Abstract
Purpose
This study aimed to evaluate the effects of photobiomodulation (PBM) on CD31 expression in pulp tissue repair of human primary teeth.
Materials and methods
Fifteen mandibular primary molars were divided into the following groups: GI—calcium hydroxide (CH), GII—PBM + CH, and GIII—PBM + zinc oxide/eugenol (ZOE). In the GII and GII groups, an indium–gallium–aluminum phosphide (InGaAlP) diode laser was used for the irradiation through 320-μm diameter optical fiber in contact with pulp tissue. The red laser diode parameters were set at 660-nm wavelength, 10-mW power output, 2.5-J/cm2 energy density, 50–60-Hz frequency, output beam area of 0.04 cm2, and irradiation time of 10 s in continuous mode. After pulpotomy treatment, clinical and radiographic follow-ups were accomplished until the teeth achieved regular exfoliation period. Teeth were extracted for histological analysis and immunolocalization of CD31. Histopathological statistical analyses were performed by Kruskal-Wallis followed by Dunn test to determine statistically significant differences (p < 0.05).
Results
Although GII showed no inflammation or discreet inflammatory cells, the comparison among the groups using a score system revealed no statistically significant differences regarding inflammation and the amount of blood vessels. The immunohistochemistry analysis revealed positive CD31 expression in the blood vessels of all the studied groups. Immunostaining was observed in large blood vessels associated with inflammatory cells on GI and GIII, whereas GII showed discreet immunostaining predominantly in small blood vessels scattered throughout the pulp tissue with none or rare inflammatory cells.
Conclusion
The CD31 expression was greater in the group PBM when associated with calcium hydroxide. Photobiomodulation therapy followed by calcium hydroxide helped in repairing and seemed to regulate angiogenesis and leukocyte migration processes in the remaining pulp tissue after primary teeth pulpotomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pulpotomy consists of removing the infected and inflamed coronal pulp and maintaining the vital radicular pulp, aiming at preserving the primary tooth until the ideal period of exfoliation [1, 2]. Pulp capping materials recover the remaining pulp to stimulate dentinal barrier formation [2]. The ideal capping material is biocompatible and can be associated with alternative therapies, such as photobiomodulation (PBM) application [3].
The use of PBM in pulp treatment has gained space in pediatric dentistry due to its anti-inflammatory effects on pulp tissue through increasing vascularization, while decreasing edema and pain [3, 4]. Pulp responses can be evaluated clinically, radiographically, histologically, as well as, through immunohistochemistry by the expression of markers related to the repair of the remaining pulp [1, 5].
Cluster of differentiation 31 (CD31) is a protein that regulates cellular adhesion signaling, leukocyte migration, activation of platelets and T cells, and angiogenesis [6, 7]. This marker is expressed in the endothelial cells of blood vessels of the pulp tissue and is related to the inflammatory response because it participates in the repair of cell diapedesis towards the inflamed area. Additionally, CD31 is linked to angiogenesis and vasculogenesis contributing to the regenerative functions of the pulp [8, 9].
This study aimed to evaluate the effects of photobiomodulation on CD31 expression in pulp tissue repair of human primary teeth.
Material and methods
Ethical considerations
All the parents or legal guardians of the children participating in the research signed the informed consent form at pretreatment screening period, after approval by the Institutional Review Board of Bauru School of Dentistry, University of Sao Paulo regarding ethical aspects (Protocol no. #058/2011) and in accordance with the Helsinki Declaration.
Sample selection
Inclusion criteria for tooth selection were as follows: primary mandibular first or second molars compromised by deep caries with the possibility of proper restoration, vital pulp with no fistula or abscess, and absence of internal or external root resorption at radiographic examination. Exclusion criteria were related to the presence of systemic pathology and history of allergic reaction to local anesthetics or some of the constituents of the dressing materials.
Clinical procedures
Fifteen molars were divided into the study groups: GI—calcium hydroxide (CH), GII—photobiomodulation therapy followed by calcium hydroxide (PBM + CH), and GIII—photobiomodulation therapy followed by zinc oxide and eugenol (PBM + ZOE).
