Abstract
The retrieval of information from long-term memory can be associated with information regarding sources or context (recollection) or without further context (familiarity). The retrieval type depends on how information has been encoded previously, and this encoding process is supposed to be modulated by the neurotransmitters dopamine and acetylcholine. For example, acetylcholine levels in the hippocampus increase when one is confronted with novel information allowing for better encoding and, presumably, for retrieval of more detailed memories (recollection). On the other hand, a dopaminergic deficit such as in Parkinson’s disease has been shown to induce deficits in familiarity rather than in recollection-based retrieval. It is, however, unclear whether this finding arises from alterations in encoding, retrieval, or both. Moreover, other research has challenged this clear-cut dichotomy and linked dopamine to both familiarity and recollection, and acetylcholine to unspecific enhancement of memory for novel information. Thirty-nine healthy seniors (age range 62–77) participated in a remember/know task in which scenes that were presented with different repetition rates had to be encoded and retrieved on the following day. Neurotransmitter levels were modulated during encoding by administrating either levodopa (100 mg, N = 13) or galantamine (8 mg, N = 13) to one of two experimental groups. A third group received a placebo (N = 13). Across all groups, recognition memory increased as a function of stimulus repetition, and this effect was specifically pronounced for remember relative to know answers. Importantly, the drugs had no effect on recollection, familiarity, or overall recognition memory. The findings argue against a simple dichotomy of dopaminergic and cholinergic contributions to either recollection- or familiarity-based memory retrieval.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recognition refers to the ability to re-identify items, such as objects or other information, that have been stored in episodic memory. Unlike in free recall, where no cues are present, during recognition, the stored memory representations are matched to the currently experienced sensory input. It was suggested that recognition can be further subdivided into two components or modes, namely recollection and familiarity, also referred to as “remembering” and “knowing,” respectively (Yonelinas 2002). When encountering a person in the supermarket, for example, one may recognize him either as a person from a recent meeting (remember) or without such contextual details (know).
The quality and fidelity of retrieval crucially depends on the initial encoding process (Tulving and Thomson 1973), which has been closely linked to the medial temporal lobe system (Eichenbaum et al. 2007). This brain system receives strong input both from dopaminergic and cholinergic projections. However, whether dopamine and acetylcholine selectively modulate encoding in a way that later favors one retrieval mode over the other is unclear. Several studies were conducted on this issue but revealed contradictory results. Many of these studies used neurodegenerative diseases as a model for neurotransmitter depletion. Parkinson’s disease (PD), for example, is characterized by a lack of dopamine due to neurodegenerative processes in the substantia nigra. Testing PD patients, Davidson et al. (2006) observed a deficit in familiarity-based but not in recollection-based retrieval. However, PD patients treated with dopamine agonists showed deficits in recollection but not familiarity (Shepherd et al. 2013). Others proposed that the effects of the disease on recognition memory depend on disease severity (Edelstyn et al. 2010). According to this and other studies (Davidson et al. 2006; Edelstyn et al. 2007; Edelstyn et al. 2015), recollection is impaired during moderate but not mild stages of PD, which is in contrast with the results of Barnes et al. (2003), who found recollection to be unaffected in moderate cases, as well. Regarding familiarity-based recognition, impairments were found in mild PD (Davidson et al. 2006), whereas moderately affected patients were unimpaired in familiarity (Barnes et al. 2003 and Edelstyn et al. 2007).
While PD serves as a model of a dopaminergic deficit, Alzheimer’s disease (AD) and its suspected precursor, amnestic mild cognitive impairment (aMCI), have been used as a model for the lack of acetylcholine caused by neurodegeneration of the nucleus basalis of Meynert in the basal forebrain (e.g., Blatt et al. 2014). The effects of these conditions on recognition memory were summarized in a meta-analysis conducted by Koen and Yonelinas (2014). They concluded that most aMCI patients show a deficit specific to recollection, whereas both retrieval processes are impaired in AD.
Patient studies, however, entail two major problems. First, potentially explaining the often discrepant results in the studies listed above, the underlying diseases involve more than one deficit in a single neurotransmitter; instead, their brains differ from healthy ones in a number of ways. This makes neurologic patients non-ideal models to study a specific neurotransmitter’s contribution to the cognitive processes under investigation. Second, patients suffer from a permanent neurotransmitter deficit. Therefore, it remains unclear whether memory impairments result from altered retrieval or from changes in the preceding encoding and consolidation processes. These pitfalls can be avoided by testing transient drug effects in healthy subjects whereby the drugs are selectively administered prior to the process under investigation. Such drug effects may be more observable in older than in young subjects: Even during healthy aging, levels of neurotransmitters such as dopamine and acetylcholine decrease. Thus, drugs that transiently enhance neurotransmitter levels should produce more pronounced behavioral effects in the elderly than in young participants with more optimal neurotransmitter levels.
