Abstract
The aim of this paper is to assess the feasibility of the co-digestion of olive mill industrial waste with urban sewage sludge from wastewater treatment plants (WWTP). These wastes include olive mill solid waste (OMSW) and olive mill effluent (OME). This co-digestion process enables the energetic exploitation of OMSW and/or OME and introduces an environmental solution for their highly pollutant compounds. The behaviour of both co-substrates was studied using biochemical methane potential assays. To determine the optimal proportions of each co-substrate, different quantities of OME and OMSW were added to a constant quantity of substrate (urban sewage sludge) and put to test. A small amount of digested sludge from WWTP was used as an inoculum to accelerate the pace of the reactions. Thus, the optimal proportions between substrate and co-substrate were defined. The samples containing OMSW showed a biodegradability around 23%, slightly above that of the substrate on its own (21.3%). Moreover, biogas production in samples containing OMSW was significantly superior to samples containing substrate only. Nevertheless, the samples containing OME as a co-substrate presented an inferior biodegradability (20.3%) to that of the substrate on its own. Similarly, biogas production in OME co-digestion remained within the usual values of urban sewage sludge biogas production. In all cases, the system remained stable and the energetic efficiency of the process was improved in comparison to the digestion of substrate only. Therefore, co-digestion with urban sewage sludge proves to be a cost-effective method for OMSW and OME environmental management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Two-phase olive manufacturing produces three identifiable and separable waste streams, namely primary wash water, olive mill solid waste (OMSW) and secondary wash water. These wash waters originate from the primary and secondary centrifugation of olives and are collectively referred to as olive mill effluents (OME) or alpechín. OMSW, also known as alperujo, is an aqueous solid waste generated during primary centrifugation (Hodaifa et al. 2008; Stoller and Chianese 2006). This two-phase manufacturing method involves minimizing water consumption. This leads to OMSW having a steep organic matter concentration, which is the reason why it is a very hazardous pollutant and cannot be handled via traditional treatments. OME and OMSW cause serious environmental concern because of their elevated organic load and low biodegradability (Danellakis et al. 2011; Hodaifa et al. 2013; Karaouzas et al. 2011; Ntougias et al. 2013). The volume of OME per processed olive kg is ≈ 0.25 dm3 (Borja et al. 2006).
This is a very concerning issue in Mediterranean countries (Khoufi et al. 2015). It has been demonstrated that OMSW and OME can be treated via anaerobic digestion at mesophilic temperature (Kosobucki et al. 2008). Anaerobic digestion is an efficient and widespread treatment option due to its excellent waste stabilization and high energy recovery (Pagés-Díaz et al. 2014) (Kelessidis and Stasinakis 2012). In the case of OMSW, COD removal efficiencies in the range of 96.8–82.9% have been achieved in continuous stirred tank reactors (CSTR) with methane yield coefficients in the range of 0.30–0.20 dm3 CH4 g−1 COD removed (operating at OLR of 0.8–15.0 g COD dm−3 day−1 and HRT of 50–10 days). When processed in immobile cell bioreactors, OME reached COD removal efficiencies within the range of 72–89% and an average methane yield coefficient of 0.3 dm3 CH4 g−1 COD removed (OLR of 0.8–5.3 g dm−3 d−1 and HRT of 25–4 days) (Borja et al. 2006).
Although the co-digestion of olive mill waste is not a new idea—the basic process was defined 20 years ago by Lebrato (Lebrato et al. 1995)—it has seen major development only during the last 10 years. Nowadays, most WWTP have oversized anaerobic reactors. This fact makes it possible for tanks to contain additional co-substrate volume without compromising digestion stability.
Biochemical methane potential (BMP) assay is a widespread and reliable tool for the feasibility assessment of different substrate-to-co-substrate ratios (Khoufi et al. 2015) (Veluchamy and Kalamdhad 2017). BMP assays constitute an easy and inexpensive way to assess digester behaviour as well as the biodegradability and methanogenic potential of a certain waste. This allows to take preventive and/or corrective actions (Goncalves Ferreira 2013) (Elbeshbishy et al. 2012). Therefore, this is the method used to determine to what extent the treatment of OMSW and OME is feasible.
Since in a co-digestion process more organic matter is fed to the reactor than in standard digestion, an increase in biogas production and, consequently, in electric energy generation shall occur.
