Abstract
Citrus anthracnose caused by Colletotrichum gloeosporioides is an economically important disease around the world affecting the pre- and postharvest stages. While fungicides have been used to control this disease, integrated management systems associated with biological control techniques are a sustainable alternative. In the present study, we report the in vitro effect of leaf aqueous extracts (at 1, 2 and 4% w/v) of Argemone mexicana, Datura discolor and Amaranthus palmeri collected from northern Sinaloa (Mexico), against the growth of C. gloesporioides. The D. discolor extract inhibited fungal mycelial growth by 52–73% and did not differ from the chemical treatment (carbendazim-1 ppm). In addition, the D. discolor application at 4% reduced anthracnose in Persian lime fruit similar to fungicide treatment. These results indicate that the aqueous extract of D. discolor has the potential to control citrus anthracnose in Persian lime fruits. Our findings thus open the pathway for future research focusing on strategies to manage citrus anthracnose caused by C. gloeosporioides.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Colletotrichum includes important plant pathogenic fungi in the world (Dean et al. 2012). Also, Colletotrichum spp. cause citrus fruit anthracnose, one of the major disease in many global citrus-growing regions (Pérez-Mora et al. 2021). Colletotrichum gloeosporioides (Penz.) Penz. and Sacc. is considered the most aggressive species to affect leaves, flowers, fruit, and twigs of citrus (Guarnaccia et al. 2017). Anthracnose disease losses in fruits can reach up to 100% under favorable conditions (Rojo-Báez et al. 2017); assessment of postharvest losses by C. gloeosporioides in India revealed that the pathogen contributed 21–26% of the total losses (Naqvi 2004). In Mexico, losses caused by C. acutatum in Mexican lime in Colima, under field conditions reached 40–60% in winter (Orozco-Santos et al. 2006); however, the losses caused by C. gloeosporioides on citrus orchards in Sinaloa (Pérez-Mora et al. 2021) have not determined.
Although fungicides are widely used to control anthracnose, there is increasing concern regarding the resistance of Colletotrichum spp. strains to these compounds (Forcelini et al. 2016; Luo et al. 2021).
Plants content a lot of chemical compounds with direct antimicrobial activity or that induce systemic resistance resulting in reduction of disease development (Kagale et al. 2004); e.g. PAL, phenolics, PR-proteins, terpenes and saponins as well as phytohormones. Furthermore are easily biodegradable (Qasem and Abu-Blan 1966).Thus they can provide us a source of natural agrochemicals for an eco-friendly disease management of plant-pathogens.
Plant-based fungicides have therefore gained interest, and several studies have focused on the antifungal activity of plant extracts against anthracnose in different fruits. For example, weeds include a broad group of plants with a wide distribution, and their extracts present potential antifungal activity (Mushatq et al. 2012). Ademe et al. (2013) studied the in vitro effect of nineteen plant ethyl acetate extracts against C. gloeosporioides, and found that Echinops sp. and Lantana camara L. extracts were the most effective in inhibiting the pathogen’s mycelial growth and reducing the spore germination. In addition, aqueous extracts of Echinops sp. at concentrations of 10% and 25% exerted an effective control against postharvest anthracnose on papaya fruit. Alemu et al. (2014) evaluated the effect of twenty plant extracts, including several weed species, in which methanolic extracts from Datura stramonium L. inhibited in vitro conidial germination and reduced mycelial growth of the pathogen. Furthermore, the aqueous extract of D. stramonium at 50% was shown to reduce the incidence and severity of anthracnose in mango. Finally, Karim et al. (2017) reported that extracts of Datura metel L. caused significant reduction of mycelial radial growth in the same fungus in vitro.
The objectives of the present study were to: (a) determine the in vitro effect of aqueous extracts of the weeds Argemone Mexicana L., Datura discolor Bernh., and Amaranthus palmeri S. Wats. (collected in northern Sinaloa) against C. gloeosporioides; and (b) evaluate the efficacy of the extract in the control of anthracnose in Persian lime fruit (Citrus × latifolia).
