Abstract
Root-knot nematodes (RKNs), Meloidogyne spp., are globally important plant-parasitic nematodes with a very broad host range including cucumber. In this research, we evaluated the nematicidal efficacy of commercial Abamectin-CAP (Vertimec 1.8% EC) versus Iranian-produced Abamectin-IAP (Vertimec 2% EC) compared with Cadusafos (Rugby), Trichoderma harzianum T-22 (Tricuran-P), and chicken manure against the cucumber RKN, Meloidogyne javanica, in commercial greenhouses. We also analyzed the effect of the products and amendment on several soil enzymes because of their significant roles in increasing the rate of decomposition and release of plant available nutrients. The results showed highly significant differences among treatments. The highest reduction of second-stage juveniles (J2) in the soil was recorded for Abamectin-CAP and Abamectin-IAP (93–95%), followed by Tricuran-P (90%), Rugby (82%) and chicken manure (65%). Similar results were obtained for the number of J2 and eggs in the root (94%), root gall indexes (94%), egg mass indexes (74–79%), and reproduction percentage (5.4–8.3%) in the Abamectin-CAP and Abamectin-IAP treatments. Enzyme activity assays showed that Rugby and chicken manure both caused a significant decrease in urease activity, followed by Abamectin-CAP and Abamectin-IAP. The highest alkaline phosphatase activity was observed for Abamectin-IAP and Abamectin-CAP, whereas the highest acidic phosphatase activity was in the Abamectin-CAP treatment. The results form a basis for developing integrated pest management strategies for RKN in cucumber.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cucumber, Cucumis sativus L., is one of the most important vegetable crops worldwide and ranks fourth in global vegetable production (Huang et al. 2009; Vercelli et al. 2017). Cucumber is very popular among consumers for a wide range of food uses (e.g., salads, pickles and digestive aids) (Sadeghpoor et al. 2023). Cucumber production is affected by various plant pathogens including plant-parasitic nematodes, of which the most destructive genus is Meloidogyne (root-knot nematode, RKN) (El-Marzoky et al. 2022). RKN has a high fertility rate, short generation time, and wide host range. The related hosts included more than 2000 plant species, such as vegetables, fruit trees, oil and fiber crops, grain crops, and forage crops (Oka et al. 2020), of which are considered as secondary hosts to nematodes in addition to the weed hosts (Resquín-Romeroet al. 2023). RKNs are sedentary endoparasites that attack the root, leading to host nutrient deprivation and impaired water transport, causing aboveground symptoms of stunting, wilting, chlorosis, and reduced crop yields (Resquín-Romero et al. 2023).
Conventionally, RKN control has been performed by soil chemical fumigation, but bans and restrictions on the use of most chemical fumigants, such as methyl bromide, 1.3-dichloropropene, chloropicrin or metam-sodium, have brought serious concerns regarding nematode control in intensive horticulture (Rodrigues et al. 2017). For this reason, the search for new efficient nematicides with different modes of action to the long-time used organophosphate and carbamate nematicides is presently a priority in nematological research (Li et al. 2021). In this regard, Abamectin is one of the suggested alternative biorational tools; this compound belongs to the avermectin group, macro-cyclic lactone metabolites produced via natural fermentation by an actinomycete, Streptomyces avermitilis. Abamectin is marketed under the trade name Vertimec EC1.8% (Hebei Enge Biotech), and its mixture contains more than 80% avermectin B1a and less than 20% avermectin B1b (Hoti et al. 2023). Abamectin is used as an insecticide, acaricide and nematicide on vegetables, fruits and field crops (Sasanelli et al. 2021). This pesticide is formulated as emulsifiable concentrate (EC) in Iran (Hoti et al. 2023). Abamectin is effective in controlling M. javanica and M. incognita in many crops including vegetables such as cucumber, pumpkin, and tomato(Zhang et al. 2023).
Nematode control strategies include a variety of methods such as removing infected roots, flooding the field, deep plowing the soil, using trap plants, steaming, and solarization (Gine et al. 2016; Roth et al. 2020; Sasanelli et al. 2021). Applying biological control agents, such as species of Trichoderma or Gliocladium, is another potential tool to suppress RKNs (Hesar et al. 2011, 2022; Dhillon et al. 2023). Moreover, the addition of organic fertilizers including chicken manure to the soil not only changes its physical and chemical properties, but also reduced the population of M. javanica in cucumber (Nasr Esfahani and Ahmadi 2014, 2020) and M. incognita in eggplant (Osman et al. 2018). Many researchers confirmed the efficacy of organic manures in managing RKNs associated with improvement of plant growth and crop yield (Osman et al. 2018; Dhillon et al. 2023). The addition of chicken manure to the soil caused a change in nematode movement by altering soil physical properties, especially increasing the hydraulic conductivity and porosity of the soil (Forghani et al. 2021). Previous research documented that chicken manure and T. harzianum improved disease-control efficacy and crop yields and were economically as well as environmentally acceptable (Halalat et al. 2017; El-Remaly et al. 2022; Liu et al. 2023).
Soil enzymes play a key role in maintaining the ecology, physical and chemical properties, fertility and health of soils (Koçak 2020; Badawy et al. 2022). These enzymes regulate overall organic matter decomposition in the soil system, of which urease has an important role in the accessibility of N for plant growth in the N cycle (Halalat et al. 2017; Abdel-Nasser et al. 2018). Phosphomonoesterase, i.e., alkaline and acid phosphatases, are the most active soil phosphatases, hydrolyzing phosphomonoesters and in some cases phosphodiesters to release assimilable phosphates for microbes and plants, playing an important role in organic P cycling (Abdel-Nasser et al. 2018; Uwituze et al. 2022). To date, there are few studies asserting the effect of nematicide application on soil enzyme activities (Fig. 1).
