Abstract
Purpose
This clinical trial aims to evaluate in vivo the efficacy of a fluoride gel, a low-level laser (LLL), and a resin varnish at the treatment of dentin hypersensitivity (DH). Treatments assessed for their effectiveness, immediate analgesia, and duration of desensitization.
Material and methods
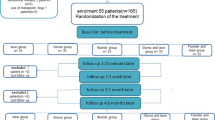
A total of 78 patients (one tooth per patient) with a clinical diagnosis of dentin hypersensitivity were included in this clinical trial. Dentin sensitivity in response to an air blast stimulus was assessed, and a Numeric Rating Scale (NRS) for pain from 0 to 10 was chosen to quantify pain at baseline and 15 min, 1 month, and 3 months after the first application. Patients were randomly divided into three groups. In the first group (treatment A, 26 patients), a fluoride gel (Calmodent Professional, Intermed, Greece) was applied. In the second group (treatment B, 26 patients), teeth were irradiated by a 670-nm InGaAlP continuous wave, red diode laser (MED-701, Lasotronic, Switzerland) with an output power of 180 mW, energy of 5.4 J, and irradiation time of 30 s. In the third group (treatment C, 26 patients), a resin varnish with giomer technology (PRG Barrier Coat, Shofu, Japan) was applied.
Results
The main analysis of the results was done with a linear mixed model (algorithm MIXED, IBM Statistics SPSS 21.0), while pairwise comparisons were conducted with the Bonferroni method. The statistical significance for all tests was set at p < 0.05. The main effects of time and group were found to be statistically significant. The time × group interaction effect was also statistically significant, and finally, a significant reduction (p < 0.05) of DH was recorded in all groups, compared with baseline.
Conclusion
All three treatments offered satisfactory and prolonged results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dentin hypersensitivity (DH) is a widespread and painful situation for the patients. The most recent definition is coming from the Canadian Advisory Board on dentin hypersensitivity and describes DH as the “Pain derived from exposed dentin in response to chemical, thermal, tactile or osmotic stimuli which cannot be explained as arising from any other dental defect or disease” [1,2,3].
Exposed dentin is the major problem. In the bibliography, this situation is called “lesion localization” [4]. The most common reasons for dentin exposure are as follows: anatomical abnormalities at enamel-dentin conjunction, gingival recession, erosion (acidic diet, bulimia, gastric regurgitation), attrition (occlusal abnormalities), abrasion (vigorous toothbrushing, abrasive toothpaste), abfraction (parafunctional activity), and tooth malposition (lack of antagonist).
Each of these situations separately or more often a combination of all usually causes tooth wear and eventually DH. However, not all exposed teeth are sensitive. In order for an exposed dentin to be sensitized, the tubule plugs and the smear layer should be removed. This condition is not rare, as we are talking about a thin, different in structure, and probably under-calcified smear layer [4]. At the same time, the results of the scanning electron microscope (SEM) indicate a modification at the structure of sensitive dentin [5, 6]. The width and the number of the tubules are particularly relevant to fluid flow which subsequently results in the activation of sensory nerves [7]. This situation is called “lesion initiation” and is the second phase of DH pathogenesis [4]. The age, the formation of secondary or tertiary sclerotic dentin or the occlusion of the tubules by other environmental factors may prevent the second phase of pathogenesis [8]. On the other hand, we should not forget that pain is a subjective feeling and it is related to patients’ tolerance and also to emotional and physical factors.
Differential diagnosis of DH may be a challenging task as it is based on exclusion. For this reason, detailed medical and dental history is necessary [9]. Even though information from patients’ anamnesis could be enlightening, however, the personal character of the replies makes the clinical examination and, furthermore, the radiographic examination necessary. In clinical testing, cold air and probe could be used to locate DH. Differential diagnosis should be made from dental caries and defective feelings which can cause postoperative sensitivity due to marginal leakage. Moreover, DH should be distinguished from fractured fillings, fractured teeth, enamel cracks, and reversible pulpitis [7]. All these situations could also cause instant and sharp pain.
Treatments about DH are numerous and diverse. Classification of all these agents is a challenging task, and one standard way to categorize them is according to their mechanism of action. So, there are those who decrease neural response to pain and those who occlude dentinal tubules. Tubule occlusion may succeed either by sealing with coating mechanism or by altering tubule content through coagulation, protein precipitation, and creation of insoluble calcium complexes [10,11,12]. However, there is not yet a “gold standard” treatment. So, dental professionals continue to look for more-effective, faster-acting, and longer-lasting treatments.