Material and methods
Molecular identification of weeds used to obtain leaf extracts
Samples of Mexican prickly poppy (A. mexicana), desert thorn-apple (D. discolor) and palmer amaranth (A. palmeri) were collected at the flowering stage during July and August 2019 in the municipality of Ahome, Sinaloa, Mexico (25°54–55’ N and 108–109°02–55’ W).
Genomic DNA was extracted using the CTAB method (Doyle 1990). Subsequently, the ITS region was amplified using the ITS1 (5’-TCCGTAGGTGAACCTGCGG-3’)/ITS4 (5’-TCCTCCGCTTATTGATATGC-3’) primer set (White et al. 1990). The PCR mix contained 1 μL (30–50 ng) of DNA, 1X of reaction buffer, 1 mM of MgCl2, 0.5 mM of each primer, 500 μM of deoxynucleotide triphosphate (dNTPs) and 0.5 U of Taq DNA polymerase (Invitrogen, CAS: 10342–046) in a total volume of 25 μL. Amplification was performed in a C1000 thermal cycler (Bio-Rad®, Germany) under the following conditions: initial denaturation at 94 °C for 5 min, followed by 35 cycles of denaturation at 95 °C, 58 °C for 30 s, 72 °C for 40 s and a final extension at 72 °C for 5 min. The PCR products were separated by agarose gel electrophoresis (2%) at 80 V for 40 min in 0.5 × Tris–acetate-EDTA (TAE) buffer and visualized with UV light after ethidium bromide staining. The products were subsequently purified and then sequenced by Macrogen (Seoul, South Korea).
Sequences were edited in BioEdit v 7.0.5.3 (Hall 1999) and compared to sequences in GenBank using the BLASTn algorithm. MEGA X (Kumar et al. 2018) was used for alignment and the phylogenetic analysis of each genus. Multiple sequence alignment was performed using the MUSCLE alignment program (Edgar 2004) implemented in MEGA X. The Akaike information criterion (AIC) was used for substitution model selection, and the phylogenetic trees were constructed using the maximum likelihood (ML) method. The Argemone tree was constructed using the Tamura-Nei model with among-site rate modeling (four gamma categories; TN93 + G). The General Time Reversible model with among-site rate modeling (four gamma categories) and invariant sites (GTR + G + I) was used for phylogenetic reconstruction of Datura, and Amaranthus phylogeny was constructed using the Tamura 3-parameter model with among-site rate modeling (four gamma categories; T92 + G). Tree topology support was assessed by 1000 bootstrap replicates. All trees were edited in FigTree (Rambaut 2010).
Preparation of aqueous extracts
A. mexicana, D. discolor and A. palmeri leaves were disinfested with sodium hypochlorite (1%; v/v), rinsed three times with distilled water, and dried for 19 h at 60 °C. Next, 4 g of leaf samples were ground and mixed with 40 ml of distilled water to obtain a 1:10 dilution of each extract. Subsequently, each extract was boiled at 120 °C for 10 min and centrifuged at 4,500 rpm for 10 min. Finally, the supernatant was recovered and stored at 4 °C until further use (Baka and Mousa 2020).
Pathogenicity test
In order to determine the pathogenicity of C. gloeosporioides (isolate FAVF355), six Persian lime fruits, at maturity stage, were first washed with neutral soapy water, rinsed with tap water, and then immersed in 70% ethanol for 3 min. This was followed by immersion in 1% NaCl for 5 min, and then rinsing three times with sterile distilled water. Subsequently, three wounds were made on each fruit with a sterile toothpick. The fruits were then inoculated with 0.5 mL of conidial suspension (1 × 106 conidia/mL), while control fruits were treated with 0.5 mL of sterile distilled water. The fruits were placed in a humidity chamber, incubated at 28 °C with 95% relative humidity (Baka and Mousa 2020). Seven days post inoculation, pathogenicity was determined by measuring the diameter and the depth of the lesions. In order to fulfill Koch’s postulates, fungal colonies were re-isolated from the lesions of inoculated fruits the morphology of the colonies and conidia were similar to the original isolates. The treatments were arranged in a completely randomized design, and the experiment was performed twice.