Age-related changes in episodic memory have previously been demonstrated. In contrast to the young, older subjects display lower recognition accuracy whereby recollection is more affected than familiarity (Mäntylä 1993; Perfect and Dasgupta 1997; Naveh-Benjamin 2000). However, whether these deficits result from altered retrieval or encoding and whether they are indeed reflecting neurotransmitter deficits is not yet clear. An attempt to investigate these questions was made by Morcom et al. (2010), who modulated dopamine levels in young and elderly subjects via administration of dopaminergic agonists (bromocriptine) or antagonists (sulpiride). Compared to the administration of a placebo, drug treatment had no effect on recognition performance. In a different study (Chowdhury et al. 2012), healthy older adults were medicated with l-dopa and showed a recollection improvement 6 h after encoding.
Studies that address the influence of cholinergic modulation on recognition memory in healthy elderly persons have not yet been conducted (to the authors’ current knowledge). However, a study investigating the modulatory effect of acetylcholine administered before encoding on recognition memory in young subjects revealed selective deficits in familiarity (Eckart et al. 2016). Knowing that nicotinergic stimulation enhances the encoding of new information (Froeliger et al. 2009; Hasselmo 2006), increasing acetylcholine activity may lead to more detailed memories and, therefore, favor recollection. Alternatively, both recollection and familiarity may be enhanced. Another study investigated the effects of dopaminergic and cholinergic modulation on recognition memory in young subjects and found no behavioral drug effects (Bunzeck et al. 2014).
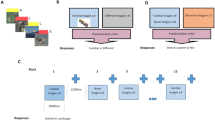
The aim of this study was to investigate the effects of the neurotransmitters dopamine and acetylcholine during encoding on later recognition memory in healthy seniors. Subjects took part in a remember/know paradigm including a study phase in which scenes had to be categorized into indoor and outdoor scenes under medication with levodopa (100 mg), galantamine (8 mg), or placebo. The scenes were presented once, twice, or three times during the study phase in order to induce different levels of familiarization. In the test phase, which occurred 24 h after studying, the formerly presented scenes were mixed with novel scenes and subjects had to classify them as new or old. In case of “old” decisions, they had to further indicate whether they remembered the scene, including associations or whether the scenes were only familiar, with no further associations. A third response option let subjects report whether they were just guessing. After judging a scene as “new,” subjects had to report whether they were sure or guessing.
The purpose of the study was to test whether the drugs used had an effect on recognition memory performance. Given the mixed results in previous work, we further explored possible differential effects on the rate of remember vs. know responses and whether this was further modulated by the degree of familiarization, i.e., how often scenes had been presented. One hypothesis was that galantamine, which increases acetylcholine levels in the brain by reversibly blocking the enzyme acetylcholinesterase, should foster encoding processes that later result in recollection-based recognition, i.e., increase the rate of remember responses, especially for less familiar stimuli. This assumption was based on the previously reported role of this neurotransmitter during the encoding of novel information, which is assumed to entail a more detailed memory representation in the hippocampus, biasing recognition towards recollection. However, levodopa, which is converted into dopamine after passing the blood-brain barrier, should modulate encoding in a way that later favors familiarity-based retrieval, even for repeatedly presented scenes. As stated above, this hypothesis—dopamine favors familiarity, acetylcholine supports recollection—is not unambiguously supported by the literature; therefore, the nature of the present study was exploratory.
Materials and Methods
Experimental Design
Subjects aged 62 to 77 years were first screened for cognitive or affective deficits by means of test batteries, including the CERAD (Consortium to Establish a Registry for Alzheimer’s Disease, Welsh et al. 1994) and GDS (Geriatric Depression Scale, Yesavage et al. 1983). Only subjects with normal scores (CERAD: deviation of 1.5 standard deviations from the normal sample for each subtest; GDS: 9 = cutoff score) in these batteries and without a history of severe neurological or psychiatric diseases were included in the study. The subjects were randomly assigned to one of three groups (placebo, galantamine, or dopamine). After single blind drug administration and a delay matched to the specific drug’s kinetics (see below), all subjects took part in the same computer-based experiment. They were presented with a number of scenes and were asked to indicate the indoor vs. outdoor status using the index and middle fingers. One day later, the subjects were presented with old and new scenes and were asked to indicate whether the scenes were either old or new (see below for details).