Laser technology is a relatively new and alternative treatment for DH. Near- and mid-infrared lasers such as Nd:YAG (1064 nm), Er:YAG (2940 nm), and CO2 (10,600 nm) have been tested for many years. Even though the application of this type of laser has been tested by many researchers with positive results, however, a significant difference between them and the placebo groups has not been noticed. Sgolastra ascribed these positive results to the Hawthorne effect [13]. Kimura also compared treatments with low-output-power and middle-output-power laser and concluded that positive results range from 5.2 to 100%. Moreover, he noticed a better performance of laser to cases with moderate pain [14].
During last years, low-level laser devices which emit at red and infrared spectrum range (600–1000 nm) seem to win the interest of scientists as they combine low cost, easy handling, non-invasive, nonthermal, and painless application with rare side effects. Low-level laser devices typically emit from 600 to 1000 nm, with an output power ranging from 1 up to 500 mW in pulsed or continuous mode. Typical intensity ranges from 500 mW/cm2 to 5 W/cm2 and treatment time from 30 to 60 s per point [15].
The term low-level laser therapy (LLLT) is a familiar term to the majority of the patients and the most frequently used in medical articles. Other famous names for this kind of therapy are cold laser, soft laser, low-level light therapy, low-intensity laser therapy, low-power laser therapy, photobiostimulation (first mentioned by Endre Mester in 1967), and finally photobiomodulation (PBM). Growing interest in the topic of DH during the last 10 years has led research into new treatments, and PBM is a novel and promising method for treating DH. In 2014, the “North American Association for Light Therapy” and the “World Association for Laser therapy” tried to distinguish PBM from other forms of phototherapy like photodynamic therapy (PDT) and optogenetics [16]. Actually, in photodynamic therapy, light and photosensitive dyes, which are exogenous chromophores, are used to kill cells while in PBM, endogenous chromophores are directly stimulated to heal [17]. The term PBM has recently been added to Medical Subject Headings (MeSH) 2016. According to this new definition, PBM is “A form of light therapy that utilizes non-ionizing forms of light sources, including lasers, LEDs and broadband light, in the visible and infrared spectrum.” It is a nonthermal process involving endogenous chromophores eliciting photophysical and photochemical events at various biological scales. This process results in beneficial therapeutic outcomes including, but not limited to, the alleviation of pain or inflammation, immunomodulation, and promotion of wound healing and tissue regeneration [16]. The purpose of this clinical study is the comparative evaluation of a fluoride desensitizing gel, a red wavelength diode laser device (670 nm), and a resin varnish with giomer technology, for the treatment of DH. This longitudinal, randomized, clinical trial is trying to estimate the immediate effect of desensitizers, the duration of desensitization, and finally the level of desensitization. It is hypothetized that there is no difference among treatment groups, there is no difference among time moments of the treatment, and finally treatment results are not influenced by the time (group × time interaction).
Materials and methods
At the beginning of this clinical trial, detailed medical and dental anamnesis from every patient involved was obtained. Patients with the following characteristics were excluded: carious lesions, defective restorations, enamel cracks, active periodontal disease, periodontal surgery (last 6 months), reversible pulpitis, analgesic or anti-inflammatory treatment (last 72 h), bleaching procedure (last 3 months), desensitizing products (last 6 weeks), pregnant and lactating women.
A total of 78 teeth from 78 patients aged from 30 to 60 with a diagnosis of DH were treated. Approval by Ethics Committee was obtained, and the patients signed written consent. The trial took place at the Operative Dentistry Department of Dental School of the Aristotle University of Thessaloniki.
Prior to their first visit, patients received dietary counseling and oral hygiene instructions. Same type of toothbrushes and tubes of toothpaste, without fluoride, were distributed to use twice daily for 2 weeks before and during the trial for 3 months. At first visit, adjacent teeth were isolated by cotton rolls. Air from the same dental equipment was applied for 3 s, at a distance of 2 mm and perpendicularly to the root surface. The same operator performed all air applications.