Two previously calibrated pediatric dentists performed pulpotomy procedures, at one single session as described previously [4]. In CH group, the remaining pulp tissue was dressed with calcium hydroxide alone after hemostasis (Biodinâmica Química e Farmacêtica Ltda., Ibiporã, PR, Brazil). In the PBM groups, an indium–gallium–aluminum phosphide (InGaAlP) diode laser (Twin Flex Evolution, MMOptics®—São Carlos/São Paulo, Brazil) was used for the irradiation through 320-μm diameter optical fiber in contact with pulp tissue [3]. The red laser diode parameters were set at 660-nm wavelength, 10-mW power output, 2.5-J/cm2 energy density, 50–60-Hz frequency, output beam area of 0.04 cm2, and irradiation time of 10 s in continuous mode as previously described [3, 4]. Prior to laser application, the output power was checked by a radiometer (Laser Check, MMOptics®—São Carlos/São Paulo, Brazil). After laser irradiation in PBM + CH and PBM + ZOE groups, powdered calcium hydroxide and zinc oxide and eugenol paste covered the pulp remaining, respectively. All teeth were restored with reinforced zinc oxide-eugenol (IRM, Dentsply, Petropolis, RJ, Brazil) followed by resin-modified glass ionomer cement (Vitremer, 3M ESPE, São Paulo, SP, Brazil). Then, the patients were dismissed and recalled at periodic follow-ups at 3-month intervals, until the treated teeth achieved the regular exfoliation period to be extracted for histological and immunohistochemistry analyses [1, 3].
Histological analysis
After extraction, the teeth were immediately fixed in 10% neutral formalin solution for 24 h, and decalcified in 4% EDTA solution for 45–60 days. Then, 5-μm thick serial sections were prepared to be routinely assessed after hematoxylin and eosin staining and submitted to immunohistochemical procedures [1].
To perform the immunohistochemical analysis, 5-μm thick serial tissue sections were digested with Proteinase K (Dako North America, Carpinteria, CA, USA) for 25 min, and the endogenous peroxidase activity was blocked with 3% hydrogen peroxide solution in methanol (0.01 M) (Easy Path (EP) 12-205222, São Paulo, SP, Brazil) for 10 min. Sections were incubated with polyclonal goat anti-human CD31 antibody (Santa Cruz—(M-20) sc—1506, Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 1:100 dilution for 30 min, and then sections were rinsed with phosphate-buffered saline and incubated with a horseradish peroxidase (HRP)-conjugated secondary antibody (Advance, DAKO North America Inc., Carpinteria, CA, USA) for 30 min. Further staining was performed with 3,3′-diaminobenzidine tetra hydrochloride (DAB) solution and Lillie-Mayer’s hematoxylin was used to counterstained the sections for 60 s.
The histological evaluation was based on the scores previously described for inflammation [3]: 0 (none), 1 (mild), 2 (moderate), 3 (intense), and 4 (necrosis) and vascularization: 0 (absent), 1 (regular amount of blood vessels), and 2 (great amount of blood vessels). For the immunohistochemical evaluation, CD31 expression in the dentin-pulp complex was descriptively analyzed under light microscope. Kruskall-Wallis followed by Dunn test was used to determine statistically significant differences (p < 0.05).
Results
During the evaluation period, all groups demonstrated clinical and radiographic success. Histological analysis of the sections confirmed that the remaining radicular pulp tissue of all teeth remained vital, and the comparison among the groups using the score system revealed no statistically significant difference regarding inflammation and the amount of blood vessels, as shown in Table 1 (p > 0.05).
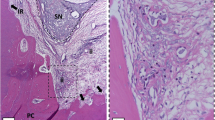
Microscopic descriptive evaluation of GI revealed a variable but mainly mild mononuclear inflammatory infiltrate within dense connective tissue showing regular vascularization. GII presented a dense connective tissue highly vascularized with no inflammatory infiltrate. GIII showed in most of the cases mild to moderate inflammatory infiltration with a loose and highly vascularized connective tissue (Fig. 1a–c).
Representative images of HE staining (a–c) and CD31 immunostaining (d–f) for GI (CH), GII (PBM + CH) and GIII (PBM + ZOE). GI (CH) shows dense connective tissue with regular vascularization and mild mononuclear inflammatory infiltrate (a) in association with CD31-immunostained blood vessels (➔; (d). GII (PBM + CH) shows dense connective tissue with regular vascularization and no inflammatory infiltrate (b), revealing discreet CD31-immunostained blood vessels (➔) scattered by the pulp tissue with none or rare inflammatory cells (e). GIII (PBM + ZOE) shows loose connective tissue highly vascularized with moderate and mainly perivascular inflammatory infiltrate (c), and CD31-immunopositivity in large blood vessels (➔; f). Scale bars indicate 20 μm (d, e), 50 μm (a) and 100 μm (b, c and f); ii means inflammatory infiltrate
The immunohistochemistry analysis revealed positive CD31 expression in the blood vessels of all the studied groups. Notwithstanding, GI and GIII immunostaining was observed in large blood vessels associated with inflammatory cells, whereas GII showed discreet immunostaining predominantly in small blood vessels scattered by the pulp tissue with none or rare inflammatory cells (Fig. 1d–f).