Participants
Four subjects were excluded from the analysis because they were not able to perform the recognition session clearly above the chance level. For the remaining subjects, no differences between groups were detected regarding their average performance in the screening tests. Thus, 39 older adults (age 62–77 years, 21 females) were randomized to a dopamine (N = 13), galantamine (N = 13) or placebo (N = 13) group using the website www.randomization.com (Table 1). All participants were right-handed and healthy (i.e., without any severe internal or neuropsychiatric disease) with normal or corrected-to-normal vision.
Sample size was calculated via statistical power calculation using data from previous own unpublished research. Based on the number of groups (three) and measurements (six, repeated measures ANOVA), power analysis was conducted on a moderate effect size (Cohen’s f = .30), assuming a α level of .05 and a desired power (1 − β) of .80. Thus, a sample size of N = 39 was determined.
The participants were paid volunteers and provided written informed consent before participation. The study was approved by the local ethics committee of the Medical Faculty of the Otto von Guericke University.
Treatment
Galantamine
An 8-mg single dose of galantamine (REMINYL R, Janssen-Cilag GmbH) was administered orally in the form of a tablet. Because the retarded form of galantamine reaches maximum release approx. After 4 h, the waiting time between drug administration and the experiment was 2 h for this drug.
Dopamine
A single dose of 100 mg levodopa (NACOM R 100, Janssen-Cilag GmbH) in combination with 25 mg carbidopa was orally given in the form of a tablet. Because levodopa reaches a maximum plasma concentration after approximately 0.7 h, which then remains stable for 2 to 4 h (based on pharmacokinetic data), levodopa was administered 1 h before the encoding session started.
Placebo
The placebo capsules for the placebo group were composed of magnesium (Abtei Pharma GmbH) and administered 1 h before the encoding session started.
Experimental Task
Study Phase
After the waiting time elapsed, subjects were presented with 240 images consisting of 120 different scenes that had to be categorized into indoor and outdoor via a button press (Fig. 1). All images were gray-scaled and normalized into a mean gray value of 127 and a standard deviation of 75.
While 40 of 120 scenes were presented once, 40 were presented twice and 40 were presented three times. Each image was shown for 1.5 s with interstimulus intervals of 1.5 s in which a fixation cross was shown on a gray screen. To ensure that the participants understood the instructions, all participants completed one short practice session (16 trials) before the main session. Participants were informed that another test would follow the next day, but it was not revealed to them that the same stimuli would be used.
Test Phase
After 24 h (23.72 ± .09 SEM), subjects completed a second session in which all 120 previously presented images were shown again together with 40 novel images (Fig. 2). Subjects were asked to rate every scene as either seen before (“old”) or unseen (“new”) by button press. When subjects rated the image as old, they additionally had to report whether the old image was “remembered,” “familiar,” or whether they were “guessing.” Similarly, after rating an image as “new,” subjects had to report whether they were “sure” or “guessing.” Every image was presented for 6 s, in which subjects had to make the initial old/new judgment, followed by another 6 s for the subsequent judgment, both by button press. The test phase was performed around 24 h after encoding to avoid an impact of the drugs on retrieval.
Data Analysis
Study Phase
To analyze the performance during studying, the proportion of correct answers and corresponding response times were entered into a repeated measures ANOVA with the between factor treatment and the within factor scene type (indoor, outdoor).
Test Phase
First we run a repeated measures ANOVA (rANOVA) on uncorrected remember, know, and guessing hit rates and response times with the within factors presentation rate (p1, p2, p3) and response type (remember, know, guess) and the between factors treatment (placebo vs. galantamine; placebo vs. dopamine). As the ANOVA revealed no significant differences between the treatment groups (uncorr. hit rates: F(2,36) = .085; p = .918; η2 = .003; uncorr. response times: F(2,20) = .520; p = .602; η2 = .021), we conducted two separate rANOVAS (drug vs. placebo) to give a full picture of our data. A second ANOVA included the factors response type and treatment but was carried out on false alarm rates and response times. Again an omnibus ANOVA revealed no significant group effects (false alarm rates: F(2,36) = .314, p = .733; η2 = .009; response times: F(2,25) = 1.428, p = .259; η2 = .056); therefore, we calculated single rANOVAS.