Illustration of the effects of various soil treatments on cucumber plants in root-knot nematode infested soil in the greenhouse. A. Untreated controls; B. infected roots in untreated controls; C. Tricuran-P (Trichoderma harzianum T22); D. Commercial Abamectin-CAP; E. Iranian Abamectin-IAP; E. Rugby (Cadusafos); F. chicken manure
The specific objective of this study was to evaluate the efficacy of commercial Abamectin-CAP (Vertimec 1.8% EC), Iranian-produced Abamectin-IAP (Vermectin® 2% EC), the acetylcholinesterase inhibitor nematicide Cadusafos (Rugby); T. harzianum T22 (Tricuran-P), and chicken manure against Meloidogyne javanica, the dominant RKN species on cucumber in Iran in infested commercial greenhouses in two successive years, 2020/21 and 2021/22. In addition, we analyzed crop biomass growth parameters and soil enzyme activities in all treatments.
Materials and methods
Experimental design and treatment application
Two trials were conducted in a loamy soil (36% clay, 39% sand, 25% silt) over the two consecutive growing seasons (2020/21 and 2021/22) in commercial greenhouses at localities in Dorcheh, Isfahan (latitude: 32°39′8.86′′ N: longitude: 51°40′28.63′′ E) in the central part of Iran. In this greenhouse production system, cucumbers are traditionally grown in a rotation with tomato and pepper, all of which are particularly susceptible to RKN (Khader et al. 2023; Li et al. 2020). The trials utilized natural RKN populations present in the soils. Environmental conditions were characterized by temperature 22–27 ± 2 °C, humidity 50–60%, and 16 h daylight.
Forty-day-old Nunhems cucumber seedlings produced by Rexvan (the Netherlands) were transplanted into the soil in the greenhouse with spacing of 0.5 m between plants and 1.0 m between rows. Plots were 30 m2 in area (60 plants/plot) and arranged in a randomized complete block design with three replicates. Cucumber plants were amended through irrigation water with Knop’s nutrient solution (10 mg FeCl3; 0.25 g KH2PO4; 0.25 g KNO3; 0.25 g MgSO4•7H2O and 1 g NaNO3 per liter of tap water; Columbus Chemical Industries, Columbus, WI, USA). General pest and disease management followed suggested prescriptions (Nasr Esfahani et al. 2023).
At the same time, the treatments were applied in the greenhouse as follows: Cadusafos (30 g/m2; Rugby, FMC Corporation, Philadelphia, PA, USA); chicken manure (40 t/ha; Sepahan Poultry, Isfahan, Iran); and T. harzianum T22 (1.5 kg/ha Tricuran-P; Koppert, Bengaluru, India) were applied directly into the soil, whereas Abamectin-CAP (Vertimec 1.8% EC; Hebei Enge Biotech, Shijiazhuang, China) and Abamectin-IAP (Vermectin® 2% EC, Gyah Co., Tehran, Iran) were applied as a drench at 8 L./ha each (2.5 ml/plant). All of these applications were repeated as a drench thrice at an interval of 3 weeks. The cucumber plants were harvested 140 days after planting for further studies. The nutrient composition of the poultry litter was as follows (on a dry-weight basis): organic matter 73.6%; total nitrogen 3.61%; ash 48.4%; pH 7.50; EC 46.00 dS/m; P 1.99%; K 1.66%; Na 0.31%; Ca 7.09%; Mg 0.89%.
To determine the effect of the treatments on plant growth parameters, fresh and dry weights of stems and roots were measured with a digital scale, and the lengths and diameters of the stems and roots as well as root volumes (in an measuring cylinder of 1000 cc filled with tape water) for three plants per replicate were measured at the time of harvesting. Dry weights were determined after placing tissues in a dryer at 70 °C for 48 h (Tehrani et al. 2020; 2021).
Soil samples were taken at the beginning and at the end of each trial to determine RKN populations as well as soil fertility variables including soil enzymes. Details of the sampling process are described in subsequent sections. The soil samples were analyzed at the Esfahan Agriculture and Natural Resource Research and Education Center, Esfahan, AREEO, Iran.
Nematode analysis
Initial population densities of M. javanica were determined prior to planting from 200-g subsamples of well-mixed soil from each row according to Brennan et al. (2020). Randomized quadrat sampling from the related soils were taken up to a depth of 30 cm. The initial populations were recorded to be 8.9 and 6.4 J2/g for the first and second trial, respectively.
Three months after treatment application, five cucumber plants along with their roots were removed gently from the soil for each replicate. After washing the roots, the number of egg masses and gall index on each root were determined as described by El-Kelany et al. (2020). Gall index (GI) and egg mass index (EMI) of the root system of each plant were scored as follows: 0 = roots without gall or egg masses; 1 = presence of 1 to 2 galls or egg masses; 2 = presence of 3 to 10 galls or egg masses; 3 = presence of 11 to 30 galls or egg masses; 4 = presence of 31 to 100 galls or egg masses; and 5 = presence of more than 100 galls or egg masses on the root system (Taylor and Sasser 1978). To count the J2 per 3 g of root, the roots were cut into 1–2-cm pieces and poured into a blender. Then, about 300 ml of 0.9% sodium hypochlorite solution (10% commercial Vitex bleach) was added, roots were crushed at maximum speed for 40 s, and J2 were counted for each replication. Furthermore, the collected soil samples from around the roots were extracted by sieve and centrifuge (Jenkins 1964). The number of J2 per 200-g subsamples of well-mixed soil was counted as described by El-kelany et al. (2020) in 1 ml suspension using a counting slide under the light microscope at 10 × magnification. Means were based on five counts and the population density expressed per g of soil per replication (El-Kelany et al. 2020; Khan et al. 2022; Moatamedi et al. 2018; 2023).
The nematode reproduction factor (Rf = Pf/Pi) was calculated by dividing the final (after 3 months) and initial (prior to planting) RKN population numbers. The data were transformed to √(x + 1) for analysis to meet the assumptions of normality and homoscedasticity, but original values are presented in the manuscript.
Morphological and molecular characterization of nematode species
Nematode identification was based on morphological and morphometric data of the adult females, perineal pattern, and J2 (Sasser & Carter 1985). Females were carefully excised from the cucumber root tissue, and the perineal patterns were cut according to Adam et al. (2007) and cleaned with lactic acid. Finally, the perineal patterns were mounted in glycerin on glass slides and viewed. J2 were isolated, killed and fixed using Hussey and Barker's methods (Hussey and Barker 1973; Ye et al. 2015). After preparing the slides, the characteristics of the J2 and the perineal pattern were examined using an Olympus microscope at 40× magnification (Hawk 2019; Taylor & Sasser 1978).
