Abstract
There are thousands of commercially available dyes which represent 7 × 105 tons produced annually worldwide. Discharges of these dyes from dyeing industry effluents have the potential to reach aquatic environments, affecting many life forms. Methylene Blue (MB) is the most common dye used in textile industries. Congo Red (CR) is an azo-based dye that can be metabolized in benzidine, a human carcinogen agent. In this study, polymeric beads made of chitosan and cellulose were used to remove simultaneously a cationic dye (MB) and a anionic dye (CR). The sorption capacity of the beads was evaluated using different dye concentrations under individual (MB or CR) and simultaneous (MB and CR) conditions. Dyes’ concentration in solution was determined by HPLC–DAD. The results evidenced that both dyes can be adsorbed individually and simultaneously on the polymeric beads. The sorption process fitted the Langmuir’s isotherm. The qmax values for CR and MB simultaneous adsorption were 1.6 and 0.68 µmol/g, respectively. The chitosan/cellulose beads exhibited a large adsorption capacity for CR on individual and simultaneous experiments. Adsorption mechanism involves electrostatic interactions, hydrogen bonds and dipole-dipole forces between the functional groups of the polymeric beads and the dyes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pollutants such as infectious agents and toxic chemicals are risk factors that affect water quality. Industries such as textile, leather, paper, and other dyestuffs, use dyes to color their products. More than 100,000 commercially available dyes, representing over 7 × 105 tons, are produced annually worldwide. Discharges of these dyes from industrial effluents have the potential to reach aquatic environments and affect aquatic flora by reducing the transmission of sunlight through water thus affecting photosynthetic processes (Gupta and Suhas 2009).
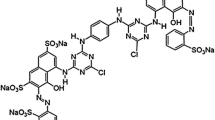
Dyes are classified into cationic (basic dyes), anionic (acid dyes) and nonionic dyes. Methylene blue (MB), a cationic dye, is a brightly blue-colored cationic thiazine dye that is mostly used for dyeing cotton, wood, and silk. MB can cause problems to human health such as skin irritation and eye burns (Rafatullah et al. 2010; Salleh et al. 2011). Congo Red (CR), an anionic azo dye, is a persistent contaminant in natural environments. This azo dye can be metabolized in benzidine, which is a human carcinogen (Chatterjee et al. 2009).
Wastewaters containing dyes and metals are difficult to treat with conventional cleaning methods (Moosavian and Moazezi 2016; Eberhard and Hamawand 2017). Dyes are also resistant to aerobic digestion and oxidizing agents. Therefore, the development of novel and biocompatible materials to remove dyes from aqueous solutions becomes indispensable. Among the methods available for water treatment, adsorption techniques are widely used due to their simplicity, high efficiency, easy recovery, and reusability of the adsorbent (Musyoka et al. 2014).
Emerging adsorption technologies like the use of biosorbents are more efficient, inexpensive, and selective than traditional ion-exchangers (Rafatullah et al. 2010; Crespo-Alonso et al. 2013; Nurchi et al. 2014). Biosorbents fabricated from chitosan and cellulose have been used to remove different water contaminants. Chitosan is a nitrogenous polysaccharide obtained from the deacetylation of chitin. It is the most promising adsorbent and has been used to remove metals, and anionic dyes from aqueous solutions (Crini and Badot 2008; Osifo et al. 2008; Mahmoodi et al. 2011; Wan Ngah et al. 2011). Cellulose is the most abundant biopolymer in nature and has similar chemical structure as chitosan. Cellulose has been reported to remove cationic dyes from aqueous samples (Musyoka et al. 2014).
The present study has evaluated the simultaneous adsorption of a cationic (methylene blue) and anionic (Congo red) dye onto a composite biosorbent made of chitosan and cellulose. Although, chitosan and cellulose (separately or mixed) have been used to remove dyes, they have never been used to remove a mixture of cationic and anionic dyes. The co-existence of functional groups on the polymeric composite allows removing simultaneously both types of dyes. Also, a HPLC method to quantify both dyes was developed.