Biogas-generated power in OECD countries grew from 3.7 TWh in 1990 to 78.8 TWh in 2015, making it the third fastest growing renewable electricity source after wind and solar energy. The leaders of this growth are European OECD countries, which accounted for almost 80% of biogas-generated power in 2015 (IEA 2016).
Biogas-based power enables energy storage via biogas storage in large quantities. This way, energy production can be adapted to the ever-varying demand patterns. This flexibility makes biogas an essential renewable energy source. Amongst other goals, this paper seeks to assess whether OME and OMSW are optimal biogas sources.
Mixed sludge from WWTP is mainly used in agriculture after its digestion. A co-digestion process with either OMSW or OME would not prevent it from becoming a reliable soil conditioner or fertilizer. In fact, both OMSW and OME have also promising applications in agriculture after undergoing a stabilization or compost process (Álvarez et al. 2010). As conditioners, OMSW and OME are valued for their ability to improve soil structure (Saveyn and Eder 2014; (Hernández et al. 2017; Dahlin et al. 2017; Weiland 2010).
The goal of this work is to determine the viability of incorporating the described co-substrates (OMWS and OME) to a conventional urban wastewater treatment process; taking advantage of the excessive capacity numerous anaerobic reactors were mistakenly designed with, while not compromising the anaerobic digestion of WWTP-generated sludge. Thus, firstly, anaerobic digestion of sludge with and without co-substrates was compared. Conclusions drawn from this comparison will clarify whether it is recommendable treating the studied co-substrates in a conventional WWTP co-digestion process, and whether this procedure improves methane yield. Moreover, different proportions of substrate and co-substrate have been essayed and eventually the optimal proportions of both have been determined.
Materials and Methods
Materials
Substrate
Mixed sludge (primary and surplus activated sludge, referred to as MS) obtained from El Copero WWTP (Seville, Spain), was the main substrate in the assays described. MS was the reference of choice in this research due to the fact it is usually treated in WWTP anaerobic digesters. It had 24.1 ± 0.6 g TS and 15.9 ± 0.7 g VS per liter. A constant amount of 50 mL of MS was used in all samples. All compounds and samples were stored at a temperature of 4 ºC prior to their use.
The good behaviour of this substrate in anaerobic digestion has been widely demonstrated in numerous studies on this subject (Zhen et al. 2017).
Inoculum
Mesophilic anaerobic digested sludge (also obtained from El Copero WWTP) was used as inoculum in order to accelerate the beginning of the process. It had 18.5 ± 0.5 gL−1 TS and 13.1 ± 0.6 gL−1 VS. The same inoculum was used in all assays to evaluate the response of the digester to different co-substrates (Goncalves Ferreira 2013). The samples, which had been stored at 4 °C, were kept in a hot camera at 35 °C for at least 3 days prior to the beginning of the assays. This protocol was followed to minimize endogenous production, readapting the sludge to assay temperature (Goncalves Ferreira 2013).
Co-substrate
OMSW and OME samples were collected from a two-phase olive oil production plant located in Jaén, Spain. The samples were stored in a freezer at 4 °C between 30 and 40 days prior to use.
The characteristics of these samples are displayed in Table 1.
The high average COD value for OMSW (380.0 ± 0.8 gL−1) in contrast to that of OME (31.9 ± 0.7 gL−1) reveals that the former might be a valuable co-substrate as far as biogas production is concerned.
Methods
Biochemical Methane Potential Test (BMP)
BMP tests were performed on each sample to determine their anaerobic biodegradabilty and the effects of different substrate and co-substrate proportions on biogas production (Angelidaki et al. 2009). BMP tests provide the volume of methane produced in normal conditions per g SV, allowing methane yield to be calculated (Angelidaki et al. 2009; Nielfa et al. 2015a, b).
Sixty transparent 250 mL borosilicate bottles equipped with rubber stoppers were used as batch reactors. These stoppers allow differential pressure measurements. Also, please notice that even though the bottles are commercially labelled to have a capacity of 250 mL, they actually have a volume of 300 mL. In total, each bottle contained 150 mL of sample volume. The remaining 150 mL were left as free space for biogas.