Molecular identification was based on polymerase chain reaction (PCR) using three Meloidogyne species-specific SCAR primer pairs: ar, inc, and jav (Table 1). The single egg masses were flash-frozen after collection and separately ground with a pestle and mortar. Genomic DNA was extracted using the DENAzist Plant DNA Isolation Kit (DENAzist, Mashhad, Iran) according to the manufacturer’s instructions (Poursakhi et al. 2023; Qalavand et al. 2023). In this way, 500 μl of DG1 buffer and 5 μl from 2-mercaptoethanol to 30 mg were added to ground tissue and mixed and centrifuged the tube at 10,000 rpm for 2 min. The supernatant was transferred into a new spin column and centrifuged at 10,000 rpm for 1 min. Then after, 500 μl from DG2 solution was added into the spin column and centrifuged at 10,000 rpm for 1 min, following addition of 700 μl from DG3 solution into the spin column and centrifuged again. Thereafter, the spin column separated from its collecting tube and placed it into a new 1.5-ml microfuge tube, following addition of 50 μl from DG4 solution onto the center of the spin column and centrifuged at 10,000 rpm for 2 min. Finally, the quantity and purity of total DNA were evaluated using a NanoDrop™ 2000/2000c spectrophotometer (Thermo Fisher Scientific). Then, based on the sequencing of the amplified regions and comparison with GenBank samples, the Meloidogyne species was determined (Mohammadbagheri et al. 2021; Nasehi et al. 2016; 2019; Maleita et al. 2021).
Soil enzyme assays
To determine enzyme activity, soil sampling was conducted by randomized quadrat sampling 3 months after treatment application, as described in Sect. "Nematode analysis". Urease activity was determined by adding 2 g of fresh soil to toluene, 20% urea, and 5 ml of citric acid buffer with a pH of 6.7, followed by incubation at 37 °C for 24 h (Javanshad et al. 2023). Then, 4 ml sodium phenol and 3 ml hypochlorite were added to determine the release of NH4+-N by colorimetry at 578 nm (Cordero et al. 2019; Nasr-Esfahani et al. 2020; Qalavand et al. 2022).
For determination of acid and alkaline phosphatase activity, 5 ml of p-nitrophenyl phosphate substrate, 1 ml of toluene, and 5 ml of pH 5.0 acetic acid buffer were added to 1 g of fresh soil and the suspension was filtered and incubated at 37 °C for 12 h. Thereafter, 1 ml of the filtrated was taken and 100 mM boric acid buffer (pH 9.0), 0.5% 4-amino alternating pyridine (0.5 M, pH 5.0), and 2.5% potassium ferricyanide (0.5 ml) were added. The produced purine nucleoside phosphorylase (pNP) was determined colorimetrically at 570 nm (Liu et al. 2023). Three replicates of each subsample for each of the three enzymes were analyzed. Moreover, substrate-free and soil-free controls were added for each sample to account for non-enzymatic substrate hydrolysis (Ciarkowska et al. 2014).
Statistical analysis
To assess the normality of the obtained data, Kolmogorov–Smirnov and Shapiro–Wilk tests were used by using SPSS 20.0 software (IBM Corporation). The homogeneity of variances within the treatment was also determined using Bartlett’s test (Gholamaliyan et al. 2021). Statistical analysis was performed via analysis of variance (ANOVA), and mean comparisons were performed using protected least significant difference (LSD) tests, with significant difference defined as p < 0.05 using SAS 9.1 software version 9.4 (SAS Institute, Cary, NC, USA) (SAS Institute 2004) (Mohammad et al. 2021; Hejazi et al. 2021; Monazzah et al. 2022).
Results
Identification of nematode species
Based on the results of morphometric studies, the characteristics of the J2, and the terminal patterns of the adult female nematode species in the greenhouse was determined corresponding to the description of Taylor and Sasser (1978) for M. javanica. In the molecular assays (Table 1), two SCAR primers, ar and inc, did not produce any bands, but specific bands were amplified with the jav marker for M. javanica, which was a 670-bp fragment consistent with the results of Hawk (2019).
Effect of treatments on nematode population parameters
Based on the analysis of variance, a significant difference was observed at p < 0.01 between the number of J2 in the root, the EMI on the root, and the reproduction factor in both experiments (Suppl. Table 1). There was no significant difference in the number of J2 in the soil and in GI between the commercial Abamectin-CAP and Iranian-Abamectin-IAP. For all investigated traits, a significant interaction effect was observed at p < 0.01 between trial and treatment. In comparison of the two experiments, the reproduction factor in the soil was significantly lower in the first year than in the second one (Table 2). The number of J2 in the root, the EMI on the root, and the reproduction factor based on the eggs and J2 in the root were significantly lower in the first experiment than in the second one (Table 2).
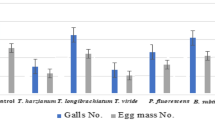
In comparison, the lowest relative number of J2 in the soil was achieved using Abamectin-CAP followed by Abamectin-IAP, 95% and 93% reductions, respectively, as compared with untreated controls. In this regard, both treatments performed significantly better than other treatments. The lowest relative number of J2 in the roots was obtained with Tricuran-P treatments at 94%, followed by Abamectin-IAP at 91% reduction, as was compared with untreated controls (Suppl. Table 2). In this regard, the two abamectin formulations performed significantly better than the controls (Table 2).
Similarly, the lowest GI was observed in the two abamectin formulations, both of which were significantly different from the other treatments and the untreated controls (p < 0.01). In fact, Abamectin-CAP and Abamectin-IAP treatments reduced the GI by 58% and 56%, respectively (Suppl. Table 2). Also, the lowest EMI values in the root were recorded in the application of Abamectin-CAP followed by Abamectin-IAP with 79% and 74% reduction, respectively (p < 0.01). The lowest percentage of J2 reproduction in the soil was in Abamectin-CAP and the highest one in the controls. In this regard, all treatments performed significantly better than the controls. The lowest reproduction factor of eggs and J2 in the root occurred in the treatment using Abamectin-CAP followed by Abamectin-IAP, and the lowest reproduction factor in the soil in the treatment of Abamectin-CAP followed by Abamectin-IAP with 5.4% -8.3%, respectively (p < 0.01) (Table 2).
