Abstract
Understanding the neural and cognitive mechanisms underlying time estimation remains a challenge. Transcranial electric stimulations, such as transcranial random noise stimulation (tRNS), are useful tools to interfere with brain activity and identifying brain areas involved in temporal processing. Here, the aim is to investigate the specific role of primary sensory cortices (either V1 or A1) in temporal processing and to further investigate if the stimulation acts on either perceived duration or temporal sensitivity. Forty-eight university students were included in the study. Twenty-four participants were stimulated over A1, and 24 participants were stimulated over V1. All participants performed a time bisection task, either in a visual or auditory modality, involving standard durations lasting 300 ms (short) and 900 ms (long). When tRNS was delivered over A1, an effect of stimulation was observed on perceived duration (temporal over-estimation) under random stimulation compared to sham in both visual and auditory modalities. When tRNS was delivered over V1, the effect of stimulation was observed only in the visual modality (temporal over-estimation). No effect of stimulation was observed on temporal sensitivity in any condition. Our results showed for the first time that tRNS acts on modulating an individual’s perceived duration, but not on temporal sensitivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ability to estimate the passage of time is fundamental for the efficient functioning of perceptual and cognitive processes. However, understanding the neural and cognitive mechanisms underlying time estimation remains a fine challenge (Bueti 2011; Grondin 2014). The idea that we time sensory signals via a single “centralised” and “amodal” clock has dominated the field of temporal cognition for decades (Gibbon et al. 1984). However, alternative positions propose that we have multiple timing mechanisms ‘distributed’ across brain areas or circuits and that the engagement of each single mechanism depends on the psychophysical task, sensory modality, and lengths of time intervals (Allman et al. 2014; Finnerty et al. 2015; Ivry and Schlerf 2008; Mauk and Buonomano 2004; Merchant et al. 2013; van Rijn et al. 2014).
It has been suggested that both modality-specific and supra-modal mechanisms underlie the estimation of temporal intervals and several of these studies used the duration bisection task. In this task, participants are presented with a ‘short’ and a ‘long’ anchor duration and are subsequently required to classify other signal durations as more similar to the ‘short’ or the ‘long’ anchors (Bausenhart et al. 2018; Mioni 2018; Penney et al. 1998). The resulting psychophysical functions (proportion of ‘long’ responses plotted against the stimulus duration) produced by humans for visual signals were shifted towards the right compared to those for auditory signals. Thus, the participants produced fewer ‘long’ responses for a given visual signal duration than for an equivalent auditory signal duration, indicating that the former was judged to be shorter than the latter. Moreover, temporal discrimination is better (lower discrimination thresholds) if auditory signal marks the temporal interval compared to visual signal (Grondin 1993; Grondin et al. 2005; Merchant et al. 2008). However, the capacity to keep in memory multiple intervals improves if the temporal signals belong to different modalities and therefore rely on different memory resources (Gamache and Grondin 2010; Penney 2003; Penney et al. 2000). Moreover, other studies showed that perceived duration of a sensory event can be distorted by modality-specific properties of the stimuli such as visual adaptation (Johnston et al. 2006; Ayhan et al. 2009), spatial, and temporal frequency (Kanai et al. 2006; Kaneko and Murakami, 2009). In addition, in the case of saccadic eye movements, the distortion of the perceived duration is limited to a single sensory domain; a compression of the perceived duration is evident for visual but not for auditory stimuli (Morrone et al. 2005; Burr et al. 2011).
Non-invasive brain stimulation techniques are used to directly interfere with brain activity and measuring behavioural outcomes (Costa et al. 2015b; Jacobson et al. 2012). A consistent number of studies have been conducted using transcranial magnetic stimulation (TMS) to investigate the involvement of specific brain areas in temporal processing (Wiener 2013; Koch et al. 2009), but as far as we know, only two studies have specifically addressed the issue of modality-specificity and supra-modal mechanisms dedicated to time perception (Bueti and Walsh 2009; Kanai et al. 2011). Specifically, Bueti et al. (2008) concluded that the right posterior parietal cortex has a key role on processing temporal information of both auditory and visual stimuli, whereas MT/V5 seems to process only the timing of visual events. Instead, Kanai et al. (2011) found that a disruption of the primary auditory cortex (A1) impaired both auditory and visual temporal stimuli and that TMS over the primary visual cortex (V1) impaired performance only in visual time discrimination. This suggests a supra-modal role (i.e. the ability to process information from different sensory modalities) of the auditory cortex and a modality-specific role of the visual cortex on time perception. Interestingly, in both cases, the authors reported that TMS modulated the sensitivity (i.e. higher temporal variability after the TMS stimulation) rather than the subjective perceived duration of the stimuli.
