and Fytianos 2006). Some factors can influence the release of P from the sediments to the water interface; potential influencing factors include redox potential (Eh), pH, temperature, dissolved oxygen (DO), salinity, and sediment resuspension (Wang et al. 2013). Moreover, not only the physico-chemical parameters reflect water quality (Ajayan and Kumar 2016), but also can be affected by the presence of periphyton biofilm. Thus, to assess and solve the risk of eutrophication in aquatic systems, it is necessary to know the conditions related to the release of endogenous P and how to control internal loading from sediments. In the overlying water, the P forms can be divided into total dissolved phosphorus (TDP) and particulate phosphorus (PP); there is a dynamic balance between the TDP and PP, and they can be transformed from one form to the other (Solórzano and Sharp 1980). Although the PP is not usually directly absorbed and utilized by biota, it could have an effect on water eutrophication over the long term. Soluble reactive phosphorus (SRP) is a component of TDP, and could be directly and readily available to periphyton biofilm (Zhang and Mei 2015).
Periphyton biofilms are biota that are ubiquitously distributed between the overlying water and the sediments; these periphyton biofilms consist of complex consortia of algae, bacteria, and other micro- and meso-organisms (Wu et al. 2010, 2012). As the basis of the food chain, periphyton biofilm is one of the most important food sources for other aquatic organisms (Wu 2016). It not only controls the assimilation, retention, and transformation of nutrients but also weakens the nutrients released from sediments and accelerates the recovery of eutrophication; thus, periphyton biofilm plays an important role in stabilizing ecosystems and promoting the recovery of aquatic plants (Wu et al. 2014, 2017). As not all forms of P present similar mobility and bioavailability, it is important to know the total phosphorus content and forms in the different types of sediments and their distribution in periphyton biofilms (Lu et al. 2014). Periphyton biofilms could adjust the ecological conditions of the aquatic environment, such as the retention of nutrients in the overlying water, which would reduce the release of sediment P to the overlying water and improve the water quality. However, thus far, the influence of periphyton biofilm on phosphorus migration in sediments with different nutritional levels has not been systematically investigated. Therefore, the primary objective of the experimental exploration was to (1) assess the effect of periphyton biofilm on changes in aquatic environmental factors and the influence on P release from the sediments; (2) systematically evaluate the changes in the P concentration and species between the overlying water and sediments with and without periphyton biofilm; and (3) explore the potential role of periphyton biofilm on phosphorus retention in sediments with different nutritional levels.
Materials and Methods
Preparation of Sediments
Two nutritional levels of surface sediments were collected from the center shallow area of a pond and a lake using Grab-type dredger (Haijing HJ-801) on December 8, 2015. The sediments were crushed to a fine consistency and homogenized after air drying for 2 days; then, any large particles and plant residues were carefully removed from the sediments (lake sediments: TP 3.51 mg g−1, IP 2.74 mg g−1, OP 0.50 mg g−1. Pond sediments: TP 1.85 mg g−1, IP 1.23 mg g−1, OP 0.25 mg g−1.)
Cultivation of the Periphyton Biofilm
Periphyton biofilms were incubated in the littoral zone of a shallow eutrophic lake in Wuhan, Hubei Province of China. Industrial soft carriers (composed of polyester fiber material, diameter of 5 cm and length of 20 cm) were immersed to an underwater depth of 0.3–0.5 m for native periphyton colonization. Then, the periphyton biofilm was collected from the coastal zone of the lake after 2 weeks and used in a follow-up experiment.
Experimental Design
The simulations of the process of N and P release from the sediments to the water were conducted in microcosms. First, 3.0 kg dry sediment was evenly spread on the bottom of glass tanks (40 × 25 × 30 cm) at a depth of approximately 5 cm. Second, six periphyton biofilms (approximately 210 g) were placed into corresponding microcosms. Finally, 22 L distilled water was slowly poured into the tanks along the inner wall to avoid resuspending the sediments. The microcosms that contained the sediments from the pond and lake were labeled the L-group and the P-group, respectively. Each microcosm had one control group and treatment group (LC and LT, PC and PT, respectively) (Table 1), and each group had three replicates. To avoid the influence of environmental conditions on the experiment, each microcosm was kept in a greenhouse, which had a temperature of 23 ± 2 °C and cool-white fluorescent light illumination of approximately 100–200 μmol photons m−2 s−1 (14 h L: 10 h D). In addition, the control was covered with black cardboard to prevent the formation of periphyton biofilm at the water–sediment interface. The entire experiment period was sustained for 48 days, and the volumes of water in the glass tanks were maintained by adding distilled water every week.