Materials and Methods
Reagents
Chitosan powder, medium molecular weight (deacetylation grade, 75–85%), and methylene blue (dye content > 82%) were provided by Sigma-Aldrich. Cellulose, microcrystalline, was purchased from Baker TLC Reagent. Congo red, triethylamine (HPLC grade), acetic acid, glacial (certified ACS), were purchased from Fisher Scientific. Formic acid (97%) was purchased from Alfa Aesar. Methyl alcohol (HPLC grade, 99.9%) and sodium hydroxide, pellets (extra pure) were provided by Acros Organics. All reagents were used as received. Deionized water was used to prepare all solutions.
Synthesis of Chitosan/Cellulose Beads
Chitosan/cellulose beads were prepared immobilizing the cellulose into chitosan. The method was developed using previous works (Chatterjee et al. 2009; Wan Ngah et al. 2011). In brief, 2.0 g of chitosan powder was dissolved into 5% (v/v) acetic acid and stirred for 1 h. Then, 2.0 g of cellulose powder was added into chitosan solution under continuous stirring, and the suspension was stirred overnight at room temperature. The obtained solution was added drop wise using a peristaltic pump into a 0.5 M NaOH solution under continuous stirring. In this step, the acetic acid is neutralized promoting the coagulation of the chitosan to produce spherical beads. The chitosan/cellulose beads were rinsed with deionized water and finally air-dried.
Characterization of Chitosan/Cellulose Beads
SEM Measurements
Surface morphology of pure and dye-loaded beads was examined by scanning electron microscopy (JEOL, JSM-5410LV) at an accelerating voltage of 25 kV. Samples were coated with gold before scanning. SEM images were taken at different magnifications (between 50× and 7,500×).
Image Stacking Photography
Image stacking photography equipment (Visionary Digitals, BK Plus Lab System) was used for low magnification observation of beads and dye-loaded beads. This optical microscopy technique was used to obtain high resolution images and determine the size of the beads.
Point of Zero Charge
The point of zero charge (PZC) of the beads was determined in presence of NaCl at different pH values. The procedure was modified from previous work (Lopez-Morales et al. 2012). In brief, 20 mL of 0.1 M NaCl solutions was added to a series of flasks. The initial pH (pH i ) was adjusted between 2 and 12 by adding 0.1 M of HCl or NaOH. About 0.15 g of composite beads were added to each flask and shaken for 48 h at 200 rpm. The final pH value (pH f ) of the supernatant in each flask was measured. The difference between pH i and pH f (ΔpH = pH i − pH f ) was plotted against pH i . The pHPZC of the material was determined as the point of intersection of the resulting curve with the x axis.
Batch Adsorption Studies
For adsorption studies, samples between 5 and 80 µM of MB and CR adjusted to 6.50 pH was used. The polymeric beads (10 g/L) were added to the solutions and shaken at 50 rpm. The adsorption of MB and CR was evaluated individually and then in presence of both dyes (simultaneous experiments). All adsorption experiments were carried out in triplicates until the equilibrium was reached.
Analyses of Solutions
An Agilent 1100 series equipped with a binary pump, auto sampler, diode array detector (DAD) and a C18 column was used to determine the concentration of dyes in the aqueous solutions. A binary gradient elution system composed of 0.1% formic acid (A) and 0.1% triethylamine in methanol (pH 6.45, adjusted with formic acid) (B) was applied as follow: 0.0–2.00 min, 60% B, with a flow rate 1.00 mL/min; 2.00–3.00 min, 60–100% B, with a flow rate 1.00–1.20 mL/min; 3.00–7.00 min, 100% B, with a flow rate of 1.20 mL/min. The injection volume was 20 µL, the column temperature was maintained at 35 °C, and the DAD detector was set at 661 nm (MB) and 510 nm (CR).
Results and Discussions
Beads Characterization
Scanning Electron Microscopy
Surface morphology of composite beads (pure and dye-loaded beads) was observed at 1500× magnification (Fig. 1). SEM images revealed that the surface of composite beads was rough and irregular. After contacting with the dyes, the beads surface became somehow smoother, which could be related to the development of a thin layer of dyes onto the surface of the beads. Similar changes in SEM micrographs have been reported for the adsorption of CR on chitosan hydro-beads (Chatterjee et al. 2009).
Image Stacking Photography
Low magnification images of dyes adsorbed beads (Fig. 2) showed a change on color from its original yellowish color to blue (MB-adsorbed) and red (CR-adsorbed) in single solutions. The color of the composite beads was purple when both dyes (MB and CR) are adsorbed. The average size for as-synthesized beads was 1.6 ± 0.2 mm.
Point of Zero Charge
Point of Zero Charge (PZC) indicates the pH conditions at which the particle surface exhibits a net positive or negative charge (Kosmulski 2014). The synthesized composite beads have a pHPZC of 6.71 ± 0.07. Under this pH, the surface should exhibit a positive charge due to the protonation of chitosan amine groups. The adsorption studies were carried out at 6.5 pH value (below pHPZC) to simulate the pH of natural water, which usually ranges between 6.50 and 8.50.
Dyes Adsorption onto Beads
MB and CR Individual Experiments
The corresponding dye removal efficiency by the chitosan/cellulose beads was determined using the following relationship:
where C0 is the initial dye concentration, and Ce is the dye equilibrium concentration in solution.
Figure 3 shows the removal efficiency of MB and CR (individually) onto chitosan/cellulose composite beads as a function of contact time. The adsorption equilibrium was established within 24 h for MB and 6 h for CR. About 58.2 and 99.9% of MB and CR, respectively, was adsorbed from starting 10 µM of each dye and 10 g/L of composite beads. Evidently, the polymeric composite was capable of adsorbing both dyes with a higher affinity for CR.
The affinity of MB and CR to the polymeric beads can be better understood from the chemical structure of the adsorbent and the dyes. Chitosan is a biopolymer with abundant –OH and –NH2 groups. At pH 6.5 (under chitosan pKa, 6.71), around 61.8% of amine groups of the chitosan are protonated (+charge) favoring the electrostatic interaction with the sulphonic groups (−charge) of the CR (anionic dye). Also, at 6.5 pH (over CR pKa, 5.5) around 91% of amine groups of the CR are deprotonated avoiding any electrostatic repulsion with chitosan (Fig. 4a). In addition, hydrogen bonds between the amine groups of the CR and the hydroxyl groups of the chitosan and cellulose can increase the affinity.
In the other hand, MB (cationic dye) has a (+) charge at pH 6.5 allowing ion–dipole interactions with the hydroxyl groups of the chitosan and cellulose. Also, at this pH (under MB pka, 5.9), around 80% of amine groups of the MB are deprotonated allowing the generation of hydrogen bonds with the hydroxyl groups of the chitosan (Luo and Zhang 2009) and cellulose (Fig. 4b). CR has the strongest bonds with the polymeric beads which support our results.
MB and CR Simultaneous Experiments
The removal efficiency of co-existing MB and CR onto the synthesized beads as a function of contact time is shown in Fig. 5. The equilibrium condition was reached within 5 h for both dyes for starting 10 µM of MB and CR. About 62% of MB and 72% of CR were adsorbed under these conditions.
Effect of Dye Initial Concentrations on Individual and Simultaneous Experiments
Table 1 shows the effect of initial dyes concentration on adsorption studies. The removal efficiency decreased with an increase in the initial dye concentration. At high concentrations of dyes, the adsorption sites on the adsorbent surface became saturated (Salleh et al. 2011). CR was higher adsorbed than MB in individual and simultaneous sorption experiments.
For simultaneous experiments, the total molar concentration for both dyes are the double than single experiments, so that, a decrease in the removal efficiency was observed due to a saturation of adsorption sites. Besides the adsorption patron, a decrease in the CR adsorption was observed. This effect could be attributed to the enhancement of the electrostatic interactions between the negative charge of CR adsorbed onto beads surface and the positive charge of MB (Fig. 6). In other words, once the CR was adsorbed onto the beads, it helped to increase the MB uptake by synergism. A similar phenomenon was observed in the adsorption of MB and methyl orange onto carboxymethyl cellulose sorbent (Yan et al. 2011).
Adsorption Isotherms
MB and CR Adsorption in Individual and Simultaneous Experiments
Adsorption tests at different initial concentrations of MB or CR were used to determine the corresponding equilibrium isotherms. Although both Freundlich and Langmuir models were evaluated, the results better fitted to the Langmuir’s isotherm relationship. Langmuir’s equation (Eq. 2) was used to calculate the maximum adsorption capacities of the beads for each type of dye (Foo and Hameed 2010).
Here, Ce is the concentration of adsorbate at equilibrium (µM), qe the solid phase concentration of adsorbate at equilibrium (µmol of dye/g of adsorbent), qmax the maximum uptake capacity for a monolayer up to saturation (µmol of dye/g of adsorbent), and b is the affinity of the adsorbate for the adsorbent (L/µmol).
Figure 7a shows the adsorption isotherm of MB for individual adsorption. In addition, Fig. 7b shows the adsorption isotherms of MB and CR for simultaneous adsorption onto chitosan/cellulose beads. The good fitting of the experimental data to the Langmuir’s model suggests the establishment of a monolayer of the corresponding dye onto the composite beads. The CR isotherm was not plotted due to all dye was adsorbed under the worked concentrations.
Langmuir constant (b) and monolayer capacity were estimated from the intercept and slope of Eq. (2), respectively. The separation factor, RL, is a dimensionless constant that helps to assess the adsorption process. This factor was calculated from the following equation and using the Langmuir’s parameter previously determined:
where b is Langmuir isotherm constant, and C0 initial MB concentration (µM). Values of: 0 < RL < 1; RL > 1; RL = 1; and, RL = 0 represents conditions at which the adsorption is favorable, unfavorable, linear, or irreversible, respectively.
Table 2 shows the qmax, b values, RL, and linear correlation for MB and CR for individual and simultaneous adsorption experiments. The qmax (maximum uptake capacity) for the MB in individual experiments was estimated at 1.5 ± 0.2 µmol/g; and, qmax for MB and CR in simultaneous experiments were 0.68 ± 0.10 and 1.6 ± 0.1 µmol/g, respectively. The parameters confirm that CR is better adsorbed than MB onto the chitosan/cellulose beads. The RL values for the adsorption of MB and CR for individual and simultaneous adsorption were between 0 and 1 suggesting the dyes adsorption onto chitosan/cellulose beads was a favorable process.
Conclusions
The capability of the chitosan/cellulose beads to simultaneously remove MB and CR species from water have been demonstrated. All adsorption data fitted the Langmuir’s model suggesting the formation of a monolayer. The composite beads can remove 58.2 and 99.9% of MB and CR, respectively, in individual experiments. When the two dyes co-existed in the same solution, the CR firstly adsorbed (72%) onto the beads surface would have helped to increase MB uptake (62%). The corresponding adsorption processes should have been promoted by electrostatic interactions between the functional groups of each polymer, chitosan or cellulose, and the corresponding functional groups in CR and MB species. Synthesized beads can be considered a biocompatible and environmentally friendly candidate for simultaneous adsorption of dyes in aqueous solutions.
References
Chatterjee S, Lee DS, Lee MW, Woo SH (2009) Congo red adsorption from aqueous solutions by using chitosan hydrogel beads impregnated with nonionic or anionic surfactant. Bioresour Technol 100(17):3862–3868
Crespo-Alonso M, Nurchi VM, Biesuz R, Alberti G, Spano N, Pilo MI, Sanna G (2013) Biomass against emerging pollution in wastewater: ability of cork for the removal of ofloxacin from aqueous solutions at different pH. J Environ Chem Eng 1(4):1199–1204
Crini G, Badot P-M (2008) Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: a review of recent literature. Prog Polym Sci 33(4):399–447
Eberhard FS, Hamawand I (2017) Selective electrodialysis for copper removal from brackish water and coal seam gas water. Int J Environ Res 11(1):1–11
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156(1):2–10
Gupta VK, Suhas VK (2009) Application of low-cost adsorbents for dye removal—a review. J Environ Manag 90(8):2313–2342
Kosmulski M (2014) The pH dependent surface charging and points of zero charge. VI. Update. J Colloid Interface Sci 426:209–212
Lopez-Morales J, Perales-Perez O, Roman-Velazquez FR (2012) Sorption of triclosan onto tyre crumb rubber. Adsorpt Sci Technol 30(12):831–845
Luo X, Zhang L (2009) High effective adsorption of organic dyes on magnetic cellulose beads entrapping activated carbon. J Hazard Mater 171(1):340–347
Mahmoodi NM, Salehi R, Arami M, Bahrami H (2011) Dye removal from colored textile wastewater using chitosan in binary systems. Desalination 267(1):64–72
Moosavian MA, Moazezi N (2016) Removal of cadmium and zinc ions from industrial wastewater using nanocomposites of PANI/ZnO and PANI/CoHCF: a comparative study. Desalin Water Treat 57(44):20817–20836
Musyoka SM, Mittal H, Mishra SB, Ngila JC (2014) Effect of functionalization on the adsorption capacity of cellulose for the removal of methyl violet. Int J Biol Macromol 65:389–397
Nurchi VM, Crespo-Alonso M, Biesuz R, Alberti G, Pilo MI, Spano N, Sanna G (2014) Sorption of chrysoidine by row cork and cork entrapped in calcium alginate beads. Arab J Chem 7(1):133–138
Osifo PO, Webster A, van der Merwe H, Neomagus HW, van der Gun MA, Grant DM (2008) The influence of the degree of cross-linking on the adsorption properties of chitosan beads. Bioresour Technol 99(15):7377–7382
Rafatullah M, Sulaiman O, Hashim R, Ahmad A (2010) Adsorption of methylene blue on low-cost adsorbents: a review. J Hazard Mater 177(1):70–80
Salleh MAM, Mahmoud DK, Karim WA, Idris A (2011) Cationic and anionic dye adsorption by agricultural solid wastes: a comprehensive review. Desalination 280(1):1–13
Wan Ngah WS, Teong LC, Hanafiah M (2011) Adsorption of dyes and heavy metal ions by chitosan composites: a review. Carbohydr Polym 83(4):1446–1456
Yan H, Zhang W, Kan X, Dong L, Jiang Z, Li H, Yang H, Cheng R (2011) Sorption of methylene blue by carboxymethyl cellulose and reuse process in a secondary sorption. Colloids Surf A Physicochem Eng Asp 380(1):143–151
Acknowledgements
The authors are thankful for the financial support from the US Department of Agriculture through the Center for Education and Training in Agricultural and Related Sciences Proposal (CETARS; award #: USDA/NIFA: 2011-38422-30835), at Inter American University of Puerto Rico at San German and University of Puerto Rico at Mayaguez. Special thanks to Mrs. Nilda Caraballo (Inter American University of Puerto Rico) and Mrs. Marta Caraballo (Fenwal: A Fresenius Kabi Company).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vega-Negron, A.L., Alamo-Nole, L., Perales-Perez, O. et al. Simultaneous Adsorption of Cationic and Anionic Dyes by Chitosan/Cellulose Beads for Wastewaters Treatment. Int J Environ Res 12, 59–65 (2018). https://doi.org/10.1007/s41742-018-0066-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41742-018-0066-2