In vitro assay
In order to determine the in vitro effect of aqueous leaf extracts of A. mexicana (Am), D. discolor (Dd), and A. palmeri (Ap), extracts were diluted to a concentration of 1%, 2%, or 4% (v/v) in autoclaved potato dextrose agar (PDA; Bioxon, Cuautitlán Izcalli, Estado de Mexico, Mexico) at 45 °C. The medium was poured into 90-mm diameter Petri dishes. Next, one mycelial plug (5 mm in diameter) from an 8-day-old colony of C. gloeosporioides was transferred to the center of plates containing the different extract concentrations. An additional treatment consisting of the fungicide carbendazim (1 ppm) was included, and Petri dishes containing PDA without any plant extracts or fungicide were included as a control. Five replicates (i.e. five Petri dishes) were used per treatment and incubated at 28 °C, and the growth of the colony was recorded every 24 h. Experiments were concluded once mycelial growth in the control plates reached 90 mm in diameter. The percentage of inhibition (PI%) was calculated as PI% = [(C-T)∕(C)] × 100, where C is the radius of the fungus in the control plate and T is the radius of the fungus in the presence of the extract or fungicide (Paneerselvam et al. 2012). The treatments were arranged in a completely randomized design, and the experiment was repeated once.
In vivo assay
Healthy detached Persian lime fruits uniform in size and comparable in color were used in the study. Six fruits were superficially disinfected and inoculated as in the pathogenicity test assay. Fifteen hours post inoculation with C. gloeosporioides, fruit were sprayed with 1%, 2% or 4% (w/v) aqueous leaf extract of D. discolor or carbendazim (1 ppm). The control fruits were sprayed with sterile distilled water (Koomen and Jeffries 1993). A second application of the treatment was performed seven days post inoculation.
The efficacy of the extract and fungicides was determined at seven- and fourteen-days post inoculation, by measuring the diameter of the lesion caused by the pathogen on the fruit surface as well as the depth of the lesions with a TRUPER® digital caliper (CALDI-6MP). The treatments were arranged in a completely randomized design, and the experiment was carried out twice.
qPCR quantification
Samples of the infected areas (1 cm3 of damaged tissue) were taken from each fruit. Subsequently, genomic DNA was extracted using the CTAB method (Doyle 1990). DNA was quantified on a Thermo Scientific NanoDrop™ One Spectrophotometer (Thermo Fisher Scientific, USA). Three damaged areas from each fruit were pooled and considered as a single sample, and three fruits per treatment were assessed.
PCR products were amplified using a CFX96™ thermal cycler (Bio-Rad, Germany). The thermocycling program consisted of one cycle at 95 °C for 3 min followed by 50 cycles at 95 °C (30 s) and 60 °C (30 s). The reaction mixture in each well contained 10 µL of Supermix (SsoAdvanced Universal Probe; Bio-Rad, cat. no. 1725281), 6 µL of nuclease-free water, 1 µL to 2 µM of each primer/probe and 1 µL (10 ng) of DNA; a negative control and a healthy control sample were also included in each qPCR run. Three biological replicates with three technical replicates were made for each treatment. A concentration curve, extending from 0.01 to 100 ng, was made with DNA from C. gloeosporioides. The ColF3/ColR1/ColP1 primers used for pathogen detection (Rahman et al. 2019) are shown in Table 1. In addition, the COXfpr primers, based on the citrus mitochondrial cytochrome oxidase gene, were used as an endogenous control (Hu et al. 2013).
Shelf life of D. discolor aqueous extract
A shelf life test of the D. discolor aqueous extract was conducted using the most effective concentration from the in vitro tests (4% aqueous extract). The extract was stored at 4 °C, and the evaluations were carried out three times on a monthly basis. These experiments were performed in the same way as in the in vitro assay described above.
Statistical analyses
All data were subjected to normality tests: for the in vitro assay, data were analyzed by the Kruskal–Wallis and Conover tests. Data obtained from fruit and the shelf life of D. discolor aqueous extract were analyzed by one-way ANOVA, and mean separation was performed using Tukey’s test (Little and Hills 1973) with a value of α = 0.05. To allow for zeros values in some treatments, lesion data from artificially inoculated fruits were transformed by √x + 1 as previously described (Gomez and Gomez 1984). All data were analyzed using SPSS software (IBM SPSS Statistics for Windows, version 25.0. IBM, Armonk, NY, USA).