Sampling and Measurement
The experiment started on December 12, 2015, and water samples were collected at 2 d, 4 d, 6 d, 8 d, 12 d, 16 d, 20 d, 24 d, 28 d, 32 d, and 48 d. The total P (TP), particulate P (PP), total N (TN), and particulate N (PN) of the water were determined according to APHA (2002). To measure whether the environmental parameters were synchronized with the N and P in the overlying water, various parameters, such as the water pH (portable pH meter, PHB-4, China), dissolved oxygen (portable dissolved oxygen meter, JPB-607, China), redox potential (ORP meter, SX712, China), and conductivity (portable conductivity meter, DDB-303A, China), were measured.
At the beginning and end of the experiment, the P fractions in the sediments and in the periphyton biofilms were subjected to sequential P fractionation according to the method of Ruban (Ruban et al. 2001). The TP was divided into two main groups: inorganic P (IP) and organic P (OP); the IP included Fe/Al–P (NaOH-extractable P, P bound to Al, Fe, and Mn oxides and hydroxides) and Ca–P (HCl-extractable P, P associated with Ca). All experiments were conducted in triplicate, and the mean results (± SD) were reported. Statistical analysis was performed using SPSS 19.0. Pearson’s correlation analysis was used to analyze the relationship between the aquatic environmental factors and P, and independent-sample t tests were used to assess various indexes between the control and treatments. All figures were produced using Origin 9.0, Excel 2010. Sample acquisition, pretreatment, analysis and testing, data processing, and result report all conform to the standards and specifications (Michener 2018).
Results and Discussion
The Influence of the Periphyton Biofilms on Water Quality and P Release from the Sediments
The influence of periphyton biofilm on water quality was obviously different in the sediments with different nutritional levels. In the lake microcosms (i.e., the L-group), the pH, DO, and Eh values were higher in the treatment groups than those in the control, but the EC values in the treatment groups were lower than those in the control. In the pond microcosms (i.e., the P-group), the DO values in the treatment groups were significantly higher than those in the control (P < 0.05); in addition, the EC values in the treatment groups were significantly lower than those in the control (P < 0.05). In the two microcosms, the pH, DO, and Eh values in the P-group were higher than those in the L-group, while the EC values in the P-group were lower than those in the L-group (Fig. 1). The Eh values in the P-group presented oxidation potential during the entire experiment, but the Eh values in the L-group presented reduction potential in the first 16 days of the experiment; after 16 days, the Eh of the water body changed to oxidation potential.
On the other hand, the TP concentrations in the LC and LT groups were 0.25 and 0.19 mg L−1, respectively, and the average daily TN removal rates were 25% in the LT treatment. This result implied that water quality can be improved with the presence of the periphyton biofilm. In the L-group, TN was positively correlated with EC (r = 0.742, P < 0.01), and TP was negatively correlated with DO (r = − 0.633, P < 0.01) and Eh (r = − 0.831, P < 0.01). Compared to the L-group, the TP concentrations in the PC and PT groups were 0.034 and 0.037 mg L−1, respectively, and the average daily TN removal rates were 52% in the PT treatment. Meanwhile, Pearson’s correlations revealed several notable relationships among the water-quality variables. In the P-group, TN was negatively correlated with pH (r = − 0.692, P < 0.05), while TP was negatively correlated with pH (r = − 0.681, P < 0.05) (Table 2). These results implied that periphyton biofilms could influence changes in the physical and chemical indexes of water. Furthermore, these changes influenced the release of P from sediments, and their degree of influence was closely related to the nutrient level of the sediments.
Some previous studies have focused on how periphyton biofilms may play an important role in the P biogeochemistry of aquatic ecosystems (Mccormick et al. 2006); however, these studies have ignored how periphyton biofilms have a stronger ability to improve water quality and adjust the P cycle. In this study, it was notable that the DO and pH of water in the treatments were substantially higher than those in the control. The reason for this observation was most likely that the periphyton biofilm consisted mainly of phototrophic algae whose intense photosynthesis could consume CO2 from the water and release O2, resulting in an increase in the water pH and DO (Zhang et al. 2013). At the same time, with the increased DO in the water body under the distribution of periphyton biofilm, the Eh values also changed. In the later stage of the experiment, the DO values in the L-group presented a large increase, and the Eh of the water body changed from a reducing penitential to an oxidation potential. The changes in the physical and chemical indexes of the water body were important factors that affected the release of P in the sediments (Kaiserli et al. 2002). Based on the results of this experiment, the influence of pH and DO on the release of P from the sediment cores was clear, and the sediments released more P under low pH than under high pH conditions; in addition, the sediments released more P under anaerobic and anoxic conditions than under aerobic and oxygen-saturated conditions. The P concentration released from the sediments was closely related to the pH and DO (Wang et al. 2008). Christophoros and Fytianos also noted that the P release from the sediments was related to the redox potential (Eh). When the water body presented a reduction potential, a higher concentration of P was released from the sediments (Christophoridis and Fytianos 2006). This view was in conformity with the results of our experiment. In this study, there was a positive or negative correlation between the P concentrations and the pH, DO, and Eh in the water in the P and L groups, suggesting that periphyton biofilms were able to change the aquatic environmental factors in the water and then influenced the P concentrations released from the sediments.
The Form and Transformation of N and P in Water
There were tremendous differences in the N and P levels and the N/P ratios of the overlying water based on the distribution of periphyton biofilm in the sediments with different nutritional levels (P < 0.05). In the L-group and P-group, the TN concentrations in the treatments were lower than those in the control throughout the experiment, and the average daily TN removal rates were 24% and 52%, respectively. One-way ANOVA indicated that there was a significant difference between the LT and LC in terms of the TN concentration and the N/P ratio (P < 0.05), but there were no differences in pond microcosms (P > 0.05). This result suggested that the role of N removal on periphyton biofilms in sediments with different N concentrations was different. The PN contents in the LC and LT were 1.9 and 1.5 mg L−1, respectively, and in the PC and PT, the values were 0.22 and 0.14 mg L−1, respectively (Fig. 2); these results implied that the periphyton biofilm had a stronger ability to detain PN.
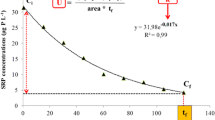
In the microcosms with the lake sediments, the TP concentrations in the LC and LT were 0.25 and 0.19 mg L−1, respectively, and the average daily TP removal rates were 20.9%. The PP concentrations in the LC and LT were 0.19 and 0.13 mg L−1, respectively, and the PP exhibited similar patterns to those of the TP in the L-group; furthermore, the proportion of the PP concentrations accounted for more than 50% of the TP (Fig. 2). The concentration of SRP in the overlying water was lower than that of the other P fractions (Fig. 3), and the SRP concentrations in the LT were higher than those in the control during the whole experiment.
In the microcosms with the pond sediments, the TP concentrations were lower than those in the L-group, but the TP concentrations in the PT had no obvious difference from those in the PC (P > 0.05). The PP accounted for approximately 45% of the TP in the P-group, and the changes in the contents of PP and TP were similar. Meanwhile, Pearson’s correlations revealed several notable relationships between the PP and TP of the water. The PP was positively correlated with the TP in the microcosms with the pond sediments [P1 (r = 0.709, P < 0.05), P2 (r = 0.790, P < 0.01)]. The SRP concentrations in the PT were also higher than those in the PC, which was consistent with the experimental results of the L-group (Fig. 3). The presence of periphyton biofilm significantly reduced the concentrations of TN, PN and PP in the sediments with different nutritional levels (P < 0.01), and the periphyton biofilm effectively reduced the TP, SRP, and N/P ratio of lake sediments (P < 0.05) (in the later stage of the experiment); however, there was no obvious difference in the removal of TP and SRP in the pond sediments (P > 0.05), but the N/P ratio decreased obviously (P < 0.05). This result suggested that periphyton biofilms acted as biological buffers for nutrient cycling between sediments and overlying water through the processes of nutrient absorption, filtration, and precipitation.
In the pond and lake microcosms, the PP accounted for nearly 50% of the TP, and the PP had positive relationships with the TP. This result was in accordance with those of past studies (Rydin and Brunberg 1998), which suggested that PP concentrations had a very important contribution to the TP. In the overlying water, the PP was divided into suspended PP and sediment PP. Typically, the PP cannot be directly absorbed and utilized by aquatic organisms, and their spatial distribution and transformation in the water body were related to the processes of biotransformation and physical sedimentation (Zhou et al. 2001). In the pond and lake microcosms, the concentration of PP increased in the first half of the experiment and began to decline in the middle stage of the experiment. The reason for this phenomenon may be that the sediment PP and suspended PP transformed between each other (House et al. 1995), and some relevant literature has presented that the physical deposition and suspension of PP commonly migrate as fluid elements between water bodies and sediments.
According to the biological activity, the SRP could be directly utilized by aquatic organisms, and it was an important source of endogenous phosphorus. The proportion of the SRP concentrations to the TP concentration fluctuated between 10 and 30% (Duhamel et al. 2011). Because the SRP could be directly and readily available to aquatic organisms, when the SRP was insufficient, the organisms needed to convert more SRP to ensure their own growth. In this study, the SRP concentrations in the treatments were higher than those in the control, suggesting that periphyton biofilms could transform more SRP during growth. The concentrations of SRP could also be used as an indicator for the utilization of phosphorus by aquatic organisms. When the concentration of the SRP decreased in the water, the periphyton biofilms effectively absorbed and utilized the P in water; however, when the concentration of the SRP increased in the water, it was likely that the periphyton biofilms did not need any more P.
The Retention of Phosphorus by Periphyton Biofilm in the Different Sediments
The TP concentrations in the water, sediments, and periphyton biofilm in the pond and lake microcosms were estimated after 48 d. There was an obvious difference in the P contents between the pond and lake sediments; for example, the P concentration in the lake sediments was greater than 104 mg, but the P content in the lake sediments was half that of the pond sediments after 48 d. Based on the analysis of the P content, we suggested that the sediments from the lake had higher nutrient contents than did the sediments from the pond (Table 3). It was also observed that the TP concentrations of the periphyton biofilms in the PT and LT were 18.42 and 20.92 mg, respectively, and the P concentrations of the periphyton biofilms at 48 d were slightly higher than those at 0 d in the PT and LT. On the other hand, there was also an obvious difference in the release of P from sediments with different nutritional levels, and the TP content of water in the L-group was significantly higher than that in the P-group (P < 0.05) (Table 3). At the same time, the TP content of water with periphyton biofilm was lower than that of the control group, which also implied that the growth of periphyton biofilm reduced the release of P from sediments.
The TP content and P forms were significantly different (P < 0.05) in sediments with different nutritional levels were significantly different. The initial P concentration of sediment in the lake microcosms was 3.51 mg g−1, and the Fe/Al–P, Ca–P, and OP concentrations were 2.22, 0.52, and 0.5 mg g−1, respectively; however, in the pond microcosms, the TP concentration in the sediment was 1.85 mg g−1, and the Fe/Al–P, Ca–P and OP concentrations were 0.72, 0.51, and 0.25 mg g−1, respectively. The TP content of the periphyton biofilm was similar in the two microcosms, but the P form composition was different. After 48 d, the total P content of the periphyton biofilm in the lake microcosms increased from 0.59 to 0.84 mg g−1, but, in the pond microcosms, the total P content of the periphyton biofilm increased from 0.59 to 0.61 mg g−1 (Table 3). This result implied that the periphyton biofilm had a large potential to remove P from the water–sediment interface and then store it. Specifically, the levels of the Fe/Al–P of periphyton biofilm in the pond and lake microcosms were highest in terms of the TP, at 41.7% and 19.7%, respectively. The OP content was next, at 14.2% and 19.6%, respectively, and the Ca–P was lowest, at 8.3% and 9.8%, respectively (Fig. 4). This result suggested that the P forms retained by the periphyton biofilms were affected by the composition of P in the sediments.
Research on the influence of periphyton biofilms on the P cycle is one of the most important topics for the restoration of the aquatic ecological environment, and periphyton biofilms have been considered a short-term sink for P by a few scholars (Cao et al. 2014). In the sediments with different nutritional levels in the microcosms, there was a difference in the TP concentrations of the periphyton biofilms. In the TP pool of the lake microcosms, the highest concentrations of P in the periphyton biofilms were found in the Fe/Al–P fractions; however, in the TP pool of the pond microcosms, the highest concentrations of P in the periphyton biofilms were found in the Fe/Al–P and OP after 48 d. The results suggested that periphyton biofilms have a stronger ability to retain phosphorus, and with the change in sediment P concentration, the ability of periphyton biofilms to retain phosphorus is different.
In the periphyton biofilm, Ca–P was considered to be a non-available phosphorus; in contrast, Fe/Al–P was considered to be an available phosphorus (De-Bashan and Bashan 2004). The presence of periphyton biofilm in waters could lead to the co-precipitation of P with CaCO3 or its adsorption onto CaCO3 crystals (Dodds 2010). The Ca–P content could account for nearly 30% of the TP in the periphyton biofilm at the end of experiment (Lu et al. 2016), and similar proportions of Ca–P in the periphyton biofilms were found in our experiments. Furthermore, most of the P in the periphyton biofilms was found in exchangeable and loosely bound forms, such as Fe/Al–P, which could account for more than 50% of the total P (Lu et al. 2016); in addition, the content of Fe/Al–P is also considered a pollution indicator (Hantke et al. 1996). In the study, the Fe/Al–P content had the highest content of TP over 48 d, which provided indirect evidence that periphyton biofilms could absorb P to ensure their own growth. As a result, the evaluation of P migration and transformations based on a sediment-periphyton biofilm-water system will improve our understanding of P cycling in aquatic ecosystems.
References
Ajayan A, Kumar A (2016) On the seasonal changes in the surface water chemistry of Museum Lake, Thiruvananthapuram, Kerala, India. Pollution 2(2):103–114
APHA (2002) Standard methods for the evaluation of water and wastewater, 22nd edn. American Public Health Association, Washington, DC
Cao J, Hong X, Pei G (2014) Removal and retention of phosphorus by periphyton from wastewater with high organic load. Water Sci Technol J Int Assoc Water Pollut Res 70(1):62–69
Christophoridis C, Fytianos K (2006) Conditions affecting the release of phosphorus from surface lake sediments. J Environ Qual 35(4):1181–1192
Das Sharma S (2019) Risk assessment and mitigation measures on the heavy metal polluted water and sediment of the Kolleru Lake in Andhra Pradesh, India. Pollution 5(1):161–178
De-Bashan LE, Bashan Y (2004) Recent advances in removing phosphorus from wastewater and its future use as fertilizer (1997–2003). Water Res 38(19):4222–4246
Dittrich M, Gabriel O, Rutzen C, Koschel R (2011) Lake restoration by hypolimnetic Ca(OH) 2 treatment: impact on phosphorus sedimentation and release from sediment. Sci Total Environ 409(8):1504–1515
Dodds WK (2010) The role of periphyton in phosphorus retention in shallow freshwater aquatic systems. J Phycol 39(5):840–849
Duhamel S, Björkman KM, Wambeke FV, Moutin T, Karl DM (2011) Characterization of alkaline phosphatase activity in the North and South Pacific Subtropical Gyres: implications for phosphorus cycling. Limnol Oceanogr 56(4):1244–1254
Hantke B, Fleischer P, Domany I, Koch M, Pleß P, Wiendl M, Melzer A (1996) P-release from DOP by phosphatase activity in comparison to P excretion by zooplankton. Studies in hardwater lakes of different trophic level. Hydrobiologia 317(2):151–162
House WA, Denison FH, Armitage PD (1995) Comparison of the uptake of inorganic phosphorus to a suspended and stream bed-sediment. Water Res 29(3):767–779
Kaiserli A, Voutsa D, Samara C (2002) Phosphorus fractionation in lake sediments–lakes Volvi and Koronia, N. Greece. Chemosphere 46(8):1147–1155
Lu H, Yang L, Zhang S, Wu Y (2014) The behavior of organic phosphorus under non-point source wastewater in the presence of phototrophic periphyton. PLoS ONE 9(1):e85910
Lu H, Wan J, Li J, Shao H, Wu Y (2016) Periphytic biofilm: a buffer for phosphorus precipitation and release between sediments and water. Chemosphere 144:2058–2064
Mccormick PV, Iii RBES, Chimney MJ (2006) Periphyton as a potential phosphorus sink in the Everglades Nutrient Removal Project. Ecol Eng 27(4):279–289
Michener WK (2018) Quality assurance and quality control (QA/QC)
Najar I, Khan A, Hai A (2017) Evaluation of seasonal variability in surface water quality of Shallow Valley Lake, Kashmir, India, using multivariate statistical techniques. Pollution 3(3):349–362
Ruban V, Lópezsánchez JF, Pardo P, Rauret G, Muntau H, Quevauviller P (2001) Harmonized protocol and certified reference material for the determination of extractable contents of phosphorus in freshwater sediments–a synthesis of recent works. Fresenius J Anal Chem 370(2–3):224–228
Rydin E, Brunberg AK (1998) Seasonal dynamics of phosphorus in Lake Erken surface sediments. Archhydrobioll Specissues Advanclimno 51:157–167
Solórzano L, Sharp JH (1980) Determination of total dissolved phosphorus and particulate phosphorus in natural waters. Limnol Oceanogr 25(4):754–758
Wang S, Jin X, Zhao H, Wu F (2006) Phosphorus fractions and its release in the sediments from the shallow lakes in the middle and lower reaches of Yangtze River area in China. Colloids Surf A 273(1–3):109–116
Wang S, Jin X, Bu Q, Jiao L, Wu F (2008) Effects of dissolved oxygen supply level on phosphorus release from lake sediments. Colloids Surf A 316(1):245–252
Wang H, Holden J, Spera K, Xu X, Wang Z, Luan J, Xu X, Zhang Z (2013) Phosphorus fluxes at the sediment-water interface in subtropical wetlands subjected to experimental warming: a microcosm study. Chemosphere 90(6):1794–1804
Wu Y (2016) Periphyton: functions and application in environmental remediation. Elsevier, Amsterdam
Wu Y, Zhang S, Zhao H, Yang L (2010) Environmentally benign periphyton bioreactors for controlling cyanobacterial growth. Biores Technol 101(24):9681–9687
Wu Y, Li T, Yang L (2012) Mechanisms of removing pollutants from aqueous solutions by microorganisms and their aggregates: a review. Biores Technol 107(2):10–18
Wu Y, Xia L, Yu Z, Shabbir S, Kerr PG (2014) In situ bioremediation of surface waters by periphytons. Biores Technol 151(1):367–372
Wu Y, Liu J, Rene ER (2017) Periphytic biofilms: a promising nutrient utilization regulator in wetlands. Biores Technol 248(Pt B):44–48
Xie LQ, Xie P, Tang HJ (2003) Enhancement of dissolved phosphorus release from sediment to lake water by Microcystis blooms—an enclosure experiment in a hyper-eutrophic, subtropical Chinese lake. Environ Pollut 122(3):391–399
Yang L, Lei K, Yan W, Li Y (2013) Internal loads of nutrients in Lake Chaohu of China: implications for lake eutrophication. Int J Environ Res 7(4):1021–1028
Zhang XF, Mei XY (2015) Effects of benthic algae on release of soluble reactive phosphorus from sediments: a radioisotope tracing study. Water Sci Eng 8(2):127–131
Zhang X, Liu Z, Jeppesen E (2013) The effect of benthic algae on phosphorus exchange between sediment and; overlying water in shallow lakes: a microcosm study using P-32 as a tracer. Hydrobiologia 710(1):109–116
Zhang Y, He F, Xia S, Zhou Q, Wu Z (2015) Studies on the treatment efficiency of sediment phosphorus with a combined technology of PCFM and submerged macrophytes. Environ Pollut 206:705–711
Zhou Q, Gibson CE, Zhu Y (2001) Evaluation of phosphorus bioavailability in sediments of three contrasting lakes in China and the UK. Chemosphere 42(2):221–225
Acknowledgements
This research was supported by the National Science Foundation of China (grant number 30970550), “the Fundamental Research Funds for the Central Universities”, the South-central University for Nationalities (Grant Number czy18019) ,and the Major Technological Innovation of Hubei Province of China (Grant No.2018ABA093).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Rights and permissions
About this article
Cite this article
Yi, Z., Yang, Y., Yan, C. et al. The Influence of Periphyton Biofilm on Phosphorus Migration in Sediments. Int J Environ Res 13, 327–335 (2019). https://doi.org/10.1007/s41742-019-00182-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41742-019-00182-z