Each patient was asked to determine the level of pain by using a Numeric Rating Scale (NRS) from 0 to 10 where 0 represented “no pain” and 10 “unbearable pain.” To standardize the sample, the level of pain should have been over 4. This measurement was characterized as “moderate” pain. The first measurement was recorded as “baseline.” Patients were randomly divided into three groups, and each group received different treatment. Treatment in each group was randomly applied as this was defined by the method of block randomization. During the trial, patients were examined four times and four measurements were recorded for each one. Before applying any of the treatments, experimental teeth were cleaned with low-speed handpiece and pumice powder without fluoride. Teeth were rinsed and dried taking extra care to be blood and saliva free.
In the first group (treatment A), a fluoride gel (Calmodent Professional by Intermed, Greece) was applied. This desensitizing gel consists of stannous fluoride (SnF2) 0.4%, amine fluoride compounds (olaflur, amine fluoride 297), micro-hydroxyapatite 20%, and 2000 ppm F−. The gel was applied once with appropriate tip for 10 min.
In the second group (treatment B), a low-power laser device was used, with the following parameters: GaAlInP 670 nm, red wavelength diode laser (MED-701, Lasotronic, Switzerland) with continuous wave (CW) mode of operation, calibrated output power at the end of the tip 180 mW, power density (intensity) 360 mW/cm2, treatment time 30s, illuminated area 0.5cm2, energy 5.4 J, and energy density (dose) 10.8 J/cm2. Laser therapy was performed in three sessions with maximum intervals of 48 h. The pain was assessed 15 min, 1 month, and 3 months after the final session of the treatment. Mechanism of action is PBM.
In the third group (treatment C), a resin varnish with giomer (glass ionomer + polymer) technology (PRG Barrier Coat—surface pre-reacted glass ionomer by Shofu, Japan) was applied. The base part consists of S-PRG fillers based on fluoro-boro-alumino-silicate glass, methacrylic acid monomers, and other ingredients while the active part of the agent includes phosphonic and methacrylic acid monomers, bis-MPEEP, carboxylic acid monomers, TEGMA, reaction initiator, and others. Mixed material was applied by appropriate tips and, after waiting 3 mins, was light-cured by using a LED unit (irradiation wavelength 440–480 nm, light intensity > 1000 mW/cm2) for 10s. Treatment was applied only once.
All teeth remained vital, and there were no adverse events during the clinical trial.
Statistical methods
The calculation of the sample size was done with a formula suitable for repeated measures that took into account an 80% power to reveal a minimum difference of 0.7 SD between groups and a 30% attrition rate. Accordingly, block randomization was used to split the 78 patients that resulted from sample size calculation into three equal groups. The data at baseline were analyzed with an analysis of variance model to study differences between groups due to random allocation of the patients. The primary analysis then was done with a linear mixed model (algorithm MIXED, IBM Statistics SPSS 21.0), while pairwise comparisons were conducted with the Bonferroni method. The choice of the appropriate residual variance-covariance matrix was based on the Schwarz’s Bayesian Criterion (BIC) while in addition to the assumptions of residual normality and homoscedasticity which were verified with the Kolmogorov-Smirnov statistic and the scatterplot between predicted values and residuals, respectively. The statistical significance for all tests was set at p < 0.05.
Results
Regarding the sample data, descriptive statistics that took time and group into account are given in Fig. 1.
The three groups were initially compared for differences at baseline with an analysis of variance model. The results revealed no statistically significant differences between them at baseline (F(2,75) = 2.093, p = 0.130).
In accordance with the previous result and after the application of a linear mixed model (algorithm MIXED of SPSS) to the data set, the main effects of time and group were found to be statistically significant (F(3,112.372) = 129.007, p < 0.001 and F(2,75.637) = 3.853, p = 0.026, respectively). Further and more importantly, the time × group interaction effect was also statistically significant (F(6,112.361) = 7.191, p < 0.001) (Table 1); therefore, it was studied in more details with the Bonferroni method, and the conclusions are presented in the following.
Regarding comparisons between groups at each time point, CALMODENT showed statistically significant higher mean value than PRG BARRIER at 15 min (MD = 2.3, 95%CI 1.1–3.5, p < 0.001) and also at 1 month (MD = 1.6, 95%CI 0.5–2.8, p = 0.003), while LASER did not differ significantly from both CALMODENT and PRG BARRIER at any time point (Table 2).