Results
Molecular identification of weeds
The ITS sequences from weeds were compared to the NCBI database. Amaranth sample showed 100% identity with the Amaranthus sequence of A. palmeri (GenBank accession numbers KY968864 and KY968865), whereas the Argemone sequence had a 99.40–100% identity with A. mexicana voucher TuTY1485 (MH768272) and MIB:SASS 0108 (MZ489728); furthermore, the sequence of Datura displayed a 99.10–100% identity with D. discolor (GenBank accession numbers MG693017 and JX467605). Phylogenetic inference confirmed the identity of each weed as A. mexicana (81.3% bootstrap, Fig. 1a), D. discolor (100% bootstrap, Fig. 1b) and A. palmeri (95.5% bootstrap, Fig. 1c), since all clustered with the respective reference sequence of each botanical species with high bootstrap support.
In vitro assay of the aqueous extracts
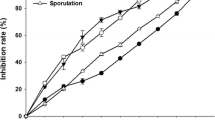
The results of the in vitro tests revealed that the Dd-1%, Dd-2% and Dd-4% aqueous leaf extracts of D. discolor, respectively, reduced the mycelial growth of C. gloeosporioides by 52%, 55% and 73% relative to the control (Fig. 2). Furthermore, the efficacy of these extracts was not significantly different (P = 0.05) from that of carbendazim (1 ppm), which exhibited 86% mycelial inhibition. The aqueous extracts of A. mexicana (Am-1%, Am-2% and Am-4%) and A. palmeri (Ap-1%, Ap-2% and Ap-4%) did not display any significant differences in the growth reduction of the pathogen relative to the control (Table 2). Therefore, these extracts were not included in subsequent studies.
Antifungal effect of the aqueous extract of D. discolor in Persian lime fruits
Fourteen days post inoculation and seven days after the second treatment of the fruits with Dd-1%, Dd-2%, Dd-4% and carbendazim-1 ppm (as well as the inoculated and non-inoculated controls), the lesion diameter ranged from 0.0 to 3.2 mm. The depth of the lesions in fruits treated with aqueous extracts and carbendazim-1 ppm varied from 1.9 to 5.2 mm. By contrast, the inoculated and non-inoculated controls presented lesions of 6.6 and 0.0 mm, respectively, with significant differences among the treatments (P = 0.05) (Fig. 3; Table 3).
Effect of Datura discolor extracts on surface and depth growth of C. gloeosporioides in Persian lime (Citrus × latifolia). A D. discolor at 1%; B D. discolor at 2%; C D. discolor at 4%; D chemical control (carbendazim-1 ppm); E fruit with pathogen; F fruit treated with water in the absence of the pathogen
qPCR quantification of C. gloeosporioides
The reduction of anthracnose in Persian lime fruit by Dd-4% was corroborated by the qPCR detection of fungus DNA copies. Concentration curve from 0.01 to 100 ng of pathogen DNA is shown in Fig. 4a. The amplification curve obtained in fruit samples infected with the pathogen (C +) was similar to the amplification of 10 ng of C. gloeosporioides (10 ng-Cg). Fruit samples treated with the chemical control carbendazim-1 ppm (Cc) and samples treated with the Dd-4% extract displayed a level of amplification similar to 0.01 ng of C. gloeosporioides (0.01 ng-Cg; Fig. 4b). The estimation of pathogen molecules revealed a smaller quantity in the Cc and Dd-4% treatments as compared to the control inoculated with the pathogen (Fig. 4c).
Specific detection of C. gloeosporioides by qPCR. A Log starting quantity of C. gloeosporioides DNA (ng). B Amplification curves of qPCR reactions performed using the ColTqF1, ColTqR1 and ColTqP1 primers sets. Samples include pathogen DNA (with values ranging from 0.01 to 100 ng-Cg), fruit with pathogen (C +), fruit without pathogen (C−), fruit treated with fungicides (Cc), fruit treated with D. discolor at 4% (Dd-4%), and no template control (NTC). C Estimation of the amount of C. gloeosporioides molecules in the specified treatments of Persian lime fruits
Shelf life of D. discolor aqueous extract
In order to determine the shelf life of the D. discolor extract, in vitro experiments were performed on a monthly basis with the aqueous extract at the most effective concentration (Dd-4%). The extract was able to reduce the growth in vitro of C. gloeosporioides up to 3 months after its preparation (Table 4), but with less effectiveness. Fresh leaf extract inhibited mycelial growth by 73% as indicated above, whereas the inhibition effect of the Dd-4% treatment over time was 24.69% (1 month), 24.37% (2 months), and 28.08% (3 months).