Surprisingly, very few studies have been conducted using transcranial electric stimulation (tES). TES is a technique that does not induce activity on resting neuronal networks, but modulates spontaneous neuronal activity; consequently, the amount and the direction of tES-related outcomes critically depend on the previous physiological state of the target neural structures (Woods et al. 2016). Vicario et al. (2013) combining transcranial direct current stimulation (tDCS) and a time reproduction task (visual stimuli between 1500 and 1900 ms) observed that cathodal stimulation over the right posterior parietal cortex (rPPC) affected temporal accuracy by leading participants to over-estimate time intervals. Oyama et al. (2017) using a time discrimination task (visual stimuli, midpoint duration 1800 ms) showed a lower threshold under the cathodal condition compared to sham condition. The authors concluded that an inhibition of the rPPC leads to an improvement in temporal discrimination performance: a result that is in contrast with Vicario et al. (2013) findings.
More germane to the present study, Mioni et al. (2016b) used both visual and auditory stimuli targeting A1 and primary visual cortex V1 trying to replicate and extend Kanai et al. (2011) results with tDCS. Compared to sham stimulation, they observed lower temporal sensitivity under anodal and cathodal stimulations when A1 was targeted in both visual and auditory modalities, whereas lower temporal sensitivity was observed under cathodal stimulation in visual modality when V1 was stimulated. In line with previous findings (Kanai et al. 2011), no effect of stimulation was observed on perceived duration but only on temporal sensitivity. A possible limitation of our study was the lack of systematic correspondence between the anodal-excitation and cathodal-inhibition effects on time perception. Although the distinction between anodal-facilitatory/cathodic-inhibitory is mainly confirmed by studies on motor functions (Antal et al. 2004a; Antal et al., 2004b), it seems not to be generalizable to all cognitive functions, since several issues, including the timing of stimulation, the excitability status of the cortical area, and the type of task, can influence the outcomes (Battaglini et al. 2017; Costa et al., 2015a; Jacobson et al. 2012).
In contrast to tDCS, transcranial random noise stimulation (tRNS) has no constraint of current flow direction sensitivity; in fact, the intensity and the frequency of the current vary in a random manner. This technique is newer than other tES applications; therefore, speculation about its possible mechanisms of action in cognition is rare to date (Miniussi and Ruzzoli 2013). The mechanisms of action of tRNS might be based on repeated subthreshold stimulations that collectively prevent the homeostasis of the system (Fertonani et al. 2011; Terney et al. 2008). The effects of tRNS may also be explained in the context of the stochastic resonance phenomenon (Miniussi et al. 2010; Moss et al. 2004; Stacey and Durand 2000); tRNS is, by definition, a stimulation that induces non-finalised random activity in the system (i.e. noise). The presence of neuronal noise might enhance the sensitivity of the neurons to a given range of weak inputs (the neurons with the same directionality as the signal), thereby introducing a functionally useful noise to the signal and increasing the signal. Importantly, less aversive sensations were reported by participants during tRNS compared to tDCS. Therefore, the application of tRNS might be better suited for placebo-controlled studies (Ambrus et al. 2010; Antal and Herrmann 2016; Fertonani et al. 2011).
Based on these observations, in a recent study, Mioni et al. (2018) used a tRNS procedure to investigate the involvement of the right parietal cortex (P4) in the processing of inter- and intra-modal temporal intervals (Mioni et al. 2018). Interestingly, they found, to our knowledge for the first time, the effect of stimulation on perceived duration but not on sensitivity. Specifically, participants stimulated over P4 generally over-estimated time in the random compared to the sham condition independently of the modality of the stimuli presented. No effect of tRNS was observed on temporal sensitivity.
Beside the specific implication regarding the involvement of P4 in temporal processing (Bueti and Walsh 2009), what was interesting in Mioni et al. (2018) study was the effect of stimulation on perceived duration rather than on temporal sensitivity, as previously observed with tDCS (Mioni et al. 2016b) and TMS (Kanai et al., 2011; Bueti et al. 2008). What is the source of this effect? It is critical to evaluate if the stimulation acts on perceived duration (i.e. changing the subjective feeling of the duration of a temporal interval producing a temporal bias) or on temporal sensitivity (i.e. Weber ratio, WR) (Bausenhart et al. 2018; Eisler et al. 2008; Kopec and Brody 2010). These considerations are of fundamental interest for a correct interpretation of the effects of stimulation on temporal processing and to evaluate the brain areas and circuits involved in temporal processing. Briefly, considering the time bisection task (Bausenhart et al. 2018; Kopec and Brody 2010) from the data collected during the task, experimenters are able to construct a psychometric curve plotting the duration of the stimuli (probes and reference stimuli) versus the subject’s probability of responding ‘long’. At some intermediate duration, the subject’s performance crosses 0.5 on the y-axis. It is this duration, referred to as the bisection point (BP; Allan and Gibbon 1991; Gibbon 1981), that they are equally likely to call ‘long’ or ‘short’. This single, seemingly trivial, point actually offers significant insight to how time is represented and processed in the brain, because, at this duration, the decision process used to compare temporal stimuli to temporal values stored in memory must be equal for both options. This is an index of subjective experience of time. Aside from the BP itself, it is also possible to determine the degree of discriminability (temporal sensitivity) the subject uses to parse the probe trials into the ‘short’ and ‘long’ categories, from the psychometric function. A participant with a high degree of discriminability would produce a psychometric curve that appears very step-like, resulting in a low WR, while another subject with a poorer discriminability would produce a more gradual psychometric function, resulting in a higher WR.