Regarding changes over time for each group, CALMODENT showed a mean reduction over time. The mean NRS value at baseline was significantly higher than that at 15 min (MD = 2.7, 95%CI 1.9–3.4, p < 0.001), at 1 month (MD = 2.6, 95%CI 1.6–3.6, p < 0.001), and at 3 months (MD = 3.8, 95%CI 2.6–5, p < 0.001). Further, the mean NRS value at 15 min was more significant than that at 3 months (MD = 1.1, 95%CI 0.2–2.1, p = 0.015) and the mean NRS value at 1 month was greater than that at 3 months (MD = 1.2, 95%CI 0.4–2, p < 0.001).
Regarding LASER, the mean NRS value at baseline was significantly higher than that at 15 min (MD = 2.8, 95%CI 2–3.6, p < 0.001), at 1 month (MD = 2.7, 95%CI 1.7–3.7, p < 0.001), and at 3 months (MD = 2.7, 95%CI 1.5–3.8, p < 0.001), while no other difference between time points was observed (p > 0.05).
Finally, PRG BARRIER revealed a similar pattern to LASER, the mean NRS value at baseline was significantly greater than that at 15 min (MD = 4.5, 95%CI 3.7–5.3, p < 0.001), at 1 month (MD = 3.9, 95%CI 2.8–4.9, p < 0.001), and at 3 months (MD = 3.5, 95%CI 2.4–4.7, p < 0.001), while there were no other differences between time points (p > 0.05).
The previous results are shown in Fig. 2.
Discussion
Calmodent Professional (treatment A) is a new desensitizing gel. The mechanism of action is altering tubule content through the creation of insoluble calcium complexes. At the same time, amine fluoride compounds in combination with stannous fluoride prevent adherence of bacteria, restrict the production of dental plaque, and reduce inflammation. Finally, micro-hydroxyapatite and fluoride reinforce remineralization of dentin by creating fluorapatite and by increasing the thickness of hydroxyapatite [11, 18,19,20,21]. According to Fig. 1, Calmodent Professional acted in a slower way (7.6 to 4.9) but gradually reduced pain level satisfactorily.
PRG Barrier Coat (treatment C) is a resin varnish. The mechanism of action is sealing by coating. Besides sealing, the release of F, Sr, and B ions prevents the growth of Streptococcus mutans while at the same time they decrease pH of oral environment and protect dentin from erosion [22]. Finally, all the ions (Al, F, B, Sr, Si) of PRG fillers inhibit demineralization and promote remineralization of sensitive dentin [23, 24]. PRG Barrier acted immediately (7.7 to 2.7) with also satisfactory final result; however, we detected a relapse (2.7 to 3.7) during the post-treatment evaluation period.
Red wavelength diode laser MED-701 (treatment B) is a GaAlInP 670-nm laser device. The mechanism of action is PBM. PBM’s purpose is to improve the condition of damaged tissues by stimulating cellular metabolism. Photons start the healing process. After the irradiation, the photons are absorbed by the main target of PBM, the cytochrome C (C-C) complex. Stimulation of C-C increases production of adenosine triphosphate (ATP), as light is absorbed. ATP is responsible for cellular energy and signaling. In addition to ATP, light stimulation also produces free nitric oxide (NO) which is also a signaling molecule that improves cellular function and blood circulation by relaxing blood vessel walls. Finally, an increase in reactive oxygen spicies (ROS) which affect many important physiological signaling pathways including the inflammatory response is noticed [15, 25]. Production of these signaling molecules triggers a series of downstream effects that promote stimulation of metabolic activity. In more detail, these molecules induce growth factor production, increase cell proliferation and motility, promote the cellular metabolic activity of odontoblasts (regenerative effect), and induce tertiary dentin production. Recently, Mooney and Arany [26] proved that stimulation of ROS with an 810-nm diode near-IR laser at low doses (3 J/cm2) activates a latent complex of a growth factor known as transforming growth factor-β1 (TGF-β1). ΤGF-βs are key biological mediators that stimulate dental steam cells and create a new form of reparative dentin called tertiary or osteodentin.
Finally, NO improves microcirculation and oxygenation of the tissues.
In addition to tissue repair, production of ATP, NO, and ROS induces analgesia. The increase of these molecules can also increase the nerve ending threshold for pain, increase serotonin and β-endorphin, decrease bradykinin, and increase synaptic activity of acetylcholine esterase. Besides, the increase inhibits Na+-K+-ATPase, blocks depolarization of Aδ and C fibers, and changes neuronal transmission. These changes lead to an immediate analgesic effect which, according to the literature, is reversible and may last from 48 h to 6 months depending on the irradiation parameters. Moreover, Chow has proved that analgesic effect may be succeeded only with intensity over 300 mW/cm2. In DH, limitation of acute pain is the first and crucial stage before the regenerative stage starts. However, the way that PBM interrupts neural activity remains a subject of continuing research [14, 17, 27].