Each reactor contained a sample consisting of 50 mL of substrate and different amounts of either OMSW or OME as co-substrate, in accordance to a ratio of 0.5 g VS (Substrate + Co-substrate)/g VS of inoculum. This unified ratio for all samples makes it possible to compare them. Biodegradability values obtained in the BMP test are strongly affected by the value of this relation (Moreno-Andrade and Buitrón 2003)—the higher the (Substrate + Co-substrate)/Inoculum ratio, the more velocity in biogas production. Taking this into account, such a high g VS (Substrate + Co-substrate)/g VS of inoculum ratio was chosen for our purposes (Angelidaki et al. 2009).
Reactors were kept in mesophilic temperature (35–36 °C) and stirred at 150 rpm over a period of 20–26 days with aid of a New Brunswick Scientific G10—Gyratory Shaker, as described by Soto et al. (1993) and Fdz-Polanco et al. (2005).
The produced gas volume was measured daily (Angelidaki et al. 2009). Biogas composition was determined by taking 20 mL of volume from the reactor with aid of a syringe and injecting it into an NaOH 6 N solution, causing CO2 to precipitate as carbonate. The displaced NaOH volume corresponds to methane volume (Veluchamy and Kalamdhad 2017).
In order for a biodegradability test to be carried out, the environment needs to have all nutrients that microorganisms need to increase their population. Therefore, FeCl3.4H2O, CoCl2·6H2O, MnCl2·4H2O, CuCl2·2H2O, ZnCl2, H3BO3, EDTA, NH4Cl, KH2PO4, CaCl2·H2O and MgSO4·4H2O were added (Angelidaki et al. 2009). In total, these compounds represent 1 mL of volume per liter of inoculum.
HCl or NaOH was added to the reactors to bring pH levels to 7.5. Since the sample decomposition process liberates H+ ions into the medium, a buffer solution was also put into the reactors with the aim to maintain pH constant. Furthermore, the batch digesters were sealed to sustain anaerobic conditions until the end of the test. Finally, the reactors were set into the stirring equipment and kept under controlled temperature. These conditions were intended to emulate the behaviour of an actual WWTP digester.
Biogas volume could be calculated from pressure measurements:
where VBiogas is the biogas volume obtained (mL), Vgas camera is the volume of the gas camera of BMP (150 mL), T is the reference temperature (273 K), P is the reference pressure (1 atm), Tassay is the assay temperature (309 K), and Pmeasured is the measured pressure in the assays (mbar).
Calculations of methane volume are allowed to calculate COD. In turn, COD data are enabled to calculate biodegradability (Soto et al. 1993) (Fdz-Polanco et al. 2005) (Angelidaki et al. 2009). All calculations were carried out by triplicate with the aim to minimize errors.
At the end of the tests, methane yields for all reactors were calculated by dividing either accumulated biogas or total methane volume by the initial substrate VS mass. Methane yield represents the volume of CH4 (mL) produced per mass of added volatile solids (mL CH4 g−1 added VS).
Analytical Methods
Characterization of all substrates and inoculums regarded the following parameters: pH, conductivity, COD, total solids (TS), volatile solids (VS), fixed solids (FS), alkalinity and volatile fatty acids (VFA).
These analytical tests follow the methodology described hereinafter:
pH (activity of H+ ions) was measured with a Hatch HQ40D potentiometer (accuracy ± 0.01). Conductivity was measured with a EUTECH PCD 650 multi-parametric measurer (accuracy ± 0.0001 mS). COD was measured by titration of ferrous ammonium sulphate after a total digestion with Ag2SO4 and potassium dichromate at 150 °C for two hours (APHA 1998). The TS were analysed by weighting the samples before and after being dried at 105 °C. The VS were determined weighting samples after being burned at 550 °C for 20 min (APHA 1998). Alkalinity and VFA were measured according to the standard procedure given in (APHA 1998). Biogas volume was calculated from pressure measurements with aid of an IFM PN5007 pressure transmitter with a range of measurement between 0 and 1 bar ± 1 mbar. The system was calibrated by submitting a bottle to overpressure and taking diary measurements while monitoring no significant pressure loss.
Work Procedures
With regard to COD levels (Table 1), substrate-to-co-substrate ratios have been chosen to resemble WWTP working conditions as closely to reality as possible. In all cases, a C/N ratio of 1.4 was used.