Biomass growth parameters
Based on the results of variance analysis, a significant difference was observed in the fresh and dry weight traits of stem and root between the two experiments at p < 0.01, and in the root volume trait at p < 0.05 (Suppl. Table 3) (Table 3). There was no significant difference between the two experiments in the traits of diameter and length of stem and root. The treatments had a significant effect on all traits, except the length and diameter of the stem and the diameter of the root, at p < 0.01. In all traits, except length and diameter of stem and root, a significant interaction effect was observed at p < 0.01 between the two factors of test and treatment (Suppl. Table 3). In the comparison of the two experiments, the stem fresh weight was significantly higher in the first experiment than in the second one. The mean of stem dry weight, fresh and dry weight of roots and root volume in the second experiment were significantly higher than in the first one (Table 3).
The highest stem fresh weight was recorded in chicken manure treatment. In this regard, all treatments were significantly different from the non-treated controls. The highest dry weight of the stem was recorded in the application of Abamectin-CAP followed by the Abamectin-IAP treatment. Regarding the stem length, there was no significant difference among the treatments. The highest root fresh weight was recorded for Abamectin- IAP followed by Abamectin-CAP and the controls. In this regard, the treatments were not significantly different from the control. The largest root volume was recorded for Abamectin-CAP followed by the Abamectin-IAP treatment, and the treatments were significantly different from the control. The highest root length was recorded for chicken manure and the lowest in the control (Table 3).
Activity of the selected soil enzymes
As indicated in Tables 4 and Suppl. Table 4, the treatments had significant effect on urease, alkaline phosphatase, and acid phosphatase enzyme activity. Among the treatments, Rugby and chicken manure caused a significant (p = 0.05) decrease in urease activity compared with the non-treated controls (Table 4). In contrast, the highest urease activity was in the untreated control followed by Abamectin-CAP, Abamectin-IAP, and Tricuran-P.
Phosphatase enzyme activities also underwent significant changes. The highest alkaline phosphatase activity was of Abamectin-IAP and Abamectin-CAP, followed by Tricuran-P, Rugby and chicken manure. Similar results were also observed for acid phosphatase (Table 4).
Discussion
Root-knot nematodes are a growing concern for vegetable producers because chemical nematicides are gradually reducing (Li et al. 2021). In this research, we confirmed M. javanica as the causal agent of root-knot of cucumber and analyzed the effect of soil management using chemicals, organic amendments, and biocontrol and found large and significant variations among the applied treatments. Abamectin acts by the avermectin mode of action which blocks the transmittance of electrical activity in nerves and muscle cells of the nematode larvae by stimulating the release and binding of gamma-amino butyric acid (GABA) at nerve endings (Beixing et al. 2018; Mumby et al. 2022). This causes an influx of chloride ions into the cells, which leads to hyperpolarization and subsequent paralysis of the neuromuscular systems and then death (Sasanelli et al. 2021). GABA has also been reported in the J2 of Globodera rostochiensis and Meloidogyne incognita (Khalil 2013; Hawk 2019; Li et al. 2022). Our results showed that the highest reductions of nematode population parameters were recorded in both Abamectin treatments, ranging from 93 to 95%, which is a highly desirable result as far as RKNs management is concerned.
Abamectin is a broad-spectrum insecticide and larvicide prepared from the bacterium Streptomyces avermitilis (Nasr Esfahani et al. 2014; El-Eslamboly et al. 2019), and in the management of RKNs, it offers effective control with minimal environmental pollution (Sasanelli et al. 2021). Abamectin is an effective nematicide that controls a wide range of plant parasitic nematodes such as Meloidogyne spp., Rotylenchulus reniformis and Tylenchulus semipenetrans on different crops (Cabrera et al. 2009; El-Eslamboly et al. 2019; Hawk 2019; Oka et al. 2020). Abamectin contains four major components A1a, A2a, B1a and B2a and four minor components A1b, A2b, B1b and B2b (Beixing et al. 2018). According to Li et al. (2020), Abamectin LC50 values of approximately 2 mg/L to the J2 stage of RKN and Abamectin microcapsule suspension were superior to an emulsifiable concentrate (EC) formulation. It has been suggested that Abamectin effects on female nematodes and egg masses may not match with effects on the gall index and or other parameters, which may be due to the attenuated or delayed development of adult females and reduction of their fecundity (Forghani and Hajihassani 2020).
We also found that Rugby can effectively reduce the RKN populations in the soil and in the roots. Rugby is an organophosphate insecticide used to control nematodes, ringworms, and soil insects in several crops including vegetables and fruit trees (Nasr Esfahani et al. 2014; Oka et al. 2020). The compound works by contact and will only control the mobile stages of the nematodes while they are active in the soil (Oka et al. 2020). Eggs in the soil and larvae that have already penetrated the roots are not controlled (El-Eslamboly et al. 2019). Rugby when correctly applied and incorporated will establish a barrier layer in the soil and will only provide protection for the below ground plant organs within that zone (Soltani et al. 2013). Rugby paralyzes nematodes by inhibiting the acetylcholinesterase enzyme and continuously stimulating the nerve fibers and destroys them by eliminating the possibility of movement and stopping them from feeding. There are several other reports on the efficacy of Rugby against M. incognita in cucumber (El-Eslamboly et al. 2019) and on olive (Soltani et al. 2013), and against the citrus nematode, Tylenchulus semipenetrans, consistent to our results (Cabrera et al. 2009; Oka et al. 2020).
Our results also revealed that Tricuran-P could effectively reduce RKN populations to some extent compared with the other treatments including Abamectins, with almost similar results in terms of soil and root J2, GL, and EMI. Tricuran-P contains the antagonist fungus Trichoderma harzianum, a plant-associated fungus with a high adaptability in different soils and climates. T. harzianum can act symbiotically in plants, promoting plant growth and improving nutrient utilization efficiency and host resistance (Yao et al. 2023). Based on the production of cellulose and chitin decomposing enzymes and the release of plant growth hormones, especially auxin, the fungus is an ideal treatment against soil-pathogenic fungi and to increase plant growth (Zhang et al. 2022). It also increases plant resistance against environmental stressors (Wang et al. 2022; Khan and Tanaka 2023). Species of Trichoderma are able to reduce the damage by RKNs directly by parasitism, antibiosis, and paralysis via production of lytic enzymes (You et al. 2022; Wang et al. 2022). They also modify root morphology and/or rhizosphere interactions, which is advantageous for plant-growth. In addition, Trichoderma spp. able to induce host resistance against nematodes by activating hormone-mediated (salicylic and jasmonic acids and strigolactones, among others) plant-defense mechanisms (Bagheri et al. 2021; 2022; Khan and Tanaka 2023).