For further analyses, corrected hit rates were calculated based on the assumption that recollection and familiarity rely on independent processes and “know” responses are given in the absence of recollection (Libby et al. 2013):
Corrected recollection rates (CR) were calculated as the probability of making a “remember” judgment to an old item (ROLD), corrected for the probability of making a “remember” judgment to a new item (false alarm rate for “remember” responses, RNEW) with the following formula:
Corrected familiarity rates (CF) were calculated as the probability of making a “know” judgment to an old item (KOLD), corrected for the probability of making a “know” judgment to a new item \( \left({F}_{\mathrm{NEW}}=\frac{K_{\mathrm{NEW}}}{\left(1-{R}_{\mathrm{NEW}}\right)}\right) \) and the fact that “know” responses were given in the absence of recollection \( \Big({F}_{OLD}=\frac{K_{\mathrm{OLD}}}{\Big(1-{R}_{\mathrm{OLD}\Big)}} \)), leading to the following formula:
First, an omnibus ANOVA was carried out on corrected recollection and familiarity rates with the within factor presentation rate (p1, p2, p3) and the between factor treatment (galantamine, dopamine, placebo). As no significant group differences were found (recollection rates: F(2,36) = .122, p = .886; η2 = .004; familiarity rates: F(2,36) = 1.607, p = .214; η2 = .047), further rANOVAS were carried out on placebo vs. drug groups. In cases of violations of the sphericity assumption according to Mauchly’s test, the Greenhouse-Geisser correction was applied and adjusted F- and p values were reported.
Results
Study Phase
A repeated measures ANOVA revealed no significant differences in accuracy (F(2,36) = .663, p = .521; η2 = .020) or response times (F(2,36) = .226, p = .799; η2 = .007) between treatment groups during the categorization of outdoor and indoor scenes (Fig. 3). Regarding the factor scene type (indoor vs. outdoor), no differences were found in accuracy (F(1,36) = .792, p = .379; η2 = .023) or response times (F(2,36) = .444, p = .645; η2 = .013).
Test Phase
Raw values of remember, know, and guess responses separated for each presentation rate can be depicted from Table 2.
Repeated measures ANOVAs revealed a significant effect of presentation rate (Plac vs. Gala: F(2,48) = 24.970, p = .000; η2 = .510/ Pla vs. Dopa: F(2,48) = 31.558, p = .000; η2 = .568) and response type (Plac vs. Gala: F(2,48) = 6.692, p = .003; η2 = .218/ Pla vs. Dopa: F(2,48) = 11.563, p = .000; η2 = .325) in both treatment groups compared to the placebo group. No significant effect of treatment was found between any of the tested groups (Plac vs. Gala: F(1,24) = .019, p = .891; η2 = .001/ Pla vs. Dopa: F(1,24) = .222, p = .641; η2 = .009).
A repeated measures ANOVA testing the treatment and response type effect on false alarms revealed a significant response type effect (Plac +. Gala: F(2,48) = 3.610, p = .035; η2 = .131/ Pla + Dopa: F(2,48) = 4.230, p = .020; η2 = .150) but no treatment effect (Plac vs. Gala: F(1,24) = .592, p = .449; η2 = .018/ Pla vs. Dopa: F(1,24) = .337, p = .567; η2 = .014).
Regarding response times, a significant effect was found for response type (F(1,29) = 27.780, p < .001; η2 = .534) with faster responses for remember compared to know responses. The ANOVA also revealed a significant main effect for presentation rate (F(2,58) = 4.887, p = .011; η2 = .129) but no significant presentation rate × response type interaction (F(2,58) = 1.919, p = .156; η2 = .055). The presentation rate × treatment group interaction (F(4,58) = .330, p = .857; η2 = .010), the response type × treatment group interaction (F(2,29) = .900, p = .418; η2 = .036), and the main effect of treatment group (F(2,29) = 1.656, p = .209; η2 = .064) were also not significant.
Further analysis was conducted with corrected measures of recollection and familiarity (Table 3). A repeated measures ANOVA revealed a significant effect of presentation rate (Plac + Gala: F(2,48) = 27.629, p = .000; η2 = .525/ Pla + Dopa: F(2,48) = 28.984, p = .000; η2 = .547) but again no significant treatment effect between the tested groups placebo vs. galantamine and placebo vs. dopamine (p > .05).