Discussion
Although the potential effect of weed leaf extracts against plant diseases has already been reported (Mushatq et al. 2012), research focused on this topic is still in its initial stages in Mexico. For this reason, the present study examined the antifungal potential of leaf extracts of A. mexicana, D. discolor, and A. palmeri against C. gloeosporioides, the causal agent of anthracnose in lime fruits. Datura discolor extracts significantly reduced mycelial growth of the fungus in vitro (52%, 55% and 73%; Table 2), whereas the extracts of A. mexicana and A. palmeri did not show any such effect.
Karim et al. (2017) evaluated the antifungal activity of methanolic extract from the leaves, seeds and roots of D. metel at 1.0, 1.5, 2.0, 2.5 and 3%. All concentrations significantly reduced the radial growth of C. gloeosporioides, although the seed extract at 1.5% showed the highest (80%) antifungal potential. In addition, leaf methanolic extracts of D. stramonium inhibited the mycelial growth of C. gloeosporioides, and the same extract also reduced spore germination by up to 15.7% (Alemu et al. 2014). Considering the results of the present study, further research should be focused on evaluating other D. discolor extracts, with special attention to different solvents (e.g. methanolic and ethanolic) and parts of the plant (e.g. roots and seeds). It would also be interesting to evaluate the effect of D. discolor extracts on the germination of pathogen conidia.
Previously, the aqueous extract of D. stramonium at 50% was shown to reduce anthracnose incidence and severity on postharvest mango fruit by 80% and 58.7%, respectively (Alemu et al. 2014). In this work, lesions on Persian lime fruit 14 days after inoculation reached a surface diameter of 3.2 mm and a depth of 6.6 mm in the control inoculated with the pathogen, while at the high treatment concentration (Dd-4%) the lesion had a surface diameter of 1.9 mm and a depth of 2.8 mm (Table 3). It is therefore possible that a greater reduction of anthracnose could be observed by increasing the concentration of the D. discolor extract. Furthermore, our molecular studies revealed that the amount of C. gloeosporioides DNA molecules was reduced when the Persian lime fruits were inoculated and sprayed with the 4% aqueous extract of D. discolor used to control anthracnose. This approach may also be followed to determine the effect of different concentrations of aqueous extracts of D. discolor and other plants on disease control.
Species within the genus Datura are known to produce several phytochemical compounds with ethnopharmacological and antimicrobial characteristics (Cespedes-Mendez et al. 2021), and the antifungal activity of D. discolor, D. metel and D. stramonium has been evaluated. The methanolic and ethanolic extracts of D. discolor leaves and stems have been reported to inhibit the growth of Aspergillus flavus Link, Aspergillus niger P.E.L. van Tieghem, Penicillium chrysogenum Thom, Penicillium expansum Link, Fusarium moniliforme Sheldon, and Fusarium poae (Peck) Wollenw (Tequida-Meneses et al. 2002).
Datura discolor is a native plant and one of the most widely-distributed species in Mexico (Benítez et al. 2018), phytochemical studies of this species are scarce or null. But studies in Datura confirmed the presence of bioctive compounds as Daturilin a withanolide with antifungal activity (Choudhary et al. 1995; Kagale et al. 2004) found in D. Ferox, D. metel, D. quercifolia, and D. stramonium (Siddiqui et al. 1987; Kagale et al. 2004). Also, the accumulation of PR-proteins, the increase PAL activity and phenolic compounds have been observed in rice plants treated with leaf extract of D. metel and challenged with the pathogens Rhizoctonia solani and Xanthomonas oryzae (Kagale et al. 2004). Using GC–MS analysis, previous studies identified metabolites with antimicrobial activity such as n-hexadecanoic acid, phytol and octadecanoic acid, suggesting that the high antifungal activity of methanolic extract of D. metel might be due to the presence of these compounds (Karim et al. 2017; Hanif et al. 2022). These results suggest the possibility that aqueous leaf extracts of D. discolor might contain bioactive constituents with antifungal activity against C. gloeosporioides.