Amendments from organic sources such as compost, biochar, crop residues, livestock manure, and poultry litter have been introduced to significantly control root diseases and improve soil health, and thus, they serve as an alternative method for managing plant parasitic nematodes (Khader et al. 2023). We also found that chicken manure can reduce RKN populations to some extent compared with the other treatments. Similar results on RKNs have been reported by Kasim et al. (2021) on coffee; Izuogu and Oyedunmade (2009) on fluted pumpkin (Telfairia occidentalis); and Auwal et al. (2015) on RKNs infecting rice. Chicken manures improve the soil capacity for holding nutrients and water, which improves plant vigor and therefore increases plant tolerance to nematodes; release specific compounds that may be nematicidal (Cole et al. 2020); stimulate microbial activities in the soil, including nematode antagonists; and indirectly stimulate nematode predators and parasites that depend on microbial activities (Collange et al. 2011; Kankam et al. 2014; Azim et al. 2018). Indeed, laboratory studies have shown that chicken manure controls the nematode by releasing toxic substances (Osman et al. 2018). However, such alternative nematode management strategies are unlikely to be as effective and fast-acting as nematicides. Although nematicides effectively reduce plant parasitic nematodes, other environmental and soil fertility issues arise. Therefore, sustainable management of plant-parasitic nematodes from addition of organic amendments to the soil remains an important consideration (Wachira et al. 2009).
Soil enzymes have been suggested as suitable indicators of soil quality because of their intimate relationship with soil biology, the ease with which they can be measured, and their rapid response to change in soil management and environment (Badawy et al. 2022). Soil enzymes can promote the transformation of matter and energy in soil, and the activity of soil enzymes has a close relationship with soil nutrients and their availability (Badawy et al. 2022). Acid phosphatase, alkaline phosphatase, urease, and arylsulfatase activities have been significantly correlated with redox potential (Uwituze et al. 2022). These enzymes work in biochemical processes of overall organic matter decomposition in the soil system, of which urease has an important role in the accessibility of N for plant growth in the N cycle (Abdel-Nasser et al. 2018). In our study, application of Abamectins and Tricuran -P increased urease activity in the soil, which is a positive impact of these particular treatments. Other studies (Micuti et al. 2017; Badawy et al. 2022; Uwituze et al. 2022) showed inconsistent effects of abamectin treatments on soil urease activity.
Phosphomonoesterase is the most active soil phosphatase hydrolyzes phosphomonoesters and in some cases, phosphodiesters to release assimilable phosphates for microbes and plants, playing an important role in organic P cycling (Abdel-Nasser et al. 2018; Uwituze et al. 2022). In our study, soil application of both Abamectins increased alkaline and acidic phosphatase activity. This result is consistent with the findings of other studies, which showed increased alkaline phosphatase activity following nematicide application (Curtright and Tiemann 2021; Uwituze et al. 2022).
Beside the RKN population reduction associated with the various treatments, there were also positive effects on plant growth parameters. The highest stem fresh and dry weights, stem diameter, root length and volume were in chicken manure followed by Abamectin-CAP, Abamectin-IAP, Tricuran-P, and Rugby. In another study, Abamectin recorded an increase in plant shoot and root systems length and weight (Khalil 2013). Kankam et al. (2014) showed that poultry manure significantly reduced nematode populations and significantly increased carrot yield. Contrary to our report, application of a Trichoderma species and chicken manure was ranked third and fourth in the management of M. incognita and plant growth, respectively (Ramazani 2013). According to Li et al. (2021), incorporation of chicken manure with T. harzianum is acceptable for improving the effectiveness of RKN control and eggplant yield. In another study, a significant reduction in root galls and improved growth yield on soil amended with organic manure was associated with the addition of poultry litter in a cucumber establishment (Osman et al. 2018, 2023).
Many alternatives to the use of chemical pesticides have been evaluated for their effectiveness in suppressing nematode population and for environmental compatibility. Unfortunately, most of the environmentally benign chemical products that have recently introduced in the market are not that effective in controlling nematode damage to cucumbers (Daramola et al. 2013). This research showed that Abamectins (1.8% EC, and 2% EC), Tricuran-P, and chicken manure can be considered for incorporation into an integrated pest management strategy for replacing chemical nematicides to achieve sustainable agriculture.