Relationship to Body Weight
Because several studies have shown dose-dependent effects of pharmacological drugs on cognitive performance (Knecht et al. 2004; Monte-Silva et al. 2009; Chowdhury et al. 2012), the present data were reanalyzed while taking the individual body weight into account. No significant drug effects were found when testing the linear, inverted-u-shape, quadratic and cubic relationship between body weights, adjusted doses, and behavioral data.
Discussion
In an effort to elucidate the effects of the neurotransmitters dopamine and acetylcholine on encoding and later retrieval modes, elderly subjects performed a remember/know task 24 h after encoding indoor and outdoor scenes under drug modulation (levodopa, galantamine, or placebo). The accuracy of indoor/outdoor judgments during the study phase was close to 100% in all groups, suggesting that nearly all images were properly encoded (Fig. 3). During the test phase on day 2 (Fig. 4), recognition accuracy increased as a function of picture repetition during the study, and this effect was driven by recollection but not familiarity rates. These findings support the assumption of a memory trace being strengthened by repetition, and they are in accordance with the literature (e.g., Pitarque et al. 2015).
While the abovementioned effects were expected, in contrast to our hypothesis, no impacts of either drug on recollection vs. familiarity rates were observed: all three groups showed similar accuracy rates and response times across conditions. That is, dopamine did not shift the retrieval mode towards familiarity or recollection nor did acetylcholine drive recollection-based or familiarity-based retrieval. This was true across all repetition rates: even retrieval for repeatedly presented scenes was not pushed further towards recollection or familiarity by acetylcholine, nor were scenes that had been presented only once more often classified as being recollected or familiar under dopamine.
Both the dopaminergic midbrain and the cholinergic basal forebrain project to the hippocampus and the surrounding cortex (Samson et al. 1990; Gasbarri et al. 1994; Mesulam 2004), which both play a crucial role in episodic memory (Tulving and Markowitsch 1998; Eichenbaum and Cohen 2001; Paller and Wagner 2002). Therefore, we expected that the applied drugs should interfere with retrieval from episodic memory by modulating these projections’ impact on the medial temporal lobe memory system during encoding. The question arises why no such impact was observed in the present study. Crucial issues here are sample size (in a between group design) and dosage. Regarding the first, while n = 39 (across three groups) is relatively low, it should be enough according to our power calculation (see “Materials and Methods”).
Regarding dosage, our drug treatment (8 mg galantamine or 100 mg levodopa single shot) was based on previous research (Bunzeck et al. 2014) and clinical recommendations (German S3-guidelines Dementia & Parkinson Disease, DGPPN and DGN 2017; DGN 2016). However, the used (single) drug dose may have been too low to elicit observable behavioral changes. In a study by Bunzeck et al. (2014) with young subjects and the same doses of levodopa and galantamine, both drugs also had no effect on remember/know judgments, but, importantly, showed differential effects within the mesolimbic system as assessed by fMRI. However, higher doses are associated with numerous side effects (e.g., hallucinations, nausea, hand tremor) and therefore for ethical reasons not applicable.
It is well known that dopaminergic as well as cholinergic effects on cognitive performance follow an inverted U-shaped curve (Cools and D'Esposito 2011; Störmer et al. 2012; Dumas and Newhouse 2011). This indicates that optimal cognitive performance is achieved with medium neurotransmitter levels, a situation found in young participants. Elderly subjects can be assumed to have sub-optimal levels, so the applied drugs were expected to shift the levels towards the optimal value. However, over-medication would shift the dose-response curve to suboptimal levels again. Therefore, some of our subjects, especially those with low body weight, may have been over-dosed. However, there was no significant relationship between behavior and body weight in our study. Moreover, no side effects, which are a typical sign of over-medication, were observed. Finally, we decided to perform the experiment in elderly subjects because neurotransmitter levels are known to decrease during healthy aging (Bäckman et al. 2006; Li et al. 2010). However, a current meta-analysis reports no age effects on dopamine synthesis capacity (Karrer et al. 2017), and, to further complicate the matter, Bloemendaal et al. (2018) showed detrimental cognitive effects of the dopamine precursor tyrosine in older adults.