Knowledge of the shelf life of the aqueous leaf extracts is crucial for their use in plant disease management. The results in this study demonstrate that the in vitro inhibitory effect of D. discolor aqueous leaf extracts on C. gloeosporioides mycelial growth was maintained for up to 3 months. We also observed protective activity in Persian lime fruits when aqueous leaf extracts were applied two weeks post inoculation with the fungus in a 4% concentration at a seven-day interval.
Although aqueous leaf extract of D. metel at different concentrations (100, 200, 300, 400 and 500 µg/ml) has been reported causing deleterious effect on germination, growth and biochemical parameters in seedlings of P. vulgaris and Z. mays (Komolafe et al. 2021). In the present study, the fruits of Persian lime did not exhibit phytotoxicity after two weeks post application. This is the first report on the in vitro antifungal activity of D. discolor aqueous leaf extract against C. gloeosporioides. We also reported on the efficacy of this extract for controlling postharvest anthracnose caused by the same pathogen on Persian lime. Future research lines should focus on characterizing the bioactive compounds implicated in the antifungal activity in the aqueous extract of D. discolor.
Data availability
The authors declare that the data supporting the findings of this study are available within the article.
Code availability
Not applicable.
References
Ademe A, Ayalew A, Woldetsadik K (2013) Evaluation of antifungal activity of plant extracts against papaya anthracnose (Colletotrichum gloeosporioides). J Plant Pathol Microb 4:207. https://doi.org/10.4172/2157-7471.1000207
Alemu K, Ayalew A, Woldetsadik K (2014) Evaluation of antifungal activity of botanicals for postharvest management of mango anthracnose (Colletotrichum gloeosporioides). Int J Life Sci 8(1):1–6
Baka ZAM, Mousa MMA (2020) In vitro and in vivo, biocontrol activity of extracts prepared from Egyptian indigenous medicinal plants for the management of anthracnose of mango fruits. Arch Phytopathol Plant Prot 53:715–730. https://doi.org/10.1080/03235408.2020.1794308
Benítez G, March-Salas M, Villa-Kamel A, Cháves-Jiménez U, Hernández J, Montes-Osuna N, Moreno-Chocano J, Cariñanos P (2018) The genus Datura L. (Solanaceae) in Mexico and Spain - Ethnobotanical perspective at the interface of medical and illicit uses. J Ethnopharmacol 12(219):133–151. https://doi.org/10.1016/j.jep.2018.03.007
Cespedes-Mendez C, Iturriaga-Vasquez P, Hormazabal E (2021) Secondary metabolites and biological profiles of Datura genus. J Chil Chem Soc 66(2):5183–5189. https://doi.org/10.4067/S0717-97072021000205183
Choudhary MI, Dur-e-Shahwar PZ, Jabbar A, Atta-urRahman A (1995) Antifungal steroidal lactones from Withania coagulance. Phytochemistry 40:1243–1246. https://doi.org/10.1016/0031-9422(95)00429-b
Dean R, Van Kan JA, Pretorius ZA, Hammond-Kosack KE, Di Pietro A, Spanu PD, Rudd JJ, Dickman M, Kahmann R, Ellis J, Foster GD (2012) The top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol 13(4):414–430. https://doi.org/10.1111/j.1364-3703.2011.00783.x
Doyle JJ (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. https://doi.org/10.1093/nar/gkh340
Forcelini BB, Seijo TE, Amiri A, Peres NA (2016) Resistance in strawberry isolates of Colletotrichum acutatum from Florida to quinone-outside inhibitor fungicides. Plant Dis 100:2050–2056. https://doi.org/10.1094/PDIS-01-16-0118-RE