As far as inflammation concerns, PBM reduces edema and inflammation by increasing lymphatic flow which removes waste products and cellular debris [15, 17, 25, 28,29,30,31,32,33].
The phrase “The more, the better” does not fit to the philosophy of PBM method. Instead of this, a “biphasic dose-response” has been observed. According to this theory, there is a curve which is known as “Arndt-Schultz law,” and if the applied energy is insufficient, there is no response. If energy is increased and passes a critical threshold, which is unique for every tissue, PBM begins. This critical threshold is called “therapeutic window” and is rather narrow for every cell. Finally, if we continue to increase energy, stimulation disappears and is replaced by bioinhibition [33,34,35].
Apparently, a large number of parameters must be chosen for every treatment: wavelength, pulse structure, power, time, size of the surface, size of active tip, and distance. Any change of these parameters ends up in a different result. So, inappropriate choice of light source or dosage may lead to no results or bioinhibition [33,34,35]. Besides dose (J/cm2) and light source, PBM also depends on the physiological state of the cell before irradiation. It was found that if a cell is damaged or in a reduced redox state, the cellular response to PBM is stronger [29].
Researchers present conflicting results about PBM. Corona [36] compared diode laser (660 nm) with sodium fluoride and Labalardo [37] diode laser (660 nm) with another diode laser (830 nm). They both end up with positive results. Furthermore, Labalardo noticed faster relief with 660-nm treatment comparing with 830 nm. However, the results were equally positive for all the examined groups. Dilsiz [38] tested relief from DH with Nd:YAG and 685 nm, and the superiority of Nd:YAG treatment at 60 days was reported. Gentile [39] compared 670 nm with a placebo group, and he observed a significant statistical reduction in both groups. Vieira [40] also ended up with the same results after using 660 nm, 3% oxalate potassium gel, and a placebo treatment. However, in a more recent research, Bal [41] reported superiority of 685 nm compared with a placebo group and no significant difference between 685 nm and an arginine-carbonate calcium agent. Finally, Garcia-Delaney [17] noticed a significant statistical difference between 660 nm and the control group.
The present clinical trial also ended up with positive results, as the 670-nm treatment (treatment B) succeeded immediate and satisfactory relief from pain with long-lasting results. In conclusion, null hypothesis is rejected as the main effects of time and group were found to be statistically significant and so was the time × group interaction effect. However, the three examined treatments did not reveal significant differences between them concerning the final relief.
Limitations of this study concern the lack of placebo group and the inability to record patients’ response to pain objectively with NRS.
Even though PBM is a contemporary and promising treatment in DH field, more randomized clinical trials are necessary to create protocols with the appropriate parameters. The right use of PBM devices could offer a reliable treatment option which is not influenced by external factors.
Conclusion
-
1.
Calmodent showed statistically significant higher mean value than PRG Barrier Coat at 15 min, as also at 1 month, while the 670-nm laser did not differ significantly from both Calmodent and PRG Barrier Coat at any time point.
-
2.
Calmodent showed a mean reduction over time.
-
3.
Regarding the 670-nm laser, the mean NSR value at baseline was significantly higher than that at 15 min, at 1 month, and at 3 months, while no other differences between time points were observed.
-
4.
PRG Barrier Coat revealed a similar pattern to the laser device used. The mean NRS value at baseline was significantly higher than that at 15 min, at 1 month, and 3 months, while there were no other differences between time points.
-
5.
All three treatments did not present significant differences between initial and final measurements.
In conclusion, all three treatments can offer immediate and satisfactory relief from DH pain with long-lasting results.