Discussion
The decision on which material should be placed over the remaining pulp tissue following pulpotomy is controversial because the available capping materials do not present all the requirements for an ideal material. Our study showed that PBM prior to calcium hydroxide assisted in repairing process, decreasing inflammation, and enriching vascularization. The rationale behind this affirmation is that GII sections revealed lack of both inflammatory cells and enlarged blood vessels on histological and immunohistochemistry analyses. Although calcium hydroxide is routinely used as capping material in primary teeth, many studies report different success rates [2, 10, 11]. Further, other histological studies described the variation in chronic inflammatory response to calcium hydroxide [2, 3, 12,13,14,15,16]. Thus, long-term efficacy of calcium hydroxide is considered controversial [13]. Moreover, the pulp inflammatory condition is relevant to reach success after employing calcium hydroxide as capping material in the pulpotomy of primary teeth [17].
Inflammation is part of the repair process that involves the migration of leukocytes, cell differentiation, and proliferation [18,19,20]. Some studies evaluated the expression of markers related to the process of tooth tissue formation after pulp treatment with different materials in animals [21, 22] and human teeth [1]. Lourenço Neto et al. [5] evaluated DMP-1 expression, which acts on hard tissue formation, after the use of PBM over the pulp tissue following pulpotomy and found that PBM associated with calcium hydroxide helped in repairing. As far as we are concerned, this is the first evaluation of CD31 expression after PBM on pulp tissue of pulpotomized primary teeth.
Currently, PBM is used as adjuvant to stimulate the healing process of damaged hard tissues [3, 4, 23, 24]. To reach anti-inflammatory action, the light energy is absorbed by cellular chromophores and transformed into chemical energy, resulting in alterations in cell metabolism [25]. Fibroblasts have many photoreceptor molecules, which release chemical mediators when stimulated, mainly at the beginning of the healing process [26]. The positive biostimulation induced by laser regulates different factors related to healing process: modulation of inflammation, acceleration of epithelialization, increase of collagen synthesis, and stimulation of vascular neoformation [3, 27]. The blood vessel neoformation allows providing enough nutritional supply to fibroblasts during tissue repairing process [20].
The growth of new blood vessels based on preexisting vessels is known as angiogenesis [28, 29], which is regulated by many cytokines and growth factors [28, 30, 31]. Angiogenesis can be quantified by immunohistochemical analysis of micro-vessel density, through identifying proteins expressed on the surface of endothelial cells marked by monoclonal antibodies [28]. CD31, a transmembrane glycoprotein from endothelial cells, is one of the markers involved in this process. CD31 is part of the endothelial intercellular junction and is present on the surface of the inflammatory cells, such as monocytes, macrophages, and neutrophils. Additionally to angiogenesis, endothelial cells play a fundamental role in controlling coagulation, thrombolysis, vascular permeability, and inflammation, favoring leukocyte migration [7].
The literature suggests that CD31 molecule has pro-inflammatory action, promoting inflammation by stimulating leukocyte migration; and anti-inflammatory effects, due to its capacity of recruiting cytoplasmic phosphatases that inhibit leukocyte activation; reduces the production of pro-inflammatory cytokines; and restores the integrity of the vascular barrier through mechanisms not fully understood yet [8]. Pimenta et al. [32] histologically evaluated CD31 expression in inflamed and sound pulp tissue of permanent teeth referred for serial extraction. CD31 expression was greater in the inflamed tissue when associated with the expression of the lymphatic marker VEGF-3. Notwithstanding, CD31 was also identified in sound pulp tissue. Bruno et al. [33] evaluated through immunohistochemistry the inflamed and sound pulp tissue of permanent teeth and found CD31 expression both in sound pulp tissue and in pulp tissue with pulp inflammation. The greatest density of CD31 + blood vessels was seen in the teeth with irreversible pulpitis. Similarly, Sawa et al. [34] analyzed the CD31 immunoexpression in inflamed and sound pulp tissue of third molars and found larger blood vessels in inflamed pulp, although CD31 expression was seen in blood vessels of sound pulp tissue. Nevertheless, CD31 expression was more intense especially in medium-size vessels. Large blood vessels favor the increase of tissue fluid with inflammatory cells, which corroborates this present study showing intense CD31 expression in GI and GIII, with larger blood vessels. On the other hand, in GII, small blood vessels showed discreet CD31 expression. The intense expression of adhesion molecules in vascular tissue is associated to leukocyte migration, a critical step in the beginning of the immunological response to tissue inflammation [35].