References
Abdel-Nasser G, Massoud MA, Barakat AST, Attia AM (2018) Impact of selected pesticides on some soil enzymes activity in soil cultivated with wheat crop. Alex J Soil and Water Sci 2:66–83
Adam MAM, Phillips MS, Blok VC (2007) Molecular diagnostic key for identification of single juveniles of seven common and economically important species of root-knot nematode. Plant Pathol 56:190–197
Auwal H, Galadima I, Madu J, Joseph P (2015) Evaluation of synergistic effect of neem and poultry manure on root-knot nematodes (Meloidogyne spp.) infecting rice. Open Access Lib J 2:1–4
Azim K, Soudi B, Boukhari S, Perissol C, Roussos S, Alami I (2018) Composting parameters and compost quality: a literature review. Org Agric 8:141–158
Badawy MEI, Mostafa S, Khattab MM (2022) Toxicity, joint action effect, and enzymatic assays of Abamectin and chlorfenapyr, against spider mite Tetranychus urticae. J Basic Appl Zool 83:22
Bagheri LM, Nasr-Esfahani M, Abdossi V, Naderi D (2021) Genetic diversity and biochemical analysis of Capsicum annuum in response to root and basal rot disease, Phytophthora capsici. Phytochem 190:112884
Bagheri LM, Nasr-Esfahani M, Al-Sadi AM, Hassanzadeh KH, Ghadirzadeh E (2022) Screening for resistance and genetic population structure associated with Phytophthora capsici-pepper root and crown rot. Physiol Mol Plant Pathol 119:101835
Brennan RJB, Glaze-Corcoran S, Wick R, Hashemi M (2020) Biofumigation: an alternative strategy for the control of plant parasitic nematodes. J Integr Agric 19:1680–1690
Cabrera JA, Kiewnick S, Grimm C, Dababat AA, Sikora RA (2009) Efficacy of abamectin seed treatment on Pratylenchus zeae, Meloidogyne incognita and Heterodera schachtii. J Plant Dis Prot 116:124–128
Ciarkowska K, Sołek-Podwika K, Wieczorek J (2014) Enzyme activity as an indicator of soil-rehabilitation processes at a zinc and lead ore mining and processing area. J Environ Manage 132:250–256
Cole E, Pu J, Chung H, Quintanilla M (2020) Impacts of manures and manure-based composts on root lesion nematodes and verticillium dahliae in Michigan potatoes. Phytopath 110:1226–1234
Collange B, Navarrete M, Peyre G, Mateille T, Tchamitchian M (2011) Root-knot nematode management in vegetable crop production: the challenge of an agronomic system analysis. Crop Prot 33:25–34
Cordero I, Snell H, Richard D (2019) High throughput method for measuring urease activity. Soil Biol 134:72–77
Curtright AJ, Tiemann LK (2021) Meta-analysis dataset of soil extracellular enzyme activities in intercropping systems. Data Br 38:107284
Daramola FY, Aflame SO, Idowu AA, Odeyemi IS (2013) Effects of poultry manure and carbofuran soil amendments on soil nematode population and yield of pineapple. Int J AgriSci 3:298–307
Dhillon NK, Kaur S, Buttar HS, Singh K, Khapte PS, Kumar P (2023) Management of root-knot nematodes with non-chemical methods for sustainable production of cucumber under protected cultivation. Agronomy 13:124
El-Eslamboly AASA, Abd El-Wanis MM, Amin AW (2019) Algal application as a biological control method of root-knot nematode Meloidogyne incognita on cucumber under protected culture conditions and its impact on yield and fruit quality. Egypt J Biol Pest Cont 29:18–27
El-Marzoky AM, Abdel-Hafez SH, Sayed S (2022) The effect of Abamectin on plant growth and the infection of root-knot nematode Meloidogyne incognita chitwood. Saudi J Biol Sci 29:970–974
El-Kelany US, El-Mougy NS, Abdel-Kader MM (2020) Management of root-knot nematode Meloidogyne incognita of eggplant using some growth-pro- moting rhizobacteria and chitosan under greenhouse conditions. Egypt J Biol Pest Control 30:1–7
El-Remaly E, Osman AA, El-Gawad HGA et al (2022) Bio-Management of root-knot nematodes on cucumbers using biocidal effects of some brassicaceae crops. Horticulturae 8:699
Forghani D, Bazgir E, Esfahani MN, Darvishnia M (2021) Genomic structure of novel Iranian Rhizoctonia solani AG-3PT isolates on potato, Solanum tuberosum. Sydowia 73:217–232
Forghani F, Hajihassani A (2020) Recent advances in the development of environmentally benign treatments to control root-knot nematodes. Front Plant Sci 11:1125
Gine A, Carrasquilla M, Martínez-Alonso M, Gaju N, Sorribas FJ (2016) Characterization of soil suppressiveness to root-knot nematodes in organic horticulture in plastic green-house. Front Plant Sci 7:164
Gholamaliyan A, Esfahani MN, Dababat AA (2021) Novel Iranian wheat cultivars resistant to Bipolaris sorokiniana. Sydowia 73:257–269
Hejazi R, Nasr Esfahani M, Maleki M, Sedaghatfar E (2021) Susceptibility assessment and genetic structure associated with Rhizoctonia solani -Potato stem canker disease. Physiol Mol Plant Pathol 119:101835
Halalat N, Nasr EM, Olia M (2017) The effect of organic fertilizers on population dynamics of sugar beet cyst nematode, Heterodera schachtii Schmidt 1871. J Plant Protect 31(3):475–487
Hawk T (2019) The Effects of Seed-Applied Fluopyram on Root Penetration and Development of Meloidogyne incognita on Cotton and Soybean. Ph.D. Thesis, University of Arkansas, Fayetteville, AR, USA, p 47
Hesar AM, Moghadam EM, Maafi ZT (2011) Morphometrical and genetic diversity of Meloidogyne javanica isolates from the north east of Iran. J Nemat Morph Syst 14:1–11
Hesar AM, Rostami M, Ghaderi R, Danesh YR et al (2022) Population genetic structure of Meloidogyne javanica recovered from different regions of Iran. Agriculture 12:1374
Hoti Q, Rustem DG, Dalmizrak O (2023) Avermectin B1a shows potential anti-proliferative and anticancer effects in HCT-116 cells via enhancing the stability of microtubules. Curr Mol Biol Rep 45:6272–6282
Huang S, Li R, Zhang Z, Li L, Gu X, Fan W, Lucas WJ, Wang X, Xie B et al (2009) The genome of the cucumber, Cucumis sativus L. Nat Genet 41:1275–1281
Hussey RS, Barker KR (1973) Comparison of methods for collecting inocula of Meloidogyne spp., including a new technique. Plant Dis Reporter 57:1025–1028