Direct comparison between our two previous studies is difficult considering the different areas targeted (V1/A1, Mioni et al. 2016b; P4, Mioni et al. 2018) and the different methodology adopted (tDCS) (Mioni et al., 2016b, 2018). On the one hand, it is possible that continuous and random stimulations may have a different effect depending on the targeted brain area (Costa et al., 2015b; Costa et al., 2015a). On the other hand, it is possible that the different mechanism of action of continuous and random stimulations (see Fertonani and Miniussi, 2015) may selectively affect either temporal sensitivity or temporal perception.
The present study aims to address this question. Here, we adopted the same procedure used in Mioni et al. (2016b), but instead of tDCS, we used tRNS over A1 and V1. Whether the effect of tRNS on perceived duration was due to the targeted area (P4), and not to the type of stimulation, we should expect an effect on temporal sensitivity (Mioni et al. 2016b). Otherwise, if the findings we reported on perceived duration were due to the type of stimulation, using tRNS, we should expect an effect on the perceived duration, similarly to what was reported in Mioni et al. (2018).
Method
Participants
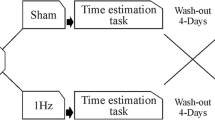
Forty-eight university students were included in the study; 24 participants were stimulated over A1 and 24 over V1, and for each group, half of the participants performed the time bisection task in auditory and the others in visual modality and 8 female were included in each subgroup (Table 1). All participants were right-handed as defined by the Edinburgh Handedness Inventory (Oldfield 1971). Exclusion criteria included a history of neurological or psychiatric illness, pregnancy, and use of drugs or alcohol 24 h prior to the experimental session.
Materials and Procedure
Online high-frequency tRNS was delivered using a battery-driven stimulator (BrainSTIM, EMS) through a pair of saline-soaked sponge electrodes, and the electrodes were kept in place with plastic bandages. The tRNS consisted of a random current of 1.5 mA intensity with a 0 mA offset applied at random frequencies. The frequencies ranged from 100 to 640 Hz. The stimulating electrode (area = 25 cm2) was placed over the right A1 or right V1 according to the international 10/20 system for EEG electrode placement (Oostenveld and Praamstra 2001), and the reference electrode (area = 35 cm2) was placed extra-cephalically on the upper right arm (Mioni et al. 2016b). After electrodes montage, participants performed the training phase (see the ‘Time Bisection Task’ section) without active stimulation followed by the experimental phase (tRNS or sham condition). The total duration of stimulation was 15 min and the total session lasted approximately 40 min. None of the participants reported experiencing pain caused by the stimulation, and all participants included in the study completed all experimental sessions. Participants were tested in two sessions (tRNS and sham) performed in two different days. Sessions were separated by at least 48 h to avoid long-lasting effects of the stimulation, and they were counterbalanced between the subjects (Nitsche et al. 2003; Nitsche et al. 2004). Participants were randomly assigned to one of the two experimental conditions (V1 or A1), depending on the stimulated area. Instruction and learning phases were conducted off-stimulation, and the stimulation started after the training phase. This procedure was adopted to avoid any effect of stimulation during the training phase. The study was approved by the ethics committee of the School of Psychology of Padova (Italy) and conducted according to the Declaration of Helsinki (59th WMA General Assembly, Seoul, 2008).
Time Bisection Task
The experimental session started with the learning phase in which participants were required to memorise the two standard durations: 300 ms (short standard) and 900 ms (long standard). Both standard durations were presented 10 times. After the learning phase, participants were required to judge different temporal intervals (300, 400, 500, 600, 700, 800, 900 ms) and to decide if the comparison interval was more similar to the short standard or to the long standard. The visual stimulus was a grey circle (filled intervals) presented on a white background, while the auditory stimulus was a white noise ramped on and off with two 10-ms raised cosine ramps. Each comparison duration was presented 8 times for a total of 56 trials in each block; participants performed 4 blocks for a total of 224 trials. The participants were asked to respond with their left and right index finger and response keys were counterbalanced between participants. After the response, there was a 1000-ms inter-trial interval. No feedback was provided.
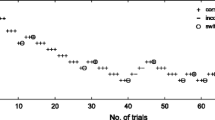
For each participant in each experimental condition, a 7-point psychometric function was traced, plotting the seven comparison intervals on the x-axis and the probability of responding ‘long’ on the y-axis (Fig. 1a, b). The cumulative normal function was fitted to the resulting curves. More specifically, we used a non-linear least squares analysis, with a Levenberg-Marquardt algorithm.
Temporal performance was analysed in terms of perceived duration (bisection point, BP) and temporal sensitivity (Weber Ratio, WR). BP is the stimulus duration at which the participants responded ‘short’ or ‘long’ with equal frequency. An observed shift of the BP can be interpreted as an indicator of differences in time processing, with smaller BP values meaning longer perceived durations. The second dependent variable was the WR, which is based on one standard deviation (SD) on the psychometric function and is an index of time sensitivity. Here, the WR was computed as the SD divided by 600 ms, which was the midpoint duration used in the experiment.