Also, data from OMSW and OME production rates in Andalusian industries were taken into account. Different amounts of co-substrate have been studied with the aim to determine the critical point in which the anaerobic process is destabilized. The samples were prepared adding different amounts of co-substrate to an identical volume (50 mL) of substrate (MS), always maintaining a ratio of 0.5 g VS (Substrate + Co-substrate)/g VS inoculum.
Table 2 displays the amounts of substrate and co-substrate present in each sample.
To contrast digestion and co-digestion processes of OMSW and OME, the assays included the digestion of these wastes on their own, as well as in co-digestion with MS. The concept of methane yield was fundamental to the processes assessment. Methane yield constituted the criteria to determine whether the co-digestion of OMSW and OME played a synergic or additive role in the overall process. Consequently, when the addition of the substrate and the co-substrate individual methane yields is significantly superior to the methane yield of substrate and co-substrate in co-digestion, an additive relationship exists between substrate and co-substrate. In this case, the increase in CH4 production during co-digestion in comparison to digestion of the substrate is indebted to a superior COD in the reactor. Providing that the co-digestion methane yield remains superior to the methane yield of the substrate on its own, this relationship between substrate and co-substrate guarantees the feasibility of the process and confirms the fact that MS and olive mill waste can indeed be processed together.
On the contrary, if the methane yield of a sample in co-digestion is approximately equal or superior to the addition of the substrate and co-substrate individual methane yields, a synergic relationship exists between substrate and co-substrate.
Results and Discussion
The results described here demonstrate, in first place, that the exploitation of OMSW and OME as co-substrates with MS is a viable process. In the light of this assessment, the behaviour of the different substrate/co-substrate samples is analysed. Finally, the optimal proportion of substrate and co-substrate is determined.
Performance in Depuration Parameters
With regard to the results obtained in the assays with MS + OMSW in three different proportions (Table 3), it is apparent that the COD removal percentage was significantly higher in MS + OMSW-2 (50.1%) in comparison to MS + OMSW-1 (43.6%) and MS + OMSW-3 (36.1%.). VS removal was similar in MS + OMSW-1 and MS + OMSW-2 samples—approximately 25% in both. Nevertheless, the MS + OMSW-3 sample experimented a dramatic rise in the VS removal rate. Even though MS + OMSW-1, the sample containing the least amount of OMSW, displayed acceptable COD and VS removal rates, its performance was the poorest amongst the OMSW samples. A superior OMSW concentration, as in the case of MS + OMSW-2, saw maximum performance levels. Further rise in OMSW proportion, as tested on the MS + OMSW-3 assay, was accompanied by a decrease in the COD removal and an increase in the VS removal rates—microbial consortia were not able to assimilate such a quantity of organic matter and the system surpassed its saturation point due to organic overload.
In fact, this is reflected in daily biogas production data (Figs. 1, 2)—from day 15 on, a decrease in daily biogas production by MS + OMWS-3 with respect to MS + OMSW-2 can be observed.
This decrease may be explained by the fact that less biomass (SV) was measured in these samples, considering that all reactors had a similar COD (0.5 g SV (Substrate + Co-substrate)/g VS inoculum ratio). Therefore, a decrease in biogas production is determined by a decrease in VS, as it can be observed in the assay results of Table 3. This confirms VS decrease as an effect of overfeeding.
These graphics point that the MS + OMSW-2 biogas production during weeks 2 and 3 was nearly equal (171 mL in week 2 and 179 mL in week 3). Nonetheless, MS + OMSW-3 had a different behaviour—unlike MS + OMSW-2, its production did not stabilize but kept growing throughout weeks 1 and 2 (305 ± 6 mL during week 1 and 258 ± 5 mL during week 2). From that point on, MS + OMSW-3 production drops dramatically, with only 118 mL in comparison to MS + OMSW-2’s 179 mL during week 3.
Therefore, the optimal proportion of MS and OMSW is represented by MS + OMSW-2, whose microbial consortia were able to adapt to the quantity of organic matter and achieve high COD removal rates while maintaining their VS removal rate.
Regarding MS + OME assays, it is noted that the COD removal rate in MS + OME-1 and MS + OME-2 was similar, but the VS removal rate was slightly superior in MS + OME-2. Therefore, a small increment in OME can induce the VS removal rate to rise and the COD removal rate to decrease, resulting in the anaerobic system reaching its saturation point. According to these results, both samples are stable in anaerobic co-digestion, with MS + OME-2 even approaching its saturation point.