Izuogu N, Oyedunmade E (2009) Pathogenicity and Control of the Root-Knot Nematode, Meloidogyne incognita on Fluted Pumpkin, Telfairia occidentalis. J Agric Res Dev 7
Javanshad R, Taylor CJ, Delavari N, Barkman TJ, Stull F, Venter AR (2023) Analysis of histidine-tagged recombinant proteins from nickel and copper coated surfaces by direct electrospray ionization and desorption electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom Suppl 1:e9516 (PMID: 37013403)
Jenkins WR (1964) A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Dis Rapt 48:692
Kankam F, Sowley ENK, Oppong NE (2014) Effect of poultry manure on the growth, yield and root-knot nematode (Meloidogyne spp.) infestation of carrot (Daucus carota L.). Arch Phytopathol 48:1–7
Kasim N, Mustari K, Iswari I, Widiayani N (2021) Effect of the application of chicken manure compost tea on the growth of certified cocoa (Theobroma cacao L.) seedlings. IOP Conf Ser Earth Environ Sci 807:042050
Khader A, Ibrahim M, Alkhathami F, Elsawy H, El-Kader NA, Shaker E, Sedky A, Mahmoud E (2023) Properties of nano-amendments and their effect on some soil properties and root-knot nematode and yield attributes of tomato plant. J Agri 13:366–377
Khan MR, Poornima K, Somvanshi VS, Walia RK (2022) Meloidogyne enterolobii: a threat to guava (Psidium guajava) cultivation. Arch Phytopathol 55:1961–1997
Khan M, Tanaka K (2023) Purpureocillium lilacinum for plant growth promotion and biocontrol against root-knot nematodes infecting eggplant. PLoS ONE 18:e0283550
Khalil MS (2013) Abamectin and Azadirachtin as eco-friendly promising biorational tools in integrated nematodes management programs. J Plant Pathol Microb 4:174–185
Koçak B (2020) Importance of urease activity in soil. V. International Scientific and Vocational Studies Congress – Science and Health (BILMES SH 2020), 12–15 December 2020, Turkey
Li B, Ren Y, Zhang D-X, Shuangyu Xu, Wei Mu, Liu F (2018) Modifying the formulation of Abamectin to promote its efficacy on southern root-knot nematodes (Meloidogyne incognita) under blending-of-soil and root-irrigation conditions. J Agric Food Chem 66:799–805
Li QQ, Li JJ, Yu QT, Shang ZY, Xue CB (2021) Mixtures of fluopyram and Abamectin for management of Meloidogyne incognita in tomato. J Nematol 52:e2020–e2129
Li J, Wang C, Bangash SH, Lin H, Tang W (2020) Efficacy of fluopyram applied by chemigation on controlling eggplant root-knot nematodes (Meloidogyne spp.) and its effects on soil properties. PLoS ONE 15:e0235423
Liu Y, Liu Y, Yang L, Zhang T, Jin Y, Liu L, Du J, Zhang D (2023) The effect of abamectin application in combination with agronomic measures on the control efficacy of cucumber root-knot nematodes and the cucumber yield. Pest Manag Sci 79:3190–3199
Maleita C, Cardoso JMS, Rusinque L, Esteves I, Abrantes I (2021) Species-specific molecular detection of the root knot nematode Meloidogyne luci. Biology 10:775
Micuti MM, Badulescu L, Israel-roming F (2017) Effect of pesticides on enzymatic activity in soil. Bull UASVM Anim Sci Biotechnol 75(2):1843–5262
Moatamedi M, Bazgir E, Nasr Esfahani M, Darvishnia M (2018) Genetic variation of bread wheat cultivars in response to cereal cyst nematode, Heterodera filipjevi. Nematol 20:859–875
Moatamedi M, Nasr-Esfahani M, Monazzah M, Faridan VG, Nasr-Esfahani A, Ghadirzadeh E (2023) Transcriptome-proteomic analysis associated with resistance to wheat cyst nematode Heterodera filipjevi. Physiol Mol Plant Pathol 125:102024
Mohammadbagheri L, Nasr-Esfahani M, Naderi D (2021) Genetic diversity and biochemical analysis of Capsicum annuum (Bell pepper) in response to root and basal rot disease Phytophthora Capsici. Phytochemistry 190:112884
Monazzah M, Nasr Esfahani M, Tahmasebi S (2022) Genetic structure and proteomic analysis associated in potato to Rhizoctonia solani AG-3PT-stem canker and black scurf. Physiol Mol Plant Pathol 122:101905
Mumby EJ, Willoughby JA Jr, Vasquez C, Delavari N, Clark CT (2022) Binding interface and electron transfer between nicotine oxidoreductase and its cytochrome c electron acceptor. Biochemistry 61:2182–2187
Nasehi A, Kadir J, Nasr-Esfahani M, Golkhandan E, Ashkani S (2016) Identification of the New Pathogen (Stemphylium lycopersici) Causing Leaf Spot on Pepino (Solanum muricatum). J Phytopath 164:421–426
Nasehi A, Al-Sadi AM, Nasr Esfahani M, Alsultan W, Javan-Nikkhah M (2019) Molecular re- identification of Stemphylium lycopersici and Stemphylium solani isolates deposited in NCBI GenBank and morphological characteristics of Malaysian isolates. Eur J Plant Path 153:965–974
Nasr-Esfahani M, Afshar Farahnaz J, Mardani S, Khankahdani HH, Yazdi MJ, Almasi H, Amini B (2023) Management of Root-Knot Nematodes, Meloidogyne spp. by Organic Manures in Pomegranate, Punica granatum. Indian J Nematol 53:45–59. https://doi.org/10.5958/0974-4444.2023.00007
Nasr Esfahani M, Ahmadi A (2014) The effect of organic and chemical fertilizers on the nematode M. javanica in cucumber. Iran Plant Pathol 41:1–17
Nasr Esfahani M, Hashemi L, Nasr Esfahani N, Nasr Esfahani A (2020) Novel Cucumis enzymes associated with host-specific disease resistance to Phytophthora melonis Katsura. Biotechnol Biotechnol Equip 34:873–884
Oka Y (2020) From old-generation to next-generation Nematicides. Agron 10:1387
Osman HA, Ameen HH, Mohamed M et al (2018) Field control of Meloidogyne incognita and root rot disease infecting eggplant using nematicide, fertilizers, and microbial agents. Egypt J Biol Pest Control 28(40):1–6
Poursakhi SR, Asadi HAG, Nasr-Esfahani M, Abbasi Z, Hassanzadeh HK (2023) Identification of novel associations of candidate marker genes with resistance to onion-fusarium basal rot interaction pathosystem. Plant Gene 100440
Qalavand F, NasrEsfahan M, Vatandoost J, Azarm DA (2022) Enzyme activity and population genetic structure analysis in wheat associated with resistance to Bipolaris sorokiniana-common root rot diseases. Phytochem 200:113208