Angiogenesis modulation through PBM has been investigated to verify a contribution to repairing process. However, the literature lacks consensus on the ideal laser parameters and expected therapeutic effectiveness. Schindl et al. [36] evaluated in vitro the effects of 670-nm LLL irradiation with different dosimetries, showing that energy densities of 2, 4, and 8 J/cm2 stimulated endothelial cell proliferation. Similarly, Góralczyk et al. [18] found that 635-nm LLL irradiations with similar energy densities stimulated cellular proliferation and positively modulated growth factors and receptors involved in the mechanism of vascular neoformation. Based on this information, the wavelength (660 nm) and energy density (2.5 J/cm2) of the red laser employed in our study probably favored the repair of the pulp tissue.
Our study showed the advantages of PBM associated with calcium hydroxide in pulp tissue repairing of primary teeth undergoing pulpotomy. Laboratorial analysis of pulp tissue repair is essential to judge the pulp capping material effectiveness [37], because although many studies reveal high clinical and radiographic success rates of pulp capping materials, inflammatory alterations of the pulp are seen histologically [2, 3, 16, 38, 39]. Thus, the histological results of this study suggest that associations of standard therapies with new technologies may be indicated aiming at advances in the pulp treatment of primary teeth.
Conclusion
The CD31 expression was greater in the group PBM when associated with calcium hydroxide. Photobiomodulation therapy followed by calcium hydroxide helped in repairing and seemed to regulate angiogenesis and leukocyte migration processes in the remaining pulp tissue after primary teeth pulpotomy.
References
Lourenço Neto N, Marques NC, Fernandes AP, Rodini CO, Sakai VT, Abdo RC, Machado MA, Santos CF, Oliveira TM (2016) Immunolocalization of dentin matrix protein-1 in human primary teeth treated with different pulp capping materials. J Biomed Mater Res B Appl Biomater 104:165–169. https://doi.org/10.1002/jbm.b.33379
Oliveira TM, Moretti AB, Sakai VT, Lourenço Neto N, Santos CF, Machado MA, Abdo RC (2013) Clinical, radiographic and histologic analysis of the effects of pulp capping materials used in pulpotomies of human primary teeth. Eur Arch Paediatr Dent 14:65–71. https://doi.org/10.1007/s40368-013-0015-x
Marques NC, Neto NL, Rodini CO, Fernandes AP, Sakai VT, Machado MA, Oliveira TM (2015) Low-level laser therapy as an alternative for pulpotomy in human primary teeth. Lasers Med Sci 30:1815–1822. https://doi.org/10.1007/s10103-014-1656-7
Fernandes AP, Lourenço Neto N, Marques NCT, Moretti ABS, Sakai VT, Silva TC, Andrade Moreira Machado MA, Marchini Oliveira T (2015) Clinical and radiographic outcomes of the use of low-level laser therapy in vital pulp of primary teeth. Int J Paediatr Dent 25:144–150. https://doi.org/10.1111/ipd.12115
Lourenço Neto N, Marques NCT, Fernandes AP, Rodini CO, Silva TC, Machado MA, Oliveira TM (2015) Expression of DMP-1 in the human pulp tissue using low level laser therapy. Laser Phys 25:095601
Clement M, Fornasa G, Guedj K, Ben Mkaddem S, Gaston AT, Khallou-Laschet J, Morvan M, Nicoletti A, Caligiuri G (2014) CD31 is a key coinhibitory receptor in the development of immunogenic dendritic cells. Proc Natl Acad Sci U S A 111:E1101–E1110. https://doi.org/10.1073/pnas.1314505111
Pusztaszeri MP, Seelentag W, Bosman FT (2006) Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J Histochem Cytochem 54:385–395
Privratsky JR, Newman DK, Newman PJ (2010) PECAM-1: conflicts of interest in inflammation. Life Sci 87:69–82. https://doi.org/10.1016/j.lfs.2010.06.001
Nakashima M, Iohara K, Sugiyama M (2009) Human dental pulp stem cells with highly angiogenic and neurogenic potential for possible use in pulp regeneration. Cytokine Growth Factor Rev 20:435–440. https://doi.org/10.1016/j.cytogfr.2009