Another possibility to explain the absence of any drug effects may relate to the notion that dopamine and acetylcholine do not have effects on retrieval when applied during encoding. Indeed, reports regarding the different roles of both neurotransmitters in episodic memory are inconsistent. For example, deficits in familiarity but not in recollection were observed in PD patients (Davidson et al. 2006). However, when treated with dopamine agonists, deficits appeared in recollection only (Shepherd et al. 2013). PD patients do not only have a lack of dopaminergic neurons as part of their pathophysiology, they may also suffer from age-related declines of the cholinergic system. Therefore, drug effects found in PD patients may also be induced by the interplay of different neurotransmitters.
A meta-analysis including 14 studies suggests a clear trend towards the impact of acetylcholine on recollection only in aMCI and on recollection and familiarity in AD (Koen and Yonelinas 2014). However, the dissociation of recognition memory into recollection and familiarity is not a main consensus among researchers. There are even reports that question the existence of two fundamentally different retrieval processes (Dunn 1996; Heathcote et al. 2006). Instead, they propose a continuum from strong to weak memory representations and that recollection simply refers to retrieving a strong memory content and familiarity to retrieving a weak memory content. Assuming this single-process hypothesis, in our study, scenes presented less often should have led to weak and often repeated scenes to strong memory traces. The former should entail more know responses and the latter more remember responses. In other words, with more repetitions, recollection rates should increase and familiarity rates should decrease. While the first assumption could be observed in the present data, the second assumption was not met. Instead, familiarity rates did not change with increasing repetition. Together with earlier studies, which also reported the repetition of study material to have stronger effects on recollection than on familiarity (Parkin et al. 1995; Dewhurst and Anderson 1999), this finding argues against single-process theories and supports the remember/know dichotomy.
In summary, while the present results support the dual-process retrieval theory (Yonelinas 2002; Yonelinas and Jacoby 2012; Wixted and Mickes 2010), they do not provide evidence of an effect of dopaminergic or cholinergic drugs on subsequent recollection or familiarity.
Further Limitations and Outlook
Apart from the limitations outlined above (sample size, dosage), this randomized controlled feasibility study has another limitation. We collected only cognitive measurements; drug effects on automatic nervous systems, biological, or basic motor parameters were not assessed. Although we could not observe a relationship between body weight and behavior in any drug group, it remains unclear whether higher or lower drug dosages may be more able to induce changes in recognition memory performance. To further address this issue, future studies could, for example, use positron emission tomography (PET) to non-invasively determine baseline levels of dopamine and acetylcholine before and after drug intake. This may help to adjust individual drug dosages.
References
Bäckman, L., Nyberg, L., Lindenberger, U., Li, S.-C., & Farde, L. (2006). The correlative triad among aging, dopamine, and cognition: Current status and future prospects. Neuroscience & Biobehavioral Reviews, 30(6), 791–807.
Barnes, L., Boubert, L., Harris, J., Lee, A., & David, A. S. (2003). Reality monitoring and visual hallucinations in Parkinson’s disease. Neuropsychologia, 41(5), 565–574.
Blatt, J., Vellage, A.-K., Baier, B., & Müller, N. (2014). The contribution of acetylcholine and dopamine to subprocesses of visual working memory—what patients with amnestic mild cognitive impairment and Parkinson’s disease can tell us. Neuropsychologia, 61, 89–95.
Bloemendaal, M., Froböse, M. I., Wegman, J., Zandbelt, B., van de Rest, O., Cools, R., & Aarts, E. (2018). Neuro-cognitve effects of tyrosine administration on reactive and proactive response inhibition in healthy older adults. eNeuro., 5(2), ENEURO.0035-17.2018. https://doi.org/10.1523/ENEURO.0035-17.2018.
Bunzeck, N., Guitart-Masip, M., Dolan, R. J., & Duzel, E. (2014). Pharmacological dissociation of novelty responses in the human brain. Cerebral Cortex, 24(5), 1351–1360 Bhs420.
Chowdhury, R., Guitart-Masip, M., Bunzeck, N., Dolan, R. J., & Düzel, E. (2012). Dopamine modulates episodic memory persistence in old age. Journal of Neuroscience, 32(41), 14193–14204.
Cools, R., & D'Esposito, M. (2011). Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biological Psychiatry, 69(12), 113–125.
Davidson, P. S. R., Anaki, D., Saint-Cyr, J. A., Chow, T. W., & Moscovitch, M. (2006). Exploring the recognition memory deficit in Parkinson’s disease: Estimates of recollection versus familiarity. Brain, 129, 1786–1779.