Qalavand F, Nasr-Esfahan M, Vatandoost J, Azarm DA (2023) Transcriptome - based analysis of resistance mechanism to Bipolaris sorokiniana - wheat common root rot disease. Plant Biol 25:119–130
Ramazani H (2013) Management of root-knot nematode, Meloidogyne incognita with some organic amendments. Plant Prot J 6:191–197
Resquín-Romero G, Mattos VS, Monteiro JMS, Lopez-Nicora HD (2023) Enzymatic and molecular identification of Meloidogyne species in tomato orchards in paraguay. Agron 13:670
Rodrigues HCS, Borges GT, Navroski R, Soares VN (2017) Effect of chemical treatment on physiological quality of seed and control of Meloidogyne javanica in watermelon plants. AJCS 11(1):18–24
Roth MG, Jacobs JL, Napieralski S, Byrne AM (2020) Fluopyram suppresses population densities of Heterodera glycines in field and green-house studies in Michigan. Plant Dis 104:1305–1311
SAS Institute (2004) SAS/STAT User's Guide. Version 9.1.3.Cary: SAS Institute Inc
Sadeghpoor N, Asadi GH, Nasr-Esfahani M, Hassanzadeh HK, Golabadi M (2023) Assessing genetic diversity and population structure of Iranian melons (Cucumis melo) collection using primer pair markers in association with resistance to Fusarium wilt. Funct Plant Biol 50:347–362
Sasanelli N, Konrat A, Migunova V, Toderas I, Iurcu-Straistaru E, Rusu S (2021) Review on control methods against plant parasitic nematodes in southern member states (C Zone) of the European Union. Agri 11:602
Sasser, J.N., Carter, C.C., 1985. An advanced treatise on Meloidogyne. North Carolina State University Graphics, p 422
Soltani T, Nejad RF, Ahmadi AR, Fayazi F (2013) Chemical control of root-knot Nematode (Meloidogyne javanica) On Olive in the Greenhouse conditions. J Plant Pathol Microb 4:183
Taylor AL, Sasser JN (1978) Biology. Identification and control of Root-Knot Nematodes. Coop. Pub. Plant Pathol. North Carolina State Univ. and U. S. Agency int. dev. Raleigh, N.C., p 111
Tehrani MM, Nasr-Esfahani M, Mousavi A, Mortezaiinezhad F, Azimi MH (2020) Regulation of related genes promoting resistance in Iris against root rot disease Fusarium oxysporum f. sp. gladioli. Genomics 112:3013–3020
Tehrani MM, Nasr-Esfahani M, Mousavi A, Mortezaiinezhad F, Azimi MH (2021) Morphogenetic characteristics and response of Iris hybrids to root rot disease by Fusarium oxysporum f. sp. gladioli. Sydowia 74:93–106
Uwituze Y, Nyiraneza J, Fraser TD, Dessureaut-Rompré J, Ziadi N, Lafond J (2022) Carbon, nitrogen, phosphorus, and extracellular soil enzyme responses to different land use. Front Soil Sci 2:814554
Vercelli M, Minuto A, Minuto G, Contartese V, Devecchi M, Larcher F (2017) The effects of innovative silicon applications on growth and powdery mildew control in soilless-grown cucumbers (Cucumis sativus L.) and zucchini (Cucurbita pepo L.). Acta Physiol Plant 39:129
Wachira FM (2009) Effect of land use and soil fertility management practices on nematode destroying fungi in Taita Taveta, Kenya. A thesis submitted in partial fulfillment for the award of doctor of philosophy (PhD) degree in mycology. http://erepository.uonbi.ac.ke/bitstream/handle/11295/6423/Wachira_Effect
Wang H, Zhang R, Mao Y, Jiang W, Chen X, Shen X et al (2022) Effects of Trichoderma asperellum 6S–2 on apple tree growth and replanted soil microbial environment. J Fungi 8:63
Yao X, Guo H, Zhang K, Zhao M, Ruan J, Chen J (2023) Trichoderma and its role in biological control of plant fungal and nematode disease. Front Microbiol 14:1160551
Ye W, Zeng Y, Kerns J (2015) Molecular characterization and phylogenetic relationships of plant-parasitic nematodes associated with turfgrasses in North Carolina and South Carolina, USA. Plant Dis 99:982–993
You J, Li G, Li C, Zhu L, Yang H, Song R et al (2022) Biological control and plant growth promotion by volatile organic compounds of Trichoderma koningiopsis T-51. J Fungi 8:131
Zhang Y, Xiao J, Yang K, Wang Y, Tian Y, Liang Z (2022) Transcriptomic and metabonomic insights into mechanism of Trichoderma asperellum M45a against watermelon Fusarium wilt. PLoS ONE 17:e0272702
Zhang K, Li Y, Wang K, Liu D, Dou S, Chen Y, He M, Ma C (2023) Response of soil enzymes to soil properties and characteristics of cyanobacteria-dominated crusts in a dryland ecosystem. J Soils Sediments 23:1–10
Zhu N, Zhou JJ, Zhang SW, Xu BL (2022) Mechanisms of Trichoderma longibrachiatum T6 fermentation against Valsa Mali through inhibiting its growth and reproduction, pathogenicity and gene expression. J Fungi 8:113
Acknowledgements
Thanks go to the Plant Protection Research Department, Isfahan Center for Agricultural and Natural Resources Research and Education, Isfahan, and the Iran Plant Protection Research Institute, Tehran, Iran, for providing facilities for the project.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Shiva Mardani is responsible for conducting the project, obtaining resources, and writing the original draft of the manuscript. Mehdi Nasr- Esfahani is responsible for supervision, conceptualization, methodology, data curation, review, and editing. Majid Olia is responsible for supervision, review, and data curation. Hamid Molahosseini is responsible for soil analysis, review, and editing. Hamed Hassanzadeh Khankahdani is responsible for methodology, data curation, and review.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
A statement confirming that consent to publish has been received from all participants should appear in the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mardani, S., Nasr-Esfahani, M., Olia, M. et al. Efficacy of nematicides, Tricuran-P (Trichoderma harzianum T-22) and chicken manure on cucumber root-knot nematode populations, plant growth and soil enzyme activities. J Plant Dis Prot (2024). https://doi.org/10.1007/s41348-024-00957-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41348-024-00957-3