For all participants, the goodness-of-fit of the psychometric function was highly satisfactory, with R2 values above .86 for the tRNS and .93 for the sham conditions. Kolmogorov-Smirnov test showed that all the variables were normally distributed. Two separate repeated measure ANOVAs were conducted with BP and WR as dependent variables separately for A1 and V1 with modality (auditory, visual) as between-subjects factor and stimulation type (tRNS, sham) as within-subjects factor (Kanai et al. 2011; Mioni et al. 2016b). The significant analyses were followed by post hoc analyses with Bonferroni’s correction to reduce the type I error rate, and the effect size was estimated with the partial eta squared index (η2p).
Sensation-Experienced Questionnaire
We included a questionnaire about the sensations experienced during the different types of stimulations (tRNS, sham) (Fertonani et al. 2015). The questionnaire includes eight possible sensations commonly experienced during stimulation. The questionnaire was introduced to evaluate whether unspecific stimulation effects related to different experimental conditions could account for differences in behavioural performance. We calculated a total score adding the scores from all questions included. Data were analysed using Wilcoxon matched-pair signed rank test for stimulation (tRNS vs sham) separately for the four conditions (A1-auditory; A1-visual; V1-auditory; V1-visual).
Results
tRNS over A1
Analyses of BP showed a significant main effect of stimulation type [F(1,22) = 7.05, p = .014, η2p = .24]. Lower BP values in the tRNS compared to the sham condition were observed indicating temporal over-estimation under tRNS condition (Table 1).Footnote 1 No effect of modality (p = .449, η2p = .03) neither interaction modality × stimulation type was found (p = .109, η2p = .11) (Fig. 2a and Fig. 3a).
Analyses of WR showed no significant main effects of modality (p = .259, η2p = .06), stimulation type (p = .716, η2p = .01), or interaction (p = .477, η2p = .12) (Fig. 4a).
tRNS over V1
Analyses of BP showed no main effect of stimulation type (p = .509, η2p = .02) but main effects of modality [F(1,22) = 4.75, p = .040, η2p = .18] and a significant modality × stimulation type interaction [F(1,22) = 7.66, p = .011, η2p = .26] (Fig. 2bFootnote 2 and Fig. 3b). Post hoc analyses showed lower BP were observed when temporal intervals were marked by visual stimuli under tRNS compared to sham stimulation indicating temporal over-estimation (p = .024, η2p = .21) but no effect of stimulation was observed when auditory stimuli were used to mark time (p = .152, η2p = .09) (Table 1); participants over-estimated time in the sham condition when the stimuli were presented in the auditory modality (p = .012, η2p = .26), and no differences were observed between modality under random stimulation (p = .286, η2p = .05).
Analyses of WR showed a significant main effect of modality [F(1,22) = 8.95, p = .007, η2p = .29], indicating higher WR in visual (M = .27) compared to auditory (M = .16). No effects of stimulation type (p = .502, η2p = .02) or interaction were found (p = .403, η2p = .03) (Fig. 4b).
Sensation-Experienced Questionnaire
Analyses of total score after stimulation over A1 showed no difference between tRNS and sham in auditory modality (Z = 0, p = 1.00) neither in the visual modality (Z = 1.68, p = .09). Similarly, after stimulation over V1, no difference between tRNS and sham in auditory modality (Z = 0.40, p = .69) neither in the visual modality (Z = 0.98, p = .33) were observed (Table 2).
Discussion
The present study was conducted to further investigate the effect of non-invasive brain stimulation on subjective time perception and to support the hypothesis that multiple mechanisms mediate time processing. Also, the present study discusses the possible effect of tRNS on perceived duration and/or on temporal variability.
Importantly, the results showed that the effect of tRNS was on perceived duration rather than on temporal variability extending, to A1 and V1, the effect observed (temporal over-estimation) when tRNS was applied over P4 (Mioni et al. 2018). Both perceived duration and temporal variability are important characteristics of temporal performance, with perceived duration reflecting the shift of the psychometric function indicating over- or under-estimation (i.e. a distance of a response to its target) and variability reflecting how spread responses are from the target. Previous studies that investigated possible areas and networks involved in temporal processing when multiple modalities are employed found increased variability after TMS and tDCS stimulation (Bueti et al. 2008; Mioni et al. 2016b; Kanai et al. 2011).
Interestingly, Vicario et al. (2013) suggested that the two parietal cortices could work jointly in the execution of a timing task by acting, in a segregated way, on different aspects such as the accuracy and variability of the participants’ performance. The study showed that temporal accuracy was affected during cathodal stimulation of the right PPC whereas cathodal stimulation of the left PPC did not affect time accuracy but only temporal variability. These results were also confirmed by Oyama and colleagues with a time discrimination task (2017).
At the behavioural level, effects of high-frequency tRNS have been usually demonstrated if applied over the visual cortex (Fertonani et al. 2011) during an orientation discrimination task. A significant enhancement in visual perceptual learning during the application of high-frequency tRNS was observed compared to anodal and cathodal tDCS as well as sham stimulation. TRNS over the lateral occipital cortex facilitated facial identity perception (Romanska et al. 2015). In contrast, tRNS to the right dorsolateral prefrontal cortex impaired categorical learning in a prototype distortion task (Ambrus et al. 2011). These results demonstrate that, depending on the involved cortical area and the type of protocols, tRNS can induce long-term changes (either positive or negative) of cognitive and brain functions.