References
Addy M, Mostafa P, Absi EG, Adams D (1985) Cervical dentine hypersensitivity. Etiology and management with particular reference to dentifrices (1985). In: Rowe NH (ed) Proceedings of Symposium on Hypersensitive Dentin. Origin and Management. University of Michigan, Ann Arbor, MI, pp 147–167
Holland GR, Narhi MN, Addy M, Gangarosa L, Orchardson R (1997) Guidelines for the design and contact of clinical trials on dentine hypersensitivity. J Clin Periodontol 24:808–813
Canadian Advisory Board on Dentin Hypersensitivity (2003) Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity. J Can Dent Assoc 69(4):221–226
Dababaneh RH, Khouri AT, Addy M (1999) Dentine hypersensitivity-an enigma? A review of terminology, epidemiology, mechanisms, aetiology and management. Br Dent J 187(11):606–611
Absi EG, Addy M, Adams D (1987) Dentine hypersensitivity. A study of the patency of dentinal tubules in sensitive and non-sensitive cervical dentine. J Clin Periodontol 14:280–284
Yoshiyama M, Masada J, Ushida A, Ishida A (1989) Scanning electron microscopic characterization of sensitive vs insensitive human radicular dentin. J Dent Res 68:1498–1502
Addy M (2002) Dentine hypersensitivity: new perspectives on an old problem. Int Dent J 52:367–375
Dowell P, Addy M (1983) Dentine hypersensitivity - a review. Aetiology, symptoms and theories of pain production. J Clin Periodontol 10:341–350
Scully C, Felix DH (2006) Oral medicine—update for the dental practitioner orofacial pain. Br Dent J 200(2):75–83
Nilam B, Bhavsar N, Sahayata V, Acharya A, Kshatriya P (2012) A double blind controlled trial comparing three treatment modalities for dentin hypersensitivity. Med Oral Patol Oral Cir Bucal May 1 17(3):483–490
Øggard B, Aim AA, Larsson E, Adfolsson U (2006) A prospective, randomized clinical study on the effects of an amine fluoride / stannous fluoride toothpaste/mouthrinse on plaque, gingivitis and initial caries lesion development in orthodontic patients. Eur J Orthod 28:8–12
Isabel PCC, Andrade AKM, Montes MAJR (2009) Diagnosis and treatment of dentinal hypersensitivity. J Oral Sci 51(3):323–332
Fabrizio S, Petrucci A, Gatto R, Monaco A (2011) Effectiveness of laser in dentinal hypersensitivity treatment: a systematic review. J Endod 37(3):297–303
Kimura Y, Wilder-Smith P, Yonaga K, Matsumoto K (2000) Treatment of dentine hypersensitivity by lasers: a review. J Clin Periodontol 27:715–721
Carroll DJ, Michael RM, Cooper PR, Hadis M, Palin WM (2014) Developments in low level light therapy (LLLT) for dentistry. Dent Mater 30(5):465–475
Juanita AJ, Lanzafame RJ, Arany PR (2015) Low-level light/laser therapy versus photobiomodulation therapy. Photomed Laser Surg 33(4):183–184
Christina G-D, Abad-Sanchez D, Arnabat-Dominguez J, Valmaseda-Castellon E, Gay-Escoda C (2017) Evaluation of the effectiveness of the photobiomodulation in the treatment of dentine hypersensitivity after basic therapy. A randomized clinical trial. J Clin Exp Dent 9(5):694–702
Banoczy J, Szoke J, Kertesz P, Toth Z, Zimmermann P, Gintner Z (1989) Effect of amine of fluoride/stannous fluoride-containing toothpaste and mouthrinsing on dental plaque, gingivitis, plaque and enamel F- accumulation. Caries Res 23:284–288
Ganss C, Schlueter N, Hardt M, Schattenberg P, Klimek J (2008) Effect of fluoride compounds on enamel erosion in vitro: a comparison of amine, sodium and stannous fluoride. Caries Res 42:2–7
S.B. Huang, S.S Gao, H.Y Yu. Effect of nano-hydroxyapatite concentration on remineralization of initial enamel lesion in vitro (2009) Biomed Mater 4 034104 (6pp)
Huang SB, Gao S, Cheng L, Yu H (2011) Remineralization potential of nano-hydroxyapatite on initial enamel lesions: an in vitro study. Caries Res 45:460–468
Ying W, Kaga M, Kajiwara D, Minamikawa H, Kakuda S, Hashimoto M, Yawaka Y (2011) Ion release and buffering capacity of S-PRG filler-containing pit and fissure sealant in lactic acid. Nano Biomed 3(2):275–281
Kotaku M, Murayama R, Shimamura Y, Takahashi F, Suzuki T, Kurokawa H, Miyazaki M (2014) Evaluation of the effects of the fluoride-releasing varnish on dentine demineralization using optical coherence tomography. Dent Mater J 33(5):648–655