Alkalinity increases in all cases towards the end of the assay, with near-optimal concentrations (Gerardi 2003) in MS + OMSW-2 and MS + OMSW-3—denoting a great buffer capacity of the solutions towards possible pH changes. This buffer solution keeps the reactor from suffering acidification and keeps pH within optimal values for methanogenic microorganisms. These VFA, alkalinity and pH values reflect the good working-state of the system as far as stability against possible variations is concerned—thus, ensuring the system’s reliability.
AGV/alkalinity ratio in all samples (acetic acid equivalent/CaCO3 equivalent) and in MS is similar and widely below limit values.
Biodegradability
The biodegradability percentage of the substrate (MS), based on methane, was of 21.3 ± 1.1%. All samples containing OMSW as a co-substrate were highly biodegradable, with values of 22.5 ± 0.9% for MS + OMSW-2 and slightly higher for MS + OMSW-1 and 3, whose values were of 23%. In all cases, the biodegradability of the samples stayed slightly above the biodegradability of the substrate MS. On the contrary, OME behaved worse in terms of biodegradability, with the same value of 20.3 ± 1.1% for both samples (MS + OME-1 and MS + OME-2). In conclusion, OMSW improved biodegradability in all three samples, whereas OME did not in any of them.
Biogas Production
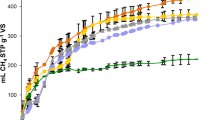
Figure 1 displays the accumulated biogas production during the MS-OMSW assay in comparison to the accumulated biogas production during the reference substrate (MS) anaerobic digestion. At first, all assays shared a similar gradient due to going through a latency phase during the first days, while the microbial consortia adapted to the different samples (Nielfa et al. 2015a, b). From day 5, biogas volumes began to differ from sample to sample. MS + OMSW-1 reached its maximum biogas production on day 17 with a total of 364 ± 8 mL. MS + OMSW-2 and MS + OMSW-3 (which contained more co-substrate quantity then MS + OMSW-1) reached maximum production on day 26 with 437 ± 8 and 423 ± 9 mL, respectively. MS + OMSW-2 achieved the highest biogas production of all three. It was, thus, verified that MS + OMSW-2 contained the optimal proportions for anaerobic co-digestion with 96 more biogas mL than the reference MS anaerobic digestion while remaining stable.
Figure 3 shows biogas production during the MS + OME assay in comparison to the biogas production accumulated in the anaerobic degradation of the reference substrate (MS). For the first 6 days of the assay, MS + OME-1 had a very similar behaviour to MS, with bacteria adapting to the sample in the same manner as to MS. As it can be observed in the Fig. 3, from day 7 the accumulated biogas volume exceeds by far the MS mark. Biogas production continued to rise and peaked on day 22 with 361 ± 23 mL.
The MS + OME-2 sample suffered a decrease in biogas production on day 4, followed by a rise from day 5 on, and surpassing the MS biogas production on day 7.
The addition of OME did not cause a significant increase in the produced biogas volume in comparison to that of the MS.
This indicates that the anaerobic co-digestion of both MS + OME-1 and MS + OME-2 is viable with approximately the same biogas quantity being produced (361 ± 24 and 363 ± 20 mL, respectively), although it should be noted that MS-OME-2 was added 2 more OME g than MS + OME-1. We would like to emphasize that even though MS + OME-2 did not produce more biogas, it did handle a bigger volume of highly pollutant waste without altering the functioning of the station.
Methane Yield
As one can see in Fig. 2 from day 5 to day 17, MS + OMSW-1 registered a higher methane yield than all other samples, peaking on day 17 with 373 ± 13 mL CH4 g−1 added VS and then decaying. Even though the gradient in MS + OMSW-2 is inferior to that of MS + OMSW-1 until day 21 and to that of MS + OMSW-3 until day 16, it is apparent that after that point the MS + OMSW-2 methane yield is significantly superior. After a 26-day study period, MS + OMSW-2 had still not reached maximum methane yield.
All three samples showed a methane yield above the reference substrate. The 443 ± 13 mL CH4 g−1 added VS methane yield from MS + OMSW-2 was a noteworthily high measurement, yet again demonstrating that MS + OMSW-2 represented the optimal substrate/co-substrate proportion for anaerobic co-digestion.