The physiological mechanisms of how tRNS generates cortical excitability are not completely understood (Antal and Herrmann 2016; Inukai et al. 2016). One potential effect of tRNS may be associated with the repetitive opening of Na+ channels (Schoen and Fromherz 2008). A recent study demonstrated that the Na+ channel blocker carbamazepine showed a tendency towards inhibiting motor evoked responses (MEP) after stimulation (Chaieb et al. 2015). In addition, the effects of tRNS may be based on other mechanisms, such as stochastic resonance (Stacey and Durand 2000). Stochastic resonance refers to the phenomenon that a signal that is too weak to exceed a threshold is amplified by adding noise. It was suggested that tRNS may increase synchronisation of neural firing through the amplification of subthreshold oscillatory activity, which in turn reduces the amount of endogenous noise (Antal and Herrmann 2016). For example, if random noise is added to the subthreshold neural oscillations in the brain, the sum of the two signals will exceed the threshold at several time points, resulting in improved cognitive performance (Miniussi and Ruzzoli 2013; Moss et al. 2004).
The results confirmed a modality-specific role of V1 confirming previous findings (Kanai et al. 2011; Mioni et al. 2016b) and suggesting some involvement of the early visual areas in visual time perception when visual stimuli are employed. At first sight, the results seem also to suggest a modality-independent role of A1 conforming that A1 is part of the modality-independent system involved in time processing. Indeed, despite some previous observation indicating that early auditory cortex is part of the modality-independent time estimation process (Kanai et al. 2011; Mioni et al. 2016b), and that visual inputs are automatically converted to an auditory representation (Franssen et al. 2006; Hickok et al., 2009), the present results do not completely confirm previous observations. We observed a main effect of tRNS over A1 that was mainly evident when auditory stimuli were employed. Our results seem to be in line with other results showing that single neuron and multiunit responses recorded in primary auditory cortex have been shown to encode information about the timing of motor responses during auditory but not visually cued behaviour (Brosch et al. 2005; Paton and Buonomano 2018). Further, studies on the role of the auditory cortex during visual time estimation will be critical to explore this possibility further.
Within the temporal domain, the dominance of the auditory system over vision has been repeatedly emphasised. Generally, participants are more accurate and less variable when discriminating auditory stimuli compared to visual one (Grondin 1993, 2014; Grondin et al. 2005; Mioni et al. 2016a). When auditory stimuli are combined with visual stimuli of the same duration, the perceived duration is dominated by the auditory stimuli (Keetels and Vroomen 2009; Stone et al., 2001). Such asymmetric dominance of audition may be due to the additional step required for visual information to be converted into an auditory representation, while auditory stimuli are encoded in its native sensory mode. The conversion of visual information to auditory information in the context of a temporal task is analogous to the automatic recruitment of the spatially superior visual cortex in auditory spatial tasks (Recanzone 2009; Zimmer et al., 2004).
To control for the possible effect of sensation induced by the stimulation on temporal performance, we included a sensation questionnaire. None of the participants reported feeling any aversive sensation after stimulation. We can conclude that the results regarding the different tRNS effects on temporal processing were due to the specific effect of the stimulation on the underlying brain areas rather than by the subjective experience of the stimulation.
Although the small sample size is indeed a limitation of the current study, our sample has similar numerosity as previous studies conducted using non-invasive brain stimulation in the study of time processing. Moreover, focality is an issue with tES. Although we cannot exclude that stimulation reached brain regions contingent to the targeted areas, here, we opted for the extra-cephalic montage over the right shoulder in order to reduce side effects of the stimulation and therefore limiting these confounding factors.
Taken together, we support the idea that multiple timing mechanisms ‘distributed’ across brain areas or circuits are involved in time processing. Interestingly, tRNS produced an effect on perceived duration but not on temporal variability, suggesting a different effect between tDCS and tRNS on time processing and on the underlying brain structures.
Notes
To further confirm this effect, separate t test were conducted between tRNS and sham in auditory and visual modality. Significant difference is observed within the auditory modality (p = .003; d = .63) but not in the visual modality (p = .563; d = .11).
Inspection of Fig. 1 might suggest the presence of an outlier in V1 group. Repeated measure ANOVA was conducted removing that subject to control the results. Analyses of BP showed significant modality × stimulation type interaction [F(1,21) = 6.75, p = .017, η2p = .24]. No main effects of stimulation type (p = .939, η2p = .01) neither modality (p = .071, η2p = .15) were found.
References
Allan, L. G., & Gibbon, J. (1991). Human bisection at the geometric mean. Learning and Motivation, 22, 39–58.
Allman, M. J., Teki, S., Griffiths, T. D., & Meck, W. H. (2014). Properties of the internal clock: first- and second-order principles of subjective time. Annual Review of Psychology, 65, 743–771.