Kunio I, Tay FR, Endo T, David HP (2008) A review of chemical-approach and ultramorphological studies on the development of fluoride-releasing dental adhesives comprising new pre-reacted glass ionomer (PRG) fillers. Dent Mater J 27(3):315–339
Hoon C, Dai T, Sharma SK, Huang Y-Y, James DC, Hamblin MR (2012) The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng February 40(2):516–533
Mooney D, Arany P (2014) Using lasers for steam cell therapies. Spie Newsroom. https://doi.org/10.1117/2.1201407.005574
Roberta CT, David MA, Patricia JA (2007) 830 nm laser irradiation induces varicosity formation, reduces mitochondrial membrane potential and blocks fast axonal flow in small and medium diameter rat dorsal root ganglion neurons: implications for the analgesic effects of 830 nm laser. J Peripher Nerv Syst 12:28–39
Chow T. Roberta. Phototherapy and the peripheral nervous system (2011) Photomedicine and Laser Surgery Vol 29, Number 9
Tina K (1989) Photobiology of low-power laser effects. Health Physical 56(5 (May)):691–704
Wakabayashi H, Hamba M, Matsumoto K, Tachibana H (1993) Effect of irradiation by semiconductor laser on responses evoked in trigeminal caudal neurons by tooth pulp stimulation. Lasers Surg Med 13:605–610
Tate Y, Yoshida K, Yosida N, Iwaku M, Okiji T, Oshima H (2006) Odontoblast responses to GaAlAs laser irradiation in rat molars: an experimental study using heat-shock protein-25 immunohistochemistry. Eur J Oral Sci 114:50–57
Hebert FSC, Navarro RL, Oltrmamari-Navarro PVP, Rodrigo FO, Oliviera DAAP, Andraus RAC, Fuirini N, Fernandes KBP (2015) Anti-inflammatory and analgesic effects of low-level laser therapy on the postoperative healing process. J Phys Ther Sci 27:1645–1648
Gerald R, Ross A (2009) Photobiostimulation: an invaluable tool for all dental specialties. J Laser Dent 17:117–124
Huang Y-Y, Chen ACH, Carroll JD, Hamblin MR (2009) Biphasic dose response in low level light therapy. Dose-Response 7:358–383
Saleem AM (2017) Laser photobiomodulation in dentistry. Adv Dent Oral Health 3:4 February
Corona SAM, Do Nascimento TN, Catirse ABE, Lizarelli RFZ, Dinelli W, Palma-Dibb RG (2003) Clinical evaluation of low-level laser therapy and fluoride varnish for treating cervical dentinal hypersensitivity. J Oral Rehabil 30:1183–1189
Pinheiro LTCCG, Pinheiro A, Campos RA d G, Junior AB, Zanin F, Albarnaz PLM, Weckx LLM (2004) Laser therapy in the treatment of dentine hypersensitivity. Braz Dent J 15(2):144–150
Alparslan D, Canakci V, Ozdemir A, Kaya Y (2009) Clinical evaluation of Nd:YAG and 685nm diode laser therapy for desensitization of teeth with gingival recession. Photomed Laser Surg 27(6):843–848
Churcre GL, Greg SLA (2004) Clinical evaluation of dentine hypersensitivity treatment with low intensity gallium-aluminium-arsenide laser-AsGaAl. J.Appl Oral Sci 12(4):267–272
Magacho VAH, Passos VF, Assis JS, Mendonca JS, Santiago SL (2009) Clinical evaluation of a 3% potassium oxalate gel and a GaAlAs laser for the treatment of dentinal hypersensitivity. Photomed Laser Surg 27(5):807–812
Mehmet BV, Keskiner II, Sezer U, Acikel C, Saygun I (2015) Comparison of low level laser and arginine-calcium carbonate alone or combination in the treatment of dentin hypersensitivity: a randomized split mouth clinical study. Photomed Laser Surg 33(4):200–205
Acknowledgments
The authors would like to express their gratitude to Karagiannis Vassilios, Research Personnel, School of Mathematics Of Aristotele University of Thessaloniki for his valuable help to statistical methods.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Approval by Ethics Committee of Aristotele University of Thessaloniki (Dental Shcool) was obtained (20 April 2016 No35), and the patients singed written consent.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Papadopoulou, A., Vourtsa, G., Tolidis, K. et al. Clinical evaluation of a fluoride gel, a low-level laser, and a resin varnish at the treatment of dentin hypersensitivity. Laser Dent Sci 3, 129–135 (2019). https://doi.org/10.1007/s41547-019-00057-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41547-019-00057-8