Dewhurst, S. A., & Anderson, S. J. (1999). Effects of exact and category repetition in true and false recognition memory. Memory and Cognition, 27(4), 664–673.
DGN. (2016). S3-Leitlinie Idiopathisches Parkinson-Syndrom: Springer. Heidelberg: Berlin.
DGPPN & DGN. (2017). S3-Leitlinie Demenzen. Berlin, Heidelberg: Springer Berlin Heidelberg.
Dumas, J. A., & Newhouse, P. A. (2011). The cholinergic hypothesis of cognitive aging revisited again: Cholinergic functional compensation. Pharmacology Biochemistry and Behavior, 99(2), 254–261.
Dunn, J. C. (1996). Remember-know: A matter of confidence. Psychological Review, 111(2), 524.
Eckart, C., Woźniak-Kwaśniewska, A., Herweg, N. A., Fuentenmilla, L., & Bunzeck, N. (2016). Acetylcholine modulates human working memory and subsequent familiarity based recognition via alpha oscillations. Neuroimage, 137, 61–69.
Edelstyn, N. M., Mayes, A. E., Condon, L., Tunnicliffe, M., & Ellis, S. J. (2007). Recognition, recollection, familiarity and executive function in medicated patients with moderate Parkinson’s disease. Journal of Neuropsychology, 1(2), 131–147.
Edelstyn, N. M., Shepherd, T. A., Mayes, A. R., Sherman, S. M., & Ellis, S. J. (2010). Effect of disease severity and dopaminergic medication on recollection and familiarity in patients with idiopathic nondementing Parkinson’s. Neuropsychologia, 48(5), 1367–1375.
Edelstyn, N. M., John, C. M., Shepherd, T. A., Drakeford, J. L., Clark-Carter, D., Ellis, S. M., & Mayes, A. R. (2015). Evidence of an amnesia-like cued-recall memory impairment in nondementing idiopathic Parkinson’s disease. Cortex, 71, 85–101.
Eichenbaum, H. E., & Cohen, N. J. (2001). From Conditioning to Conscious Recollection: Memory Systems of the Brain (Colume 35). Oxford: University Press.
Eichenbaum, H. E., Yonelinas, A. R., & Ranganath, C. (2007). The medial temporal loba and recognition memory. Annual Review of Neuroscience, 30, 123–152.
Froeliger, B., Gilbert, D. G., & McClernon, F. J. (2009). Effects of nicotine on novelty detection and memory recognition performance: Double-blind, placebo-controlled studies of smokers and non-smokers. Psychopharmacology, 205(4), 625–633.
Gasbarri, A., Packard, M. G., Campana, E., & Pacitti, C. (1994). Anterograde and retrograde tracing of projections from the ventral tegmental area to the hippocampal formation in the rat. Brain Research Bulletin, 33(4), 445–452.
Hasselmo, M. E. (2006). The role of acetylcholine in learning and memory. Current Opinion in Neurobiology, 16(6), 710–715.
Heathcote, A., Frances, R., & Dunn, J. (2006). Recollection and familiarity in recognition memory: Evidence from ROC curves. Journal of Memory and Language, 55(4), 495–514.
Karrer, T. M., Josef, A. K., Mata, R., Morris, E. D., & Samanez-Larkin, G. R. (2017). Reduced dopamine receptors and transporters but not synthesis capacity in normal aging adults: A meta-analysis. Neurobiology of Aging, 57, 36–46.
Knecht, S., Breitenstein, C., Bushuven, S., Wailke, S., Kamping, S., Flöel, A., Zwitserlood, P., & Ringelstein, E. B. (2004). Levodopa: Faster and better word learning in normal humans. Annals of Neurology, 56(1), 20–26.
Koen, J. D., & Yonelinas, A. P. (2014). The effects of healthy aging, amnestic mild cognitive impairment, and Alzheimer’s disease on recollection and familiarity: A meta-analytic review. Neuropsychology Review, 24(3), 332–354.
Li, S.-C., Lindenberger, U., & Bäckman, L. (2010). Dopaminergic modulation of cognition across the life span. Neuroscience & Biobehavioral Reviews, 34(5), 625–630.
Libby, L. A., Yonelinas, A. P., Ranganath, C., & Ragland, J. D. (2013). Recollection and familiarity in schizophrenia: A quantitative review. Biological Psychiatry, 73(10), 944–950.