Ambrus, G. G., Paulus, W., & Antal, A. (2010). Cutaneous perception thresholds of electrical stimulation methods: comparison of tDCS and tRNS. Clinical Neurophysiology, 121(11), 1908–1914.
Ambrus, G. G., Zimmer, M., Kincses, Z. T., Harza, I., Kovács, G., Paulus, W., & Antal, A. (2011). The enhancement of cortical excitability over the DLPFC before and during training impairs categorization in the prototype distortion task. Neuropsychologia, 49(7), 1974–1980.
Antal, A., Kincses, T. Z., Nitsche, M. A., Bartfai, O., & Paulus, W. (2004a). Excitability changes induced in the human primary visual cortex by transcranial direct current stimulation: direct electrophysiological evidence. Investigative Ophthalmology & Visual Science, 45, 702–707.
Antal, A., Nitsche, M. A., Kincses, T. Z., Kruse, W., Hoffmann, K. P., & Paulus, W. (2004b). Facilitation of visuo-motor learning by transcranial direct current stimulation of the motor and extrastriate visual areas in humans. The European Journal of Neuroscience, 19(10), 2888–2892.
Antal, A. & Herrmann, C. S. (2016). Transcranial alternating current and random noise stimulation: possible mechanisms. Neural Plasticity, Article ID 3616807. https://doi.org/10.1155/2016/3616807.
Ayhan, I., Bruno, A., Nishida, S., & Johnston, A. (2009). The spatial tuning of adaptation-based time compression. Journal of Vision, 9(11), 1–12.
Battaglini, L., Noventa, S., & Casco, C. (2017). Anodal and cathodal electrical stimulation over V5 improves motion perception by signal enhancement and noise reduction. Brain Stimulation, 10(4), 773–779. https://doi.org/10.1016/j.brs.2017.04.128.
Bausenhart, K., Di Luca, M., & Ulrich, R. (2018). Assessing duration discrimination: Psychophysical methods and psychometric function analysis. In A. Vatakis, F. Balcı, M. Di Luca, & Á. Correa (Eds.), Timing and time perception: Procedures, measures, and applications. Netherlands: Brill.
Bueti, D. (2011). The sensory representation of time. Frontiers in Integrative Neuroscience, 5, 34.
Bueti, D., & Walsh, V. (2009). The parietal cortex and the representation of time, space, number and other magnitudes. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 364(1525), 1831–1840.
Bueti, D., Bahrami, B., & Walsh, V. (2008). Sensory and association cortex in time perception. Journal of Cognitive Neuroscience, 20(6), 1054–1062.
Burr, D. C., Cicchini, G. M., Arrighi, R., & Morrone, M. C. (2011). Spatiotopic selectivity of adaptation-based compression of event duration. Journal of Vision, 11(2), 21.
Brosch, M., Selezneva, E., & Scheich, H. (2005). Nonauditory events of a behavioral procedure activate auditory cortex of highly trained monkeys. The Journal of Neuroscience, 25, 6797–6806.
Chaieb, L., Antal, A., & Paulus, W. (2015). Transcranial random noise stimulation-induced plasticity is NMDA-receptor independent but sodium-channel blocker and benzodiazepines sensitive. Frontiers in Neuroscience, 9, 125.
Costa, T. L., Gualtieri, M., Barboni, M. T., Katayama, R. K., Boggio, P. S., & Ventura, D. F. (2015a). Contrasting effects of transcranial direct current stimulation on central and peripheral visual fields. Experimental Brain Research, 233(5), 1391–1397.
Costa, T. L., Lapenta, O. M., Boggio, P. S., & Ventura, D. F. (2015b). Transcranial direct current stimulation as a tool in the study of sensory-perceptual processing. Attention, Perception, & Psychophysics, 77(6), 1813–1840.
Eisler, H., Eisler, A. D., & Hellström, Ǻ. (2008). Psychophysical issue in the study of time perception. In S. Grondin (Ed.), Psychology of time (pp. 76–109). Bingley: Emerald Group.
Franssen, V., Vandierendonck, A., & Van Hiel, A. (2006). Duration estimation and the phonological loop: articulatory suppression and irrelevant sounds. Psychological Research, 70(4), 304–316.
Fertonani, A., & Miniussi, C. (2015). Transcranial electrical stimulation: what we know and do not know about mechanisms. Neuroscientist, 23(2), 109–123. https://doi.org/10.1177/1073858416631966.
Fertonani, A., Ferrari, C., & Miniussi, C. (2015). What do you feel if I apply transcranial electric stimulation? Safety, sensations and secondary induced effects. Clinical Neurophysiology, 126(11), 2181–2188.
Fertonani, A., Pirulli, C., & Miniussi, C. (2011). Random noise stimulation improves neuroplasticity in perceptual learning. The Journal of Neuroscience, 31(43), 15416–15423.
Finnerty, G. T., Shadlen, M. N., Jazayeri, M., Nobre, A. V., & Buonomano, D. V. (2015). Time in cortical circuits. The Journal of Neuroscience, 35(41), 13912–13916.
Gamache, P. L., & Grondin, S. (2010). The lifespan of time intervals in reference memory. Perception, 39(11), 1431–1451.
Gibbon, J. (1981). On the form and location of the psychometric bisection function for time. Journal of Mathematical Psychology, 24, 58–87.
Gibbon, J., Church, R. M., & Meck, W. H. (1984). Scalar timing in memory. Annuals New York Academic Society, 423, 52–77.
Grondin, S. (1993). Duration discrimination of empty and filled intervals marked by auditory and visual signals. Perception & Psychophysics, 54(3), 383–394.
Grondin, S. (2014). Why studying intermodal duration discrimination matters. Frontiers in Psychology, 5, 628.
Grondin, S., Roussel, M.-F., Gamache, P.-L., Roy, M., & Ouellet, M. (2005). The structure of sensory events and the accuracy of time judgments. Perception, 34, 45–58.
Hickok, G., Okada, K., & Serences, J. T. (2009). Area Spt in the human planum temporale supports sensory-motor integration for speech processing. Journal of Neurophysiology, 101(5), 2725–2732.
Inukai, Y., Saito, K., Sasaki, R., et al. (2016). Comparison of three non-invasive transcranial electrical stimulation methods for increasing cortical excitability. Frontiers in Human Neuroscience, 10, 668.
Ivry, R. B., & Schlerf, J. E. (2008). Dedicated and intrinsic models of time perception. Trends in Cognitive Sciences, 12(7), 273–280.
Jacobson, L., Koslowsky, M., & Laviodor, M. (2012). tDCS polarity effects in motor and cognitive domains: a meta-analytical review. Experimental Brain Research, 216(1), 1–10.
Johnston, A., Arnold, D. H., & Nishida, S. (2006). Spatially localized distortions of event time. Current Opinion in Biology, 16, 472–479.
Kanai, R., Lloyd, H., Bueti, D., & Walsh, V. (2011). Modality-independent role of the primary auditory cortex in time estimation. Experimental Brain Research, 209, 465–471.
Kanai, R., Paffen, C. L., Hogendoorn, H., & Verstraten, F. A. (2006). Time dilation in dynamic visual display. Journal of Vision, 6, 1421–1430.
Kaneko, S., & Murakami, I. (2009). Perceived duration of visual motion increases with speed. Journal of Vision, 9(7), 14.
Keetels, M. & Vroomen, J. (2009). Perception of synchrony between the senses. In M. M. Murray & M.T. Wallace. The Neural Bases of Multisensory Processes, (Chapter 9). Boca Raton: CRC Press/Taylor & Francis.
Koch, G., Oliveri, M., & Caltagirone, C. (2009). Neural networks engaged in milliseconds and seconds time processing: evidence from transcranial magnetic stimulation and patients with cortical or subcortical dysfunction. Philosophical Transactions of the Royal Society B, 364, 1907–1918.
Kopec, C. D., & Brody, C. D. (2010). Human performance on the temporal bisection task. Brain and Cognition, 74(3), 262–272.
Mauk, M. D., & Buonomano, D. V. (2004). The neural basis of temporal processing. Annual Review of Neuroscience, 27, 307–340.
Merchant, H., Zarco, W., & Prado, L. (2008). Do we have a common mechanism for measuring time in the hundreds of millisecond range? Evidence from multiple-interval timing tasks. Journal of Neurophysiology, 99(2), 939–949.
Merchant, H., Harrington, D. L., & Meck, W. H. (2013). Neural basis of the perception and estimation of time. Annual Review of Neuroscience, 36, 313–336.
Miniussi, C., & Ruzzoli, M. (2013). Transcranial stimulation and cognition. Handbook of Clinical Neurology, 116, 739–750.
Miniussi, C., Ruzzoli, M., & Walsh, V. (2010). The mechanism of transcranial magnetic stimulation in cognition. Cortex, 46, 128–130.
Mioni, G. (2018). Methodological issues in the study of prospective timing. In A. Vatakis, F. Balcı, M. Di Luca & Á. Correa (Eds.). Timing and time perception: procedures, measures, & applications, (pp. 79–97). Biology E-Books Online, Brill Open E-Book Collection. https://doi.org/10.1163/9789004280205_005
Mioni, G., Grondin, S., Mapelli, D., & Stablum, F. (2018). A tRNS investigation of the sensory representation of time. Scientific Reports, 8, 10364. https://doi.org/10.1038/s41598-018-28673-7.
Mioni, G., Grondin, S., Forgione, M., Fracasso, V., Mapelli, D., & Stablum, F. (2016b). The role of primary auditory and visual cortices in temporal processing: a tDCS approach. Behavioural Brain Research, 313, 151–157.
Mioni, G., Grassi, M., Tarantino, V., Stablum, F., Grondin, S., & Bisiacchi, P. S. (2016a). The impact of a concurrent motor task on auditory and visual temporal discrimination tasks. Attention, Perception & Psychophysics, 78, 742–748.
Morrone, M. C., Ross, J., & Burr, D. (2005). Saccadic eye movements cause compression of time as well as space. Nature Neuroscience, 8, 950–954.
Moss, F., Ward, L. M., & Sannita, W. G. (2004). Stochastic resonance and sensory information processing: a tutorial and review of application. Clinical Neurophysiology, 115(2), 267–281.
Nitsche, M. A., Liebetanz, D., Antal, A., Lang, N., Tergau, F., & Paulus, W. (2003). Modulation of cortical excitability by weak direct current stimulation −technical: safety and functional aspects, Suppl. Clinical Neurophysiology, 56, 255–276.
Nitsche, M. A., Grundey, J., Liebetanz, D., Lang, N., Tergau, F., & Paulus, W. (2004). Catecholaminergic consolidation of motor cortical neuroplasticity in humans. Cerebral Cortex, 14(11), 1240–1245.
Oldfield, R. C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia, 9(1), 97–113.
Oostenveld, R., & Praamstra, P. (2001). The five percent electrode system for high-resolution EEG and ERP measurements. The Clinical Neuropsychologist, 112, 713–719.
Oyama, F., Ishibashi, K., & Iwanaga, K. (2017). Cathodal transcranial direct-current stimulation over right posterior parietal cortex enhances human temporal discrimination ability. Journal of Physiological Anthropology, 36, 41.
Paton, J. J., & Buonomano, D. V. (2018). The neural basis of timing: distributed mechanisms for diverse functions. Neuron, 98(4), 687–705. https://doi.org/10.1016/j.neuron.2018.03.045.
Penney, T. B. (2003). Modality differences in interval timing: Attention, clock speed, and memory. In W. H. Meck (Ed.), Functional and neural mechanisms of interval timing (pp. 209–234). Boca Raton: CRC Press.
Penney, T. B., Allan, L. A., Meck, W. H., & Gibbon, J. (1998). Memory mixing in duration bisection. In D. A. Rosenbaum & C. E. Collyer (Eds.), Timing of behavior: Neural, computational, and psychophysical perspectives (pp. 165–193). Cambridge: MIT Press.
Penney, T. B., Gibbon, J., & Meck, W. H. (2000). Differential effects of auditory and visual signals on clock speed and temporal memory. Journal of Experimental Psychology. Human Perception and Performance, 26, 1770–1787.
Recanzone, G. H. (2009). Interactions of auditory and visual stimuli in space and time. Hear Research, 258(1–2), 89-99. https://doi.org/10.1016/j.heares.2009.04.009.
Romanska, A., Rezlescu, C., Susilo, T., Duchaine, B., & Banissy, M. J. (2015). High-frequency transcranial random noise stimulation enhances perception of facial identity. Cerebral Cortex, 25(11), 4334–4340.
Schoen, I., & Fromherz, P. (2008). Extracellular stimulation of mammalian neurons through repetitive activation of Na+ channels by weak capacitive currents on a silicon chip. Journal of Neurophysiology, 100(1), 346–357.
Stacey, W. C., & Durand, D. M. (2000). Stochastic resonance improves signal detection in hippocampal CA1 neurons. Journal of Neurophysiology, 83(3), 1394–1402.
Stone, J. V., Hunkin, N. M., Porrill, J., et al. (2001). When is now? Perception of simultaneity. Proceedings of the Biological Sciences, 268(1462), 31–38.
Terney, D., Chaieb, L., Moliadze, V., et al. (2008). Increasing human brain excitability by transcranial high-frequency random noise stimulation. The Journal of Neuroscience, 28, 14147–14155.
van Rijn, H., Gu, B. M., & Meck, W. H. (2014). Dedicated clock/timing-circuit theories of time perception and timed performance. Advances in Experimental Medicine and Biology, 829, 75–99.
Vicario, C. M., Martino, D., & Koch, G. (2013). Temporal accuracy and variability in the left and right posterior parietal cortex. Neuroscience, 245, 121–128.
Wiener, M. (2013). Transcranial magnetic stimulation studies of human time perception: A primer. Timing and Time Perception, 2(3), 233–260.
Woods, A. J., Antal, A., Bikson, M., et al. (2016). A technical guide to tDCS, and related non-invasive brain stimulation tools. Clinical Neurophysiology, 127(2), 1031–1048.
Zimmer, U., Lewald, J., Erb, M., Grodd, W., & Karnath, H. O. (2004). Is there a role of visual cortex in spatial hearing? The European Journal of Neuroscience, 20, 3148–3156.
Acknowledgement
The author gratefully thanks Nicola Cellini for his comments and help in editing the final version and Prof Simon Grondin and Prof Franca Stablum for their theoretical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the ethics committee of the School of Psychology of Padova (Italy) and conducted according to the Declaration of Helsinki (59th WMA General Assembly, Seoul, 2008).
Conflict of Interest Statement
The author declares that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• The first study using tRNS to test modality-specific and modality-independent timers
• No effect of stimulation on temporal variability but an effect of stimulation on perceived duration
• Modality-specific role of V1 and modality independent role of A1 on time processing
Electronic supplementary material
ESM 1
(XLS 22 kb)
Rights and permissions
About this article
Cite this article
Mioni, G. Modulating Subjective Time Perception with Transcranial Random Noise Stimulation (tRNS). J Cogn Enhanc 4, 71–81 (2020). https://doi.org/10.1007/s41465-019-00128-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41465-019-00128-5