Gomez KA, Gomez AA (1984) Statistical procedures for agricultural research, 2nd edn. Wiley, New York
Guarnaccia V, Groenewald JZ, Polizzi G, Crous PW (2017) High species diversity in Colletotrichum associated with citrus diseases in Europe. Persoonia 39:32–50. https://doi.org/10.3767/persoonia.2017.39.02
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hanif S, Jabeen K, Akhtar N, Iqbal S (2022) GC-MS analysis & antifungal activity of Datura metel L. against Rhizoctonia solani Kuhn. An Acad Bras Cienc 94(1):e20200851. https://doi.org/10.1590/0001-3765202220200851
Hu H, Davis MJ, Brlansky RH (2013) Quantification of live Candidatus Liberibacter asiaticus populations using real-time PCR and propidium monoazide. Plant Dis 97(9):1158–1167. https://doi.org/10.1094/PDIS-09-12-0880-RE
Kagale S, Marimuthua T, Thayumanavan B, Nandakumara R, Samiyappana R (2004) Antimicrobial activity and induction of systemic resistance in rice by leaf extract of Datura metel against Rhizoctonia solani and Xanthomonas oryzae pv. oryzae. Pathology 65(2):91–100. https://doi.org/10.1016/j.pmpp.2004.11.008
Karim M, Jabeen K, Iqbal S, Javaid A (2017) Bioefficacy of a common weed Datura metel against Colletotrichum gloeosporioides. Planta Daninha v 35:e017164676. https://doi.org/10.1590/S0100-83582017350100040
Komolafe IJ, Fajobi AO, Dare CA, Morakinyo AE, Oyedapo OO (2021) Phytotoxic activities of aqueous leaf extract of Datura metel on germination and seedlings of Zea mays and Phaseolus vulgaris. Egypt Acad J Biolog Sci (H Botany). 12(2):165–177
Koomen I, Jeffries P (1993) Effects of antagonistic microorganisms on the post-harvest development of Colletotrichum gloeosporioides on mango. Plant Pathol 42:230–237. https://doi.org/10.1111/j.1365-3059.1993.tb01495.x
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35(6):1547–1549. https://doi.org/10.1093/molbev/msy096
Little TM, Hills FJ (1973) Agricultural experimentation and analysis. Wiley, New York
Luo Q, Schoeneberg A, Hu M (2021) Resistance to azoxystrobin and thiophanate-methyl is widespread in Colletotrichum spp. isolates from the mid-atlantic strawberry fields. Plant Dis. 105(8):2202–2208. https://doi.org/10.1094/PDIS-09-20-2048-RE
Mushatq S, Haider MS, Ali A, Javed S, Khokhar I, Mukhtar I (2012) In vitro comparative screening of antibacterial and antifungal activities of some common weeds extracts. Pak J Weed Sci Res 18(1):15–25
Naqvi SAMH (2004) Diagnosis and management of pre and post-harvest piseases of Citrus fruit. In: Naqvi SAMH (ed) Diseases of Fruits and Vegetables, vol I. Springer, Dordrecht. https://doi.org/10.1007/1-4020-2606-4_8
Orozco-Santos M, Medina-Urrutia VM, Robles-González M, Orozco-Romero J, Pérez-Zamora O, Velázquez-Monreal JJ, Timmer LW, Guzmán-González S (2006) Biología y manejo integrado de actracnosisi del limón mexicano en el trópico seco de México. SAGARPA, INIFAP, CIRPAC. Campo Experimental Tecomán. Folleto Técnico Num. 2. 73
Paneerselvam A, Kumar D, Thenmozhi R, Anupama P, Nagasathya A, Thajuddin N (2012) Selection of potential antagonistic Bacillus and Trichoderma isolates from tomato rhizospheric soil against Fusarium oxysporum f. sp. lycoperscisi. J Microbiol Biotechnol Res 2:78–89
Pérez-Mora JL, Mora-Romero GA, Beltrán-Peña H, García-León E, Lima NB, Camacho-Tapia M, Tovar-Pedraza JM (2021) First report of Colletotrichum siamense and C. gloeosporioides causing anthracnose of Citrus spp. in Mexico. Plant Dis 105(2):496–496. https://doi.org/10.1094/PDIS-08-20-1743-PDN
Qasem JR, Abu-Blan HA (1966) Fungicