Abstract
This study investigates the removal rate of divalent ions during partial desalination of brackish water using electrodialysis. An experiment was conducted with a benchtop PCCell electrodialysis instrument in batch mode with a non-ion selective membrane. The removal rate of total copper, a valuable plant micronutrient, was analysed. Both copper chloride and copper sulphate removal compared to sodium chloride removal were studied. The copper and the sulphate content in the dilute declined logarithmically with a removal rate of around 98% for copper in both experiments and 100% for sulphate over 3 h at a starting temperature of 23 °C. Copper and sulphate were removed faster than sodium chloride at 72%. The temperature of the dilute increased by 15% during the 3-h run. The loss of water from the dilute was approximately 10%, limiting brine production. Modelling indicated that the mass/charge ratio of ions could be an indicator of the removal rate of anions, especially if they have, like sulphur, a large effective radius, whereas the Effective Ionic Radius can be an indicator for the removal of cations. The smaller the ionic radius, the faster the removal rate of the cation. This model can be used to customise nutrient concentration in the water end product. The customised water has a potential to be used for fertigation, saving the farmer money by retaining beneficial plant nutrients in the water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Most elements either have a positive or a negative impact on the environment, the soil, the water and plants. Their impact depends on their concentration and the resilience of the systems they are acting on. Not all irrigation applications require total removal of these elements, in fact, it is often better to leave them in the water where they can act as soil improvers, fertilisers and nutrients. In water desalination, it is currently standard practice to remove as many cations from water as possible, without considering the environmental effects of such practice, which could be soil degradation by compaction, and nutrient imbalances in crop plants. The possibility of retaining beneficial plant micronutrients in water treatment has not been studied extensively. If beneficial cations like calcium, potassium, magnesium, and to a certain extent plant micronutrients like copper, were retained in the water, a saving, in fertiliser and soil improvement costs, and energy would result. Calcium and magnesium in water prevent heart disease in humans (Burton 2008). In soils, they prevent sodium from causing too much damage (hard setting) by keeping the sodium adsorption ratio (SAR) of soil water low. SAR describes the ratio of sodium to calcium and magnesium in the ground and irrigation water. Usually, the higher the SAR, the more the soil structure is damaged by this water (Stevens et al. 2004). However, some soils can tolerate more salt than others, for example, clay soils. Likewise, many plants and soils can be irrigated with slightly brackish water. Numerous desalination studies deal with salt removal, for example, Goodman et al. (2013), Wood (1960) and Veza et al. (2004). Copper is present in rocks, soil and natural water. It is essential to human nutrition as a trace element. Copper is also a plant micronutrient and fertiliser (Malhi et al. 2005). The leachate obtained from chicken manure piles is high in nutrients like copper, but also high in sodium chloride (Ksheem et al. 2015). Copper is a transition metal and can have multiple oxidation states, ranging from 1+ to 4+. In a transition metal, the d orbitals can contain different amounts of electrons which result in several stable ions (Greenwood and Earnshaw 1997). Copper deficiency can impact crops in many ways; for instance, in legumes it leads to a reduction in nitrogen binding, in many other crops to wilting and infertility caused by lignin deficiency (Marschner 2012). While copper is an essential plant micronutrient, copper toxicity in crops sometimes occurs which can lead to reduced growth and yield. The main sources of copper contamination are fungicides like copper sulphate. Human and animal wastewaters, especially from chickens and pigs, and inputs from polluted air and industry contamination also play a major role (Marschner 2012). Copper contamination in water systems can lead to poisoning of aquatic organisms. Surface water and groundwater near coal mines are often contaminated with copper (Shi 2013). This study aims to contribute solutions to some of the problems stated above by using electrodialysis water treatment (ED).
Electrodialysis is a method mainly used for desalination of seawater using an electrochemical generator (Xu and Huang 2008). The method was discovered in 1890 by Shaposhnik and Kesore (1997). Meyer and Straus (1940) describe the principles of ED in the 1930s. After 1940, alternating anion and cation exchange membranes were used, and different ion exchange resins were developed. Lee et al. (2009) describe the set-up of an electrodialysis plant. ED is an electromembrane process in which ions are transported through a membrane, producing a solution with low ion concentration (dilute). In the solution, the negatively charged anions drift to the anode, the positively charged cations to the cathode. Differently charged components can be separated by ED (Tularam and Ilahee 2007; Strathmann 2010). Güler et al. (2014) state that non-selective ion exchange membranes have a low ability to separate monovalent ions from multivalent ions. Van der Bruggen et al. (2004) note that separation of ions by their charge, the applied voltage (in ED) and the pressure (in nanofiltration, NF) is possible. Galama et al. (2014) studied the preferential removal of divalent ions in sea water. The lower the current, the more rapid was the removal of divalent ions compared to monovalent ions, and the boundary layer effects were lower than at higher currents. On the other hand, the lower the initial concentration of divalent Ca2+, Mg2+, SO4 2− ions and the beneficial K+ ions compared to Na+ and Cl−, the lower the concentration of these ions in the transport layer adjacent to the membrane, resulting in reduced transport. The Nernst–Planck flux equation explains the removal pattern of ions. The flux of ions depends on an ionic concentration gradient and an electric field (Kirby 2010). ED can also be utilised for the concentration and separation of acids, salts and bases from aqueous solutions. Other uses are organic acid production from whey (Huang et al. 2007), sugar demineralisation, blood and protein treatments (Xu and Huang 2008) and the elimination of fluoride and nitrate from brackish groundwater (Banasiak et al. 2007). Sadrzadeh et al. (2007), Mohammadi and Kaviani (2003) and McGovern et al. (2014a, b) all state that membrane desalination systems are energy efficient, especially for partial desalination of waters with high salinity. Korngold et al. (2004) show that the energy needed to desalinate a solution from 20% salt content to 0.4–1.8% is 1.5–7.1 kWh/m3. One major advantage of ED is that the brine component is small, amounting to only 10% of the initial volume of the dilute (Eberhard 2016). Only a small pond is needed to evaporate ED brine, compared to the huge ponds needed to evaporate brine from reverse osmosis (RO) treatments. A significant use of ED is brine concentration for example from RO applications. Also, nutrients and salts can be recovered from this concentrated brine, and their environmental impact can be limited. Brine recovery in reverse ED systems can also be used successfully for energy recovery (Kwon et al. 2015). At present many desalination units are in use worldwide; a recent example is the large EU-funded plant Aigues ter Llobregat (ATLL) in Spain (Sanz and Miguel 2013). A promising application of ED is the desalination of coal seam gas water, a waste product from fracking which can contain considerable amounts of copper. Our study investigates the possibilities of using electrodialysis for calculated removal or retention of beneficial micronutrients in brackish water and coal seam gas water, using the example of copper. Table 1 shows concentrations of copper and other contaminants in treated CSG water in QLD. The actual concentrations of untreated CSG water vary but are likely much higher.
Materials and Methods
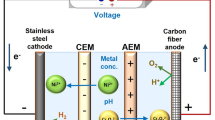
A custom-made ED instrument from PCCell GmbH, Heusweiler, Germany, was used. Figure 1 shows a diagram of the instrument set-up in the laboratory of the University of Queensland, Toowoomba, Australia. This unit provides three independent hydraulic circuits with flow meters (40–400 l/h), power supply and pumps. Table 2 shows the membrane specifications.
The experiments were conducted using the recycling batch mode. Other instruments used were: Speer Scientific handheld Conductivity-TDS-Salinity Meter from Pro Sci-Tech; TPS smartChem pH Meter with ATC temperature probe; Power supply HCS-3400/3402/3404 Laboratory Grade; and High Immunity Switching Mode Power Supply with Rotary Encoder Control. A small electric pump emptied the tanks before commencement. The membrane was flushed with distilled water for 5 min before the start of the experiment. The Dionex Ion Chromatograph ICS-2000 measured sulphate using the method described in the Dionex Application Note 154 (Dionex 2003).
In the following the expression “copper” will be used for “total copper”. Copper was measured with the AAS absorption method using a Shimadzu AA-7000 with autosampler. The samples for the atomic absorption spectroscopy (AAS) analysis were taken 10 min apart from the outlet at the top of the testing unit where the water flows back to the dilute tank. The experiment ran over 180 min; two concentrate (brine) samples were taken at the beginning and the end of the experiment. The concentrate samples and the first three dilute samples were diluted by a factor of two to achieve a concentration where they could be measured with the AAS. The lamp was a combination lamp for foundry effluents, with a measurement range of 1–25 ppm Cu. The lamp current (low peak) was 20 mA, the slit width 0.7 nm and the lamp mode BGC-D2 (background correction with a deuterium lamp). The fuel gas flow rate (L/min) was 15 L/min, the flame type Air-C2H2, the burner height 7 mm and the burner angle degree zero. The absorption wavelength of copper is 324.8 nm.
-
Dilute: 2.5 g copper II sulphate pentahydrate (CuSO4 *5H2O) in the first and 2.5 g copper chloride (CuCl2) in the second experiment, and 250 g laboratory grade NaCl, 25 L in distilled water. The dilute had an initial salt concentration of 10,000 ppm (10 g NaCl/L).
-
Concentrate: It is necessary that this solution has some preliminary conductivity (EC) to start the process. This EC was 23.7 mS/cm. The solution was a leftover from a previous experiment. The volume at the beginning was 4 L.
-
Electrolyte: This solution aids in the demineralisation by improving the rate at which the ions pass the ED membrane. The one molar electrolyte solution consisted of 14.2 g sodium sulphate/L.
-
Standards: A commercial copper standard solution (high-purity standard, from Choice Analytical, 1000 µg/L, in 2% HNO3) diluted to different concentrations with distilled water. Each diluted standard had 1% NaCl added.
The voltage of the power supply was 10 V. The amperage during the test (3 h) decreased from 1.6 to 0.8 A. The resulting pressure at the inlet and outlet of the membrane was around 0.3 bars at the inlet and 0.4 bars at the concentrate outlet. The flow rates for the dilute and concentrate streams were about 50−60 L/h. The electrolyte flow rate was approximately 125 L/h. In the following, the term EC is used interchangeably for mS/cm. All graphs were drawn with Microsoft Office Excel.
Results and Discussion
The first test using copper sulphate was run at a starting temperature of 16.7 and 23.7 °C, respectively. Initially, the copper content was 35.2 ppm, the sulphur content 21 ppm and sulphate 60.2 ppm. The copper content in the dilute went down logarithmically from the start concentration of 35.2 ppm copper to 3 ppm in 3 h (91.5%) at the lower temperature. At the higher temperature, the rate of removal was 97% (Fig. 2). These results show that the removal rates for copper are highly temperature dependent.
The conductivity of the dilute at the beginning of the experiment was 18.5 mS/cm (12.4 g total dissolved solids TDS/L, 250 g NaCl + 2.5 g CuSO4). The conductivity of the dilutate at the end of the experiment was 4.03 mS/cm (2.7 g TDS/L) (Fig. 3). The difference was 14.47 g TDS/L. The removal was logarithmic. The conductivity of the concentrate at the start was 21.1 mS/cm (14.14 g TDS/L); in the end, it was 43.8 mS/cm or 29.35 g TDS/L. The difference between start and end TDS in the concentrate was 15.21 g TDS/L. The concentrate volume increased from 7 to 9.3 L, which implies that (7.3 L * 15.21 g = 109.5 g) + (2.3 g * 29.35 g = 67.51 g) = 177 g TDS were transferred to the concentrate.
Sulphate removal was measured with an ICS-2000, at 10 V, and took place logarithmically. Its removal rate was faster than that of the total conductivity (Table 3). The starting concentration of sulphate was 46.33 ppm, and the end concentration after about 150 min was 0 ppm. The pH in the dilute went up insignificantly from 5.2 to 6.03 during the run. The temperature in the dilute increased from 16.7 to 20.7 °C. The volume of the dilute went down from 25 to 22.7 L (10%). Conversely, the volume of the concentrate increased from 7 to 9.3 L. The current went down from 2.3 to 0.9 A. There were strong positive correlations between ampere and conductivity in the dilute (r = 0.96) and ampere and copper content in the dilute (r = 0.95). The conductivity in the dilute was also correlated with the copper content in the dilutate (r = 0.97) and the conductivity in the dilute to sulphate content (r = 0.97). The correlations between conductivity and copper content and sulphate content were the same. Conversely, there were negative correlations between all these factors in the concentrate. Table 3 shows the raw data from the copper sulphate experiment.
The next section describes the energy efficiency of the ED in the copper sulphate experiment with the starting temperature of 16.7° Celsius. The conductivity went down from 18.5 to 4 mS/cm in 3 h using 10 Volts, a difference of 14.5 mS/cm. The reduction in amperage was linear and went down from 2.3 to 0.9, a difference of 1.4 A.
The following equation shows how kW can be calculated from the voltage (V) and the amperage (A) (Ranade and Bhandari 2014).
When using this formula, the starting power consumption was 0.023 kW; the final energy consumption was 0.009 kW. These kW values were averaged for each hour and added up, which leads to a total power consumption of approximately 0.05 kWh/25 L (0.002 or 2 kWh/kL) to incompletely desalinate water with half the conductivity of seawater. The agricultural cost for one kWh in 2015 in Australia was approximately $AUD 0.35/kWh. The total cost to reduce conductivity from 18.5 to 4 mS, therefore, was $AUD 0.0007/L or $AUD 0.7/kL, or $AUD 700/ML. The process was the most energy efficient in the first 60 min of the run, where 7.6 mS/L was removed with 0.022 kWh. In the following 60 min, 4.32 mS/L was removed using 0.016 kWh; then, in the next 60 min, 2.55 mS/L was removed with 0.011 kWh. The same power input removed about 35% more salt in the first hour than in the last. Therefore, the process is most efficient at higher salinities. Additionally, the temperature of the dilute increased by 15%, which is equal to around 0.028 kW in 3 h. Consequently, roughly half of the energy initially used (0.05 kW) could be recovered by re-using the heat. It would also be necessary to determine to which percentage the heat is generated by exothermal reactions compared to the kW input, but this proportion is likely low.
Desalinating seawater in Australian desalination plants currently costs around $ AUD 3–4/kL on average (Palmer 2013). This estimate provides the total price for desalination, including the plant. The total price is dependent on the starting and desired end salinity. Korngold et al. (2004) showed that the energy needed to desalinate a solution from 20% salt content to 0.4–1.8% was about 1.5–7.1 kWh/kL. Our result of 2 kWh/kL is at the lower end of this range. Plant costs, energy used by the pump and other running expenses were not included in our estimate. The electrodialysis demineralisation method is most energy and cost-efficient if the starting EC is high and the end EC (mS/cm) is also relatively high. The longer the desalination process runs, and the lower the EC becomes, the more the energy efficiency decreases due to the depleted ion current, and the longer the process takes. A customised water, which also takes into account the specific soil properties and the plants it is supposed to irrigate, could have lower energy costs than a water desalinated by standard methods. Small solar panels could provide this energy. Variability in the solar energy supply would not be a problem, as the system just works with the voltage it receives. Therefore, storage of the energy would not be necessary. Partial demineralisation could also significantly reduce the fertilisation costs. It is a need to determine the best parameters for any given water to use the partial demineralisation method most effectively.
In the following experiment, instead of copper sulphate, 2.5 g copper chloride was added to the dilute. The starting temperature was 21.2° C. Copper removal took place at a constant voltage of 10 V. The initial copper concentration in this experiment was 47 ppm, about 10 ppm higher than in the previous experiment because the copper content in copper chloride is 47% compared to 35% in copper sulphate. The copper content in the dilute went down logarithmically, and the removal rate of copper amounted to 98% (Fig. 4). The removal of copper was slightly faster than in the previous experiment, due to the higher starting concentration of the copper in this solution. Copper surpassed the membrane as the concentrate solution looked greenish-blue at the end, the colour of the copper II chloride in water solution (Greenwood and Earnshaw 1997). The dilute still had a slight bluish tinge at the end of the experiment.
Figure 5 shows the conductivity removal rate in this experiment, which was about the same as in the copper sulphate solution.
In the PCCELL instruction booklet, sodium amido sulphonate solution is recommended as the electrode rinse for a non-ion selective membrane when the pH of the dilute is neutral and mono- and divalent ions are present. However, in this experiment, a sodium sulphate solution was sufficient to remove most of the copper. As this solution is only marginally depleted over time, it is very cost-efficient. According to Galama et al. (2014), the lower the current density, the quicker divalent ions disappear compared to the monovalent ones caused by low flow rates at low current densities, which reduce permeate flux. However, at low initial concentrations of the divalent Ca2+, Mg2+, SO4 2− and the beneficial K+ compared to Na+ and Cl−, these ions are not abundant in the transport layer adjacent to the membrane. Therefore, even at higher applied current densities, boundary layer effects result in reduced transport of ions with a low initial concentration. In this experiment, the initial concentrations of copper and sulphate to sodium chloride were less than one per cent. However, the current densities were low which could be a reason for the faster removal of copper and sulphate in comparison with monovalent ions.
The pH results in this experiment were inconclusive. In the copper sulphate dilute, the pH increased slightly during the run from 5.7 to 6.18. Measuring the pH of the saline solutions proved difficult and is not recommended because the salt interferes with the measurement. At high currents, the pH in the dilute tends to go down because water is split (Strathmann 1992). As the current was low in this experiment, there likely was no water splitting in the dilute and the electrolyte solution. Water splitting can produce hydrogen gas. Hydrogen gas is an energy source. However, higher voltages than 10 V would be necessary to produce sufficient amounts. The current in the dilute went down from 2.3 to 0.9 ampere, caused by the depletion of ions in this solution. There were positive correlations between ampere to conductivity in the dilute and ampere to copper removal. On the other hand, there were negative correlations between conductivity in the dilute and pH, and current (Amp) and pH. All these correlations were not very strong. There was, however, a significant correlation between removal of salt and copper. The correlations between copper and conductivity and sulphate and conductivity were the same, which shows that the measurements in this run were correct. The dilute in each run lost about 10% of its volume. Therefore, the efficiency of the benchtop unit is in the range of industrial electrodialysis plants. In the dilute, the temperature went up by four to five degree Celsius during the runs which is due to the electrochemical membrane process. This energy can be recycled.
Membrane fouling and ageing are an issue. Further investigation of membrane fouling, for example, analysis of the membrane with a scanning electron microscope, is recommended. It is important that the membranes be in a clean state, and the testing conditions are always the same. Cleaning of the resin envelopes in an acidic ultrasound bath is possible (Wang et al. 2011). Furthermore, the membrane should never be allowed to dry out, but be stored in a saline solution at all times.
The following section discusses the possible modelling of removal rates. Figure 6 shows the calculated atomic mass/charge ratios of ions used for fertilisation (calculations by the author). Many metals are transition metals with varying charge, so only the most common charges were utilised for this calculation and the subsequent modelling. The hypothesis was that the lower the mass/charge ratio, the faster the depletion of these ions in solution when treated with the EC unit. According to the graph, boron, nitrogen and silica would be depleted from the solution very quickly. Phosphorus, magnesium, sulphur, manganese, sodium and calcium would be depleted at a slower rate and, iron, copper, molybdenum and zinc relatively slowly. The last compound to disappear from the solution would be potassium. Sodium and calcium have approximately the same removal rate.
Figure 7 shows the different Effective Ionic Radii for the most commonly used ions in fertigation (Rahm et al. 2016). In this hypothesis, the smaller the ionic radius, the faster the ion would be removed from a solution when running through the ED instrument. Boron, zinc, silicon and phosphorus would be removed from the solution very quickly, manganese, molybdenum, magnesium, copper and iron moderately fast and sodium, nitrogen, potassium and sulphur at the slowest rate. Both nitrogen and sulphur are listed as negatively charged ions in the chart, but in solution, they can be found bound to oxygen, as sulphate, or to hydrogen, in the form of ammonium, which complicates the calculation.
Two models were created in Microsoft Excel which can calculate the approximate removal time of different ions when the start and target concentrations (ppm) of an already measured ion are entered. First, the desired concentrations (ppm) of the desired nutrients are entered, and then the current levels of these nutrients (ppm) in the water to be treated are entered.
The first model (Table 4) was based on the Effective Ionic Radii of the ions. The larger this radius, the slower the removal of the ion. The radius depends on the charge of the ion, either positive or negative. Positively charged ions have smaller diameters than their respective atoms. Negatively charged ions have larger effective radii than their atoms. The reason for this is that the additional electrons lead to an expansion of the electron cloud due to the repelling of the electrons from the existing protons (Housecroft and Sharpe 2008; Jensen 2010; Oliver 1973; Shannon 1976). For this example, the concentration of the tested ion copper (35 ppm) was entered into field B1 of the Excel spreadsheet. The Excel spreadsheets for this and the following model are provided as supplementary material with this publication or can be obtained from the author. The measured time to reach the target concentration in minutes was entered into field D1. In this experiment, with a starting temperature of 16.7 °C, it took 447 min to reach the desired concentration. The radius of the measured ion, in this case, copper, was set to 100% and entered in field C3. The relative ratios of the other ionic radii compared to copper were calculated and entered in the same column below. If another ion than copper was tested, the Effective Ionic Radius of this ion would be entered in C3, and the relative Effective Ionic Radii of the other ions would change. In Column E the starting concentrations of the water to be tested were entered. Water analysis, done once or twice a year, is necessary to provide the concentrations of all ions of interest in the water. The estimated removal time for each ion from the start to the desired end concentration then appears in Column H. This model replicated the results obtained from the copper experiments quite well.
The second model (not shown here, but available in the appendix) uses the mass/charge ratio of the ions and works in the same way. It was hypothesised that the larger the mass and the smaller the charge, the slower the removal of the ion. The data were entered in the same way as above. The concentration of the tested ion copper was entered into field B1. The measured time to reach the target concentration in minutes was entered into field D1 (447 min). The mass/charge ratio of the measured ion, in this case of copper, was set to 100%, entered in field C3. The relative mass/charge ratios of the other ions compared to copper were determined and entered below. In Column E the starting concentrations of the water to be tested were entered. The estimated removal time for each ion appears in Column H. This model was not able to replicate the results obtained from the copper experiments, which implies that the individual removal times were not directly related to the mass/charge ratio of the ion. The conclusion was obtained by analysing Column F (“Removal time at the same start and end conc”). However, this model seemed to work better with anions like sulphate.
The model shown in Table 4 used the Effective Ionic Radius to estimate removal rate of ions from the solutions. The diameter of an atom is the distance from one outer boundary of the electron cloud to the other side. Ions have very different radii than the respective atoms. Interestingly, a higher charge leads to a smaller radius in the positively charged cations but a larger one in the negatively charged anions. The radius can also change depending on other interacting ions, their concentrations and charges. Na+ has a radius of 116 pm, Cl− has 181, Cu+ has 77, and Cu2+ has 73. Copper in all its oxidative stages has a much smaller Effective Ionic Radius (a function of spin and ionic charge) than sodium and chloride (Housecroft and Sharpe 2008; Jensen 2010; Oliver 1973; Shannon 1976; Wasastjerna 1923). This model explains the faster removal of copper in this experiment. The average copper concentration in a hydroponic fertigation solution is 0.08–0.2 ppm. For the copper sulphate solution with a starting concentration of 35.2 ppm, to reach a concentration of 0.2 ppm copper, the instrument would have to run approximately 447 min, when projecting the curve into the future. After this time, the salt content would be 0.335 g TDS/L or have an EC of 0.5 mS/cm. Using, for example, a starting concentration of one ppm copper, it would only take 13 min to reach a concentration of zero mS/cm. The obtained curve can then be compared to the time it takes for the removal of micronutrients. The theory behind the second model is that the removal rate rests on the mass/charge ratio of the ion/molecule. The mass is the sum of all the electrons, protons and neutrons in one static atom/molecule. The smaller the mass/charge ratio, the faster would the removal be. Sulphur itself has a lower mass/charge ratio than sodium, but in solution, it is present as sulphate. This could be the reason that the mass/charge ratio is a better indicator of the removal rate of anions, possibly because they are bound to oxygen or hydrogen. The behaviour of this sulphate at the membrane could be the topic of another study. In this experiment, the sulphate was removed logarithmically and at a slightly faster rate than the conductivity. After a 447-min run-time, the sulphate concentration would be zero when forecasting the curve. Therefore, negatively charged ions/molecules should be assessed for their removal rate by the mass/charge ratio.
The model cannot be used to estimate removal time of sodium, as the initial concentrations are usually extremely high; for example, CSG water has an EC of 6–12, leading to a steep TDS decline in the beginning and a more gradual removal later. Of course, the model also does not work if the starting concentration is lower than the desired end concentration. In this study, it was hypothesised that the Effective Ionic Radius is a better indicator of the removal rate of cations than the mass/charge ratio. The models using the Effective Ionic Radii and mass/charge ratios are very basic and need improvement. However, this study only aims to point towards future possibilities in water treatment. Other factors that influence the removal rate are the boundary layer effects on the membrane, ratios of different ions to each other which could cause the ionic radii to change size, various current densities and temperatures that affect the removal of ions differently and different membrane fouling stages. These factors were not taken into account in these models. There also was no temperature control in this experiment. However, different starting temperatures also result in different removal rates; therefore, a temperature-controlled environment should be used, or the influence of the temperature must be accounted for in a temperature correction formula. The model provides only a simplified estimation of target concentrations. These models only work when the start and end concentrations are relatively close together, as all curves follow different logarithmic curve patterns, but the model is linear. Each compounding ion would have to be tested by electrodialysis, and the various log functions applied to validate this model. The benefits of this model are that only one element has to be tested to estimate the concentrations of the others. Additional ions can be added depending on requirements. As the removal times vary widely between target concentrations and ions, the farmer or company could optimise the system to suit his needs.
Conclusions and Outlook
Investigated in this experiment were the removal rates of copper chloride and copper sulphate in a sodium chloride solution using a non-ion selective ED membrane with a sodium sulphate electrolyte solution, and modelling was undertaken to extrapolate the data obtained. So far, the retention of beneficial micronutrients in water treatment was not studied extensively. In water desalination, it is currently standard practice to remove as many sodium, calcium and other cations from the water as possible. But not all applications require total removal of these elements. If beneficial cations like calcium, potassium, magnesium and plant micronutrients like copper, are retained in the water, a saving in fertiliser and soil improvement costs would result. Calcium and magnesium in water prevent heart disease in humans (Burton 2008). In soils, they prevent sodium from causing too much damage (hard setting) by keeping the sodium adsorption ratio (SAR) of soil water low. The partial desalination of saline waters such as CSG water also leads to a saving of energy.
Relatively easy benchtop experiments can be conducted to estimate the removal rates of different micronutrients compared to salinity by extrapolation of the generated curves. While there are many studies concerning the electrodialysis water demineralisation process, the comparability of these studies is often difficult due to a large range of different testing conditions. Other studies showed that reverse osmosis treatment achieved a salt removal rate of 99.4–99.9% and a copper removal rate of 99%. Copper removal rate using ultrafiltration was about 94% and using forward osmosis 98% (Le and Nunes 2016). In this experiment only 72% of the salt was removed after a 3-h run, but previous tests in our laboratory showed that up to 99% salt removal is possible with very long running times. Almost total copper removal (98%) occurred in this experiment over 3 h. Complete elimination of these ions was not the scope of this study. The results indicate that preferential removal of total copper occurred. This preliminary study demonstrated that partial desalination and partial copper removal are possible with a relatively low energy input. If no complete desalination is needed, the process is the most energy efficient. Forecasting of desalination and demineralisation curves obtained from the benchtop ED instrument can provide valuable insights into the time necessary to get irrigation water of a certain quality. However, the complexity of ions present, and interactions between ions and other substances in the water, for example, organic matter, makes modelling challenging. Two models were tested, one using the mass/charge ratio of the ions and the other using their Effective Ionic Radii. It was found that the model using the Effective Ionic Radius worked better for cations, while the anions were better modelled by using their mass/charge ratios. The models need optimisation and validation by adding a temperature adjustment formula and by testing different voltages, currents and temperatures for their influences on selective ion removal. However, it is evident that ED methods can be used to treat CSG water and saline river water to produce a custom-made water product in an energy and resource-efficient way. Using ED for desalination and leaving the beneficial divalent ions in the treated CSG water could result in an enormous cost reduction for coal seam gas companies, farmers and the end users of farming products. Demineralisation using ED would lead to a beneficial effect on soil and human health and could help to use available resources in a more environmentally sustainable way. As different soils tolerate varying levels of salinity and require different amounts of soil improving calcium and magnesium ions, and diverse crops tolerate more or less salt in their irrigation water and require varying optimum levels of micronutrients, custom-made water products could save precious resources, especially in a global context where these are limited.
References
ABC-News (2012) Coal seam gas by the numbers. http://www.abc.net.au/news/specials/coal-seam-gas-by-the-numbers/waste/. Accessed 18 July 2016
Banasiak LJ, Kruttschnitt TW, Schäfer AI (2007) Desalination using electrodialysis as a function of voltage and salt concentration. Desalination 205(1–3):38–46
Burton A (2008) Cardiovascular health: hard data for hard water. Environ Health Perspect 116(3):A114
Dionex (2003) Dionex application note 154. Determination of inorganic anions in environmental waters using a hydroxide-selective column. Sunnyvale, CA, USA, Thermo Scientific: 10
Eberhard FS (2016) Towards a custom made water product—potential use of electrodialysis for coal seam gas water treatment using the example of copper ions. In: International conference on biological, chemical and environmental sciences (CES2016), Tokyo, 14–15 Aug 2016
Galama AH, Daubaras G, Burheim OS, Rijnaarts HHM, Post JW (2014) Seawater electrodialysis with preferential removal of divalent ions. J Membr Sci 452:219–228
Goodman NB, Taylor RJ, Xie Z, Gozukara Y, Clements A (2013) A feasibility study of municipal wastewater desalination using electrodialysis reversal to provide recycled water for horticultural irrigation. Desalination 317:77–83
Greenwood NN, Earnshaw A (1997) Chemistry of the elements. Oxford University Press, Boston
Güler E, van Baak W, Saakes M, Nijmeijer K (2014) Monovalent-ion-selective membranes for reverse electrodialysis. J Membr Sci 455:254–270
Housecroft CE, Sharpe AG (2008) Inorganic chemistry. England
Huang C, Xu T, Zhang Y, Xue Y, Chen G (2007) Application of electrodialysis to the production of organic acids: state-of-the-art and recent developments. J Membr Sci 288(1–2):1–12
Jensen BW (2010) The origin of the ionic-radius ratio rules. J Chem Educ 86(6):587–588
Kirby BJ (2010) Micro and nanoscale fluid mechanics: transport in microfluidic devices, species and charge transport. Cornell University, Kirby Research Group, Ithaca
Korngold E, Aronov L, Belayev N, Daltrophe N (2004) Electrodialysis with brine solutions over-saturated with calcium sulfate. The Institutes for Applied Research, Ben-Gurion University of the Negev, P.O. Box 653, Beer-Sheva, Israel
Ksheem AM, Bennett JM, Antille DL, Raine SR (2015) Towards a method for optimized extraction of soluble nutrients from fresh and composted chicken manures. Waste Manag 45:76–90
Kwon K, Han J, Park BH, Shin Y, Kim D (2015) Brine recovery using reverse electrodialysis in membrane-based desalination processes. Desalination 362:1–10
Le NL, Nunes SP (2016) Materials and membrane technologies for water and energy sustainability. SM&T 7:1–28
Lee H-J, Hong M-K, Han S-D, Cho S-H, Moon S-H (2009) Fouling of an anion exchange membrane in the electrodialysis desalination process in the presence of organic foulants. Desalination 238(1–3):60–69
Malhi SS, Cowell L, Kutcher HR (2005) Relative effectiveness of various sources, methods, times and rates of copper fertilizers in improving grain yield of wheat on a Cu-deficient soil. Can J Plant Sci 85(1):59–65
Marschner H (2012) Mineral nutrition of higher plants, 3rd edn. Elsevier Academic Press, London
McGovern RK, Zubair SM, Lienhard VJH (2014a) The cost effectiveness of electrodialysis for diverse salinity applications. Desalination 348:57–65
McGovern RK, Weiner AM, Sun L, Chambers CG, Zubair SM, Lienhard VJH (2014b) On the cost of electrodialysis for the desalination of high salinity feeds. Appl Energy 136:649–661
Meyer KH, Straus W (1940) La perméabilité des membranes vi. Sur le passage du courant électrique à travers des membranes sélectives. Helv Chim Acta 23(1):795–800
Mohammadi T, Kaviani A (2003) Water shortage and seawater desalination by electrodialysis. Desalination 158(1–3):267–270
Oliver J (1973) Ionic radii for spherical potential ions. Inorg Chem 12(4):780–785
Palmer N (2013) Cheaper seawater desalination. http://desalination.edu.au/2013/07/cheaper-seawater-desalination/#.V0-oevl95aQ. Accessed 18 July 2016
Rahm M, Hoffmann R, Ashcroft NW (2016) Atomic and ionic radii of elements 1–96. Chem Eur J 22(41):14625–14632
Ranade VV, Bhandari VM (2014) Chapter 1 - Industrial wastewater treatment, recycling, and reuse: an overview. Industrial wastewater treatment, recycling and reuse. V. V. R. M. Bhandari. Oxford, Butterworth-Heinemann, pp 1–80
Sadrzadeh M, Kaviani A, Mohammadi T (2007) Mathematical modelling of desalination by electrodialysis. Desalination 206(1–3):538–546
Sanz MA, Miguel C (2013) The role of SWRO Barcelona–Llobregat plant in the water supply system of Barcelona area. Desalin Water Treat 51(1–3):111–123
Shannon RD (1976) Revised Effective Ionic Radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr 32(5):751–767
Shaposhnik VA, Kesore K (1997) An early history of electrodialysis with permselective membranes. J Membr Sci 136(1–2):35–39
Shi (2013) Arsenic, copper, and zinc contamination in soil and wheat during coal mining, with assessment of health risks for the inhabitants of Huaibei, China
Stevens D, Unkovich M, Kelly J, Ying G (2004) Impacts on soil, groundwater and surface water from continued irrigation of food and turf crops with water reclaimed from sewage. CSIRO Publication, Soil, Land and Water Systems, Adelaide University, Adelaide
Strathmann H (1992) Membrane handbook. Van Nostrand Reinhold, New York
Strathmann H (2010) Electrodialysis, a mature technology with a multitude of new applications. Desalination 264(3):268–288
Tularam GA, Ilahee M (2007) Environmental concerns of desalinating seawater using reverse osmosis. J Environ Monit 9(8):805–813
Van der Bruggen B, Koninckx A, Vandecasteele C (2004) Separation of monovalent and divalent ions from aqueous solution by electrodialysis and nanofiltration. Water Res 38(5):1347–1353
Veza J, Peñate B, Castellano F (2004) Electrodialysis desalination designed for off-grid wind energy. Desalination 160(3):211–221
Wang Q, Yang P, Cong W (2011) Cation-exchange membrane fouling and cleaning in bipolar membrane electrodialysis of industrial glutamate production wastewater. Sep Purif Technol 79:103–113
Wasastjerna JA (1923) On the radii of ions. Comment Phys Math 38(1):25
Wood T (1960) Desalting of urine by electrodialysis. Nature 186(4725):634–635
Xu T, Huang C (2008) Electrodialysis-based separation technologies: a critical review. AIChE J 54(12):3147–3159
Acknowledgements
Many thanks go to Professor Jochen Bundschuh for the provision of the PCCELL testing equipment and the University of Southern QLD for the supply of the facilities and chemicals. Dr Henning Bolz and Patrick Altmeier from PCA GmbH, Heusweiler, Germany, provided much-needed advice on how to set up the ED instrument, and Portia Baskerville spent 2-week running analyses on the ED instrument as a work experience student.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Eberhard, F.S., Hamawand, I. Selective Electrodialysis for Copper Removal from Brackish Water and Coal Seam Gas Water. Int J Environ Res 11, 1–11 (2017). https://doi.org/10.1007/s41742-017-0001-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41742-017-0001-y