Despite the fact that the MS + OMSW-3 contains more OMSW, its methane yield was lower because of the microbial consortia not having adapted properly due to the high OMSW quantities.
Figure 4 illustrates the fact that, during the first 6 days, the reference substrate (MS) methane yield is superior to that of all samples. The microbial consortia adaptation period is extended in the presence of OME because it is a highly pollutant waste with many difficult-to-biodegrade polyphenols (Gunay and Karadag 2015). Since MS + OME-2 contains more OME, its methane yield is inferior to the others during the first days.
From day 7, the methane yields in both MS + OME-1 (353 ± 28 mL CH4 g−1 added VS) and MS + OME-2 (374 ± 24 mL CH4 g−1 added VS) were beyond the values of the reference substrate (333 ± 15 mL CH4 g−1 added VS).
MS-OME-2 had a higher methane yield, as it was expectable due to the bigger quantity of OME as organic matter feed for the system. Thus, despite being OME a difficult-to-biodegrade compound, the anaerobic digestion process was able to handle the large OME proportion in MS + OME-2.
Nonetheless, the difference in methane yield between the different samples is not proportional to the increase in the organic charge MS + OME-2 was subject to, in comparison to MS + OME-1. Therefore, we can conclude that a bigger volume of OME can be treated via anaerobic digestion, even though the overall biogas quantity in the reactor will not increase.
In all cases, sludge co-digestion with OMSW or OME resulted in a superior methane production in comparison to anaerobic WWTP MS digestion processes. Therefore, co-digestion increases power generation by biogas co-generation in WWTP and, thus, enhances their electrical self-sufficiency.
To determine whether this increase is indebted to an additive or synergic effect, the behaviour of each waste was analysed in digestion on its own as well as taking part in a co-digestion process.
In the light of the results obtained and taking into account the criteria explained in the “Materials and Methods” section, the following observations can be made (Table 4).
MS + OMSW samples:
In all OMSW samples, the methane yields of substrate and co-substrate combined (465 mL CH4 g−1 added VS) were superior to the co-digestion methane yield. Nevertheless, co-digestion values fit in a very similar range—especially in the case of MS + OMSW-2. Therefore, co-digestion has proven feasible and the registered biogas production can be explained by a synergic effect. In all samples, co-digestion offered a higher biogas production in comparison to MS-only digestion in WWTP.
MS + OME samples:
In all OME samples, the methane yields of substrate and co-substrate combined (706 mL CH4 g−1 added VS) are superior to the co-digestion methane yield level. In all cases, co-digestion has proven to be feasible and its methane yield was above that of MS-only digestion. Substrate and co-substrate have an additive relationship and no synergic effect is present.
Notice that both co-substrates could be digested without negatively affecting MS digestion in any way. That is to say, it is possible to co-digest olive mill wastewater (OMSW) in conventional WWTP via anaerobic digestion with MS. Moreover, OMSW co-digestion in WWTP increases the power the plant can generate for its own use.
Conclusions
The studied co-substrates, namely olive mill solid waste (OMSW) and olive mill effluents (OME), have proven to have a similar biodegradability level to the mixed sludge that is commonly found in wastewater treatment plants.
The addition of OMSW as co-substrate caused a significant increase in the biogas production, between 10 and 30% depending on sample compositions. On the contrary, the addition of OME did not modify biogas production.
Both co-substrates increased methane yield, despite the small decrease of biodegradability caused by OME.
MS + OMSW-2 and MS + OME-2 were the samples with best overall behaviour. They produced the highest biogas values, namely, 437 ± 8 and 363 ± 20 mL, respectively. They were also the ones to have the highest productivity with, respectively, 443 ± 13 mL CH4 g−1 added VS and 374 ± 24 mL CH4 g−1 added SV while keeping the digester stable.
This research demonstrates the potential of co-digestion of olive oil production waste with mixed sludge from urban wastewater treatment plants and its capability to achieve a great sustainability of this industrial sector in the Mediterranean area.
This technology eliminates polluting waste from the environment while exploiting their methane yield in order to generate electric energy that WWTP can take advantage of. The produced electrical energy introduces economic savings and a decrease in CO2 emissions, making the process advantageous from both an economic and ecological perspective.