Alaçam A, Odabaş ME, Tüzüner T, Sillelioğlu H, Baygin O (2009) Clinical and radiographic outcomes of calcium hydroxide and formocresol pulpotomias performed by dental students. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 108:e127–e133. https://doi.org/10.1016/j.tripleo.2009.07.017
Sonmez D, Sari S, Cetinbas T (2008) A comparison of four pulpotomy techniques in primary molars: a long-term follow-up. J Endod 34:950–955. https://doi.org/10.1016/j.joen.2008.05.009
Accorinte ML, Holland R, Reis A, Bortoluzzi MC, Murata SS, Dezan E Jr, Souza V, Alessandro LD (2008) Evaluation of mineral trioxide aggregate and calcium hydroxide cement as pulp-capping agents in human teeth. J Endod 34:1–6
Chacko V, Kurikose S (2006) Human pulpal response to mineral trioxide aggregate (MTA): a histologic study. J Clin Pediatr Dent 30:203–209
Tabarsi B, Parirokh M, Eghbal MJ, Haghdoost AA, Torabzadeh H, Asgary S (2010) A comparative study of dental pulp response to several pulpotomy agents. Int Endod J 43:565–571. https://doi.org/10.1111/j.1365-2591.2010.01711.x
Tunc ES, Saroglu I, Sari S (2006) The effect of sodium hypochlorite application on the success of calcium hydroxide pulpotomy in primary teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 102:e22–e26
Waterhouse PJ, Nunn JH, Whitworth JM, Soames JV (2000) Primary molar pulp therapy—histological evaluation of failure. Int J Paediatr Dent 10:313–321
Huth KC, Paschos E, Hajek-Al-Khatar N, Hollweck R, Crispin A, Hickel R, Folwaczny M (2005) Effectiveness of 4 pulpotomy techniques—randomized controlled trial. J Dent Res 84:1144–1148
Góralczyk K, Szymańska J, Łukowicz M, Drela E, Kotzbach R, Dubiel M, Michalska M, Góralczyk B, Zając A, Rość D (2015) Effect of LLLT on endothelial cells culture. Lasers Med Sci 30:273–278. https://doi.org/10.1007/s10103-014-1650-0
Tomaszewska JM, Miskowiak B, Matthews-Brzozowska T, Wierzbicki P (2013) Characteristics of dental pulp in human upper first premolar teeth based on immunohistochemical and morphometric examinations. Folia Histochem Cytobiol 51:149–155. https://doi.org/10.5603/FHC.2013.0023
Yang H, Shin S, Ahn J, Choi Y, Kim KH, Chung CJ (2013) Local injection of pulp cells enhances wound healing during the initial proliferative phase through the stimulation of host angiogenesis. J Endod 39:788–794. https://doi.org/10.1016/j.joen.2013.01.011
Nakamura Y, Slaby I, Matsumoto K, Ritchie HH, Lyngstadaas SP (2004) Immunohistochemical characterization of rapid dentin formation induced by enamel matrix derivative. Calcif Tissue Int 75:243–252
Olsson H, Davies JR, Holst KE, Schröder U, Petersson K (2005) Dental pulp capping: effect of Emdogain Gel on experimentally exposed human pulps. Int Endod J 38(186):194
Ferreira AN, Silveira L, Genovese WJ, de Araújo VC, Frigo L, de Mesquita RA, Guedes E (2006) Effect of GaAIAs laser on reactional dentinogenesis induction in human teeth. Photomed Laser Surg 24:358–365
Godoy BM, Arana-Chavez VE, Núñez SC, Ribeiro MS (2007) Effects of low-power red laser on dentine-pulp interface after cavity preparation. An ultrastructural study. Arch Oral Biol 52:899–903
Karu TI, Pyatibrat LV, Kalendo GS (2004) Photobiological modulation of cell attachment via cytochrome c oxidase. Photochem Photobiol Sci 3:211–216
Garcia VG, de Lima MA, Okamoto T, Milanezi LA, Júnior EC, Fernandes LA, de Almeida JM, Theodoro LH (2010) Effect of photodynamic therapy on the healing of cutaneous third-degree-burn: histological study in rats. Lasers Med Sci 25:221–228. https://doi.org/10.1007/s10103-009-0694-z
Rodrigues NC, Brunelli R, de Araújo HS, Parizotto NA, Renno AC (2013) Low-level laser therapy (LLLT) (660nm) alters gene expression during muscle healing in rats. J Photochem Photobiol B 120:29–35. https://doi.org/10.1016/j.jphotobiol.2013.01.002
Artese L, Rubini C, Ferrero G, Fioroni M, Santinelli A, Piattelli A (2002) Vascular endothelial growth factor (VEGF) expression in healthy and inflamed human dental pulps. J Endod 28:20–23
Yamanaka Y, Kaneko T, Yoshiba K, Kaneko R, Yoshiba N, Shigetani Y, Nör JE, Okiji T (2012) Expression of angiogenic factors in rat periapical lesions. J Endod 38:313–317. https://doi.org/10.1016/j.joen.2011.11.009
Oliveira TM, Sakai VT, Machado MA, Dionísio TJ, Cestari TM, Taga R, Amaral SL, Santos CF (2008) COX-2 inhibition decreases VEGF expression and alveolar bone loss during the progression of experimental periodontitis in rats. J Periodontol 79:1062–1069. https://doi.org/10.1902/jop.2008.070411
Silva TC, Oliveira TM, Sakai VT, Dionísio TJ, Santos CF, Bagnato VS, Machado MA (2010) In vivo effects on the expression of vascular endothelial growth factor A165 messenger ribonucleic acid of an infrared diode laser associated or not with a visible red diode laser. Photomed Laser Surg 28:63–68. https://doi.org/10.1089/pho.2008.2403
Pimenta FJ, Sa AR, Gomez RS (2003) Lymphangiogenesis in human dental pulp. Int Endod J 36:853–856
Bruno KF, Silva JA, Silva TA, Batista AC, Alencar AH, Estrela C (2010) Characterization of inflammatory cell infiltrate in human dental pulpitis. Int Endod J 43:1013–1021. https://doi.org/10.1111/j.1365-2591.2010.01757.x
Sawa Y, Yoshida S, Shibata KI, Suzuki M, Mukaida A (1998) Vascular endothelium of human dental pulp expresses diverse adhesion molecules for leukocyte emigration. Tissue Cell 30:281–291
Bagis B, Atilla P, Cakar N, Hasanreisoglu U (2009) An immunohistochemical evaluation of cell adhesion molecules in human dental pulp after tooth preparation and application of temporary luting cements. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 107:137–144. https://doi.org/10.1016/j.tripleo.2008.09.022
Schindl A, Merwald H, Schindl L, Kaun C, Wojta J (2003) Direct stimulatory effect of low-intensity 670 nm laser irradiation on human endothelial cell proliferation. Br J Dermatol 148:334–336
Parirokh M, Asgary S, Eghbal MJ, Stowe S, Eslami B, Eskandarizade A, Shabahang S (2005) A comparative study of white and grey mineral trioxide aggregate as pulp capping agents in dog’s teeth. Dent Traumatol 21:150–154
Caicedo R, Abbott PV, Alongi DJ, Alarcon MY (2006) Clinical, radiographic and histological analysis of the effects of mineral trioxide aggregate used in direct pulp capping and pulpotomies of primary teeth. Aust Dent J 51:297–305
Percinoto C, de Castro AM, Pinto LM (2006) Clinical and radiographic evaluation of pulpotomies employing calcium hydroxide and trioxide mineral aggregate. Gen Dent 54:258–261
Acknowledgements
The authors would like to thank all the volunteers for their participation in this study, and the excellent laboratorial assistance of Daniele Santi Ceolin and Patrícia De Sá Mortágua Gemino.
Financial support
This work was supported by Sao Paulo Research Foundation (FAPESP) [grant numbers 2009/11284-4] to TMO.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All the parents or legal guardians of the children participating in the research signed the informed consent form at pretreatment screening period, after approval by the Institutional Review Board of Bauru School of Dentistry, University of Sao Paulo regarding ethical aspects (Protocol no. #058/2011) and in accordance with the Helsinki Declaration.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Marques, N.C.T., Neto, N.L., Prado, M.T.O. et al. CD31 expression in human primary teeth treated with photobiomodulation therapy. Laser Dent Sci 2, 103–108 (2018). https://doi.org/10.1007/s41547-018-0025-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41547-018-0025-4