Mäntylä, T. (1993). Knowing but not remembering: Adult age differences in recollective experience. Memory & Cognition, 21(3), 379–388.
Mesulam, M.-M. (2004). The cholinergic innervation of the human cerebral cortex. Progress in Brain Research, 145, 67–78.
Monte-Silva, K., Kuo, M.-F., Thirugnanasambandam, N., Liebetanz, D., Paulus, W., & Mitsche, M. A. (2009). Dose-dependent inverted U-shaped effects of dopamine (D2-like) receptor activation on focal and nonfocal plasticity in humans. The Journal of Neuroscience, 29(19), 6124–6131.
Morcom, A. M., Bullmore, E. T., Huppert, F. A., Lennox, B., Praseedom, A., Linnington, H., & Fletcher, P. C. (2010). Memory encoding and dopamine in the aging brain: A psychopharmacological neuroimaging study. Cerebral Cortex, 20(3), 743–757.
Naveh-Benjamin, M. (2000). Adult age differences in memory performance: Tests of an associative deficit hypothesis. Journal of Experimental Psychology: Learning, Memory, and Cognition, 26(5), 1170–1187.
Paller, K. A., & Wagner, A. D. (2002). Observing the transformation of experience into memory. Trends in Cognitive Science, 6(2), 93–102.
Parkin, A. J., Gardiner, J. M., & Rosser, R. (1995). Functional aspects of recollective experience in face recognition. Consciousness and Cognition, 4(4), 387–398.
Perfect, T., & Dasgupta, Z. R. R. (1997). What underlies the deficit in reported recollective experience in old age? Memory & Cognition, 25(6), 849–858.
Pitarque, A., Sales, A., Meléndez, J. C., & Algarabel, S. (2015). Repetition increases false recollection in older people. Scandinavian Journal of Psychology, 56(1), 28–44.
Samson, Y., Wu, J. J., Friedmann, A. H., & Davis, J. N. (1990). Catecholaminergic innervation of the hippocampus in the cynomolgus monkey. Journal of Comparative Neurology, 298(2), 250–263.
Shepherd, T. A., Edelstyn, N. M., Mayes, A. R., & Ellis, S. J. (2013). Second-generation dopamine agonists and recollection impairments in Parkinson’s disease. Journal of Neuropsychology, 7(2), 284–305.
Störmer, V. S., Passow, S., Biesenack, J., & Li, S.-C. (2012). Dopaminergic and cholinergic modulations of visual-spatial attention and working memory: Insights from molecular genetic research and implications for adult cognitive development. Developmental Psychology, 48(3), 875–889.
Tulving, E., & Markowitsch, H. J. (1998). Episodic and declarative memory: Role of the hippocampus. Hippocampus, 8(3), 198–204.
Tulving, E., & Thomson, D. M. (1973). Encoding specifity and retrieval processes in episodic memory. Psychological Review, 80(5), 352–373.
Welsh, K. A., Butters, N., Mohs, R. C., et al. (1994). The consortium to establish a registry for Alzheimer’s disease (CERAD). Part V. a normative study of the neuropsychological battery. Neurology, 44(4), 609–614.
Wixted, J. T., & Mickes, L. (2010). A continuous dual-process model of remember/know judgments. Psychological Review, 117(4), 1025–1054.
Yesavage, J. A., Brink, T. L., Rose, T. L., Lum, O., Huang, V., Adey, M., & Leirer, O. (1983). Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research, 17(1), 37–49.
Yonelinas, A. P. (2002). The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language, 46(3), 441–517.
Yonelinas, A. P., & Jacoby, L. L. (2012). The process-dissociation approach two decades later: Convergence, boundary conditions, and new directions. Memory and Cognition, 40(5), 663–680.
Acknowledgements
This work was supported by the DFG (Deutsche Forschungsgesellschaft) grant Mu1364/4-1 and Mu1364/4-2 to Prof. Dr. Müller. We thank Freya-Sophie Lenz for helping with the assessment of data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vellage, A.K., Müller, P., Graf, A. et al. Increasing Dopamine and Acetylcholine Levels during Encoding Does Not Modulate Remember or Know Responses during Memory Retrieval in Healthy Aging—a Randomized Controlled Feasibility Study. J Cogn Enhanc 3, 328–337 (2019). https://doi.org/10.1007/s41465-019-00122-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41465-019-00122-x