Abbreviations
- OMSW:
-
Olive mill solid waste
- OME:
-
Olive mill effluents
- WWTP:
-
Urban wastewater treatment plant
- I:
-
Inoculum
- S:
-
Substrate
- BMP:
-
Biochemical methane potential
- TS:
-
Total solids
- VS:
-
Volatile solids
- FS:
-
Fixed solids
- MS:
-
Mixed sludge
- COD:
-
Chemical oxygen demand
- VFA:
-
Volatile fatty acids
- OLR:
-
Organic loading rate
- HRT:
-
Hydraulic retention time
References
Álvarez JA, Otero L, Lema JM (2010) A methodology for optimising feed composition for anaerobic co-digestion of agro-industrial wastes. Bioresour Technol 101:1153–1158. https://doi.org/10.1016/j.biortech.2009.09.061
Angelidaki I, Alves M, Bolzonella D, Borzacconi L, Campos JL, Guwy AJ, Kalyuzhnyi S, Jenicek P, van Lier JB (2009) Defining the biomethane potential (BMP) of solid organic wastes and energy crops: a proposed protocol for batch assays. Water Sci Technol 59:927. https://doi.org/10.2166/wst.2009.040
APHA (1998) Standard methods for the examination of water and wastewater, 20th edn. ISBN 087553-161-X
Borja R, Rincón B, Raposo F (2006) Anaerobic biodegradation of two-phase olive mill solid wastes and liquid effluents: kinetic studies and process performance. J Chem Technol Biotechnol 81:1450–1462. https://doi.org/10.1002/jctb.1563
Dahlin J, Nelles M, Herbes C (2017) Biogas digestate management: evaluating the attitudes and perceptions of German gardeners towards digestate-based soil amendments. Resour Conserv Recycl 118:27–38. https://doi.org/10.1016/j.resconrec.2016.11.020
Danellakis D, Ntaikou I, Kornaros M, Dailianis S (2011) Olive oil mill wastewater toxicity in the marine environment: alterations of stress indices in tissues of mussel Mytilus galloprovincialis. Aquat Toxicol 101:358–366. https://doi.org/10.1016/j.aquatox.2010.11.015
Elbeshbishy E, Nakhla G, Hafez H (2012) Biochemical methane potential (BMP) of food waste and primary sludge: influence of inoculum pre-incubation and inoculum source. Bioresour Technol 110:18–25. https://doi.org/10.1016/j.biortech.2012.01.025
Fdz-Polanco F, Nieto P, Pérez Elvira S, van der Zee FP, Fdz-Polanc M, García PA (2005) Automated equipment for anaerobic sludge parameters determination. Water Sci Technol 52:479LP–485LP
Gerardi MH (2003) The microbiology of anaerobic digesters. Wiley, New York
Goncalves Ferreira LC (2013) Evaluación de la biodegradabilidad anaerobia de residuos orgánicos pre-tratados térmicamente. Valladolid
Gunay A, Karadag D (2015) Recent developments in the anaerobic digestion of olive mill effluents. Process Biochem 50:1893–1903. https://doi.org/10.1016/j.procbio.2015.07.008
Hernández B, León E, Martín M (2017) Bio-waste selection and blending for the optimal production of power and fuels via anaerobic digestion. Chem Eng Res Des 121:163–172. https://doi.org/10.1016/j.cherd.2017.03.009
Hodaifa G, Martínez ME, Sánchez S (2008) Use of industrial wastewater from olive-oil extraction for biomass production of Scenedesmus obliquus. Bioresour Technol 99:1111–1117. https://doi.org/10.1016/j.biortech.2007.02.020
Hodaifa G, Ochando-Pulido JM, Rodriguez-Vives S, Martinez-Ferez A (2013) Optimization of continuous reactor at pilot scale for olive-oil mill wastewater treatment by Fenton-like process. Chem Eng J 220:117–124. https://doi.org/10.1016/j.cej.2013.01.065
IEA (2016) Renewables information. International energy agency, pp 488. https://doi.org/10.1787/renew-2016-en
Karaouzas I, Skoulikidis NT, Giannakou U, Albanis TA (2011) Spatial and temporal effects of olive mill wastewaters to stream macroinvertebrates and aquatic ecosystems status. Water Res 45:6334–6346. https://doi.org/10.1016/j.watres.2011.09.014
Kelessidis A, Stasinakis AS (2012) Comparative study of the methods used for treatment and final disposal of sewage sludge in European countries. Waste Manag 32:1186–1195. https://doi.org/10.1016/j.wasman.2012.01.012
Khoufi S, Louhichi A, Sayadi S (2015) Optimization of anaerobic co-digestion of olive mill wastewater and liquid poultry manure in batch condition and semi-continuous jet-loop reactor. Bioresour Technol 182:67–74. https://doi.org/10.1016/j.biortech.2015.01.092
Kosobucki P, Buszewski B, Górski Ł (2008) The influence of temperature on the process of dynamic methane fermentation of sewage sludge. Polish J Environ Stud 17:369–375
Lebrato J, Pérez-Rodríguez JL, Maqueda C (1995) Domestic solid waste and sewage improvement by anaerobic digestion: a stirred digester. Resour Conserv Recycl. https://doi.org/10.1016/0921-3449(94)00011-S
Moreno-Andrade I, Buitrón G (2003) Influence of the initial substrate to microorganisms concentration ratio on the methanogenic inhibition test. Water Sci Technol 48:17–22
Nielfa A, Cano R, Fdz-Polanco M (2015a) Theoretical methane production generated by the co-digestion of organic fraction municipal solid waste and biological sludge. Biotechnol Rep 5:14–21. https://doi.org/10.1016/j.btre.2014.10.005
Nielfa A, Cano R, Vinot M, Fernández E, Fdz-Polanco M (2015b) Anaerobic digestion modeling of the main components of organic fraction of municipal solid waste. Process Saf Environ Prot 94:180–187. https://doi.org/10.1016/J.PSEP.2015.02.002
Ntougias S, Gaitis F, Katsaris P, Skoulika S, Iliopoulos N, Zervakis GI (2013) The effects of olives harvest period and production year on olive mill wastewater properties—evaluation of Pleurotus strains as bioindicators of the effluent’s toxicity. Chemosphere 92:399–405. https://doi.org/10.1016/j.chemosphere.2013.01.033
Pagés-Díaz J, Pereda-Reyes I, Taherzadeh MJ, Sárvári-Horváth I, Lundin M (2014) Anaerobic co-digestion of solid slaughterhouse wastes with agro-residues: synergistic and antagonistic interactions determined in batch digestion assays. Chem Eng J. https://doi.org/10.1016/j.cej.2014.02.008
Saveyn H, Eder P (2014) End-of-waste criteria for biodegradable waste subjected to biological treatment (compost & digestate): technical proposals. Publications Office of the European Union. http://ftp.jrc.es/EURdoc/JRC87124.pdf
Soto M, Méndez R, Lema JM (1993) Methanogenic and non-methanogenic activity tests. Theoretical basis and experimental set up. Water Res 27:1361–1376. https://doi.org/10.1016/0043-1354(93)90224-6
Stoller M, Chianese A (2006) Optimization of membrane batch processes by means of the critical flux theory. Desalination 191:62–70. https://doi.org/10.1016/j.desal.2005.07.021
Veluchamy C, Kalamdhad AS (2017) Biochemical methane potential test for pulp and paper mill sludge with different food/microorganisms ratios and its kinetics. Int Biodeterior Biodegradation 117:197–204. https://doi.org/10.1016/j.ibiod.2017.01.005
Weiland P (2010) Biogas production: current state and perspectives. Appl Microbiol Biotechnol 85:849–860. https://doi.org/10.1007/s00253-009-2246-7
Zhen G, Lu X, Kato H, Zhao Y, Li Y-Y (2017) Overview of pretreatment strategies for enhancing sewage sludge disintegration and subsequent anaerobic digestion: current advances, full-scale application and future perspectives. Renew Sustain Energy Rev 69:559–577. https://doi.org/10.1016/j.rser.2016.11.187
Acknowledgements
The authors would wish to thank the Empresa Metropolitana de Abastecimiento y Saneamiento de Aguas de Sevilla, EMASESA, for their technical support and supplies of mixed sludge and inoculum they provided us with. They would also like to thank the co-operative society AGROSEGURA for the co-substrates they provided for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Benito-Mora, C., Alonso-Contreras, A.J., Garvi, D. et al. Olive Mill Industrial Waste as Co-substrate in Anaerobic Digestion with Aim at its Energetic Exploitation. Int J Environ Res 12, 713–723 (2018). https://doi.org/10.1007/s41742-018-0123-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41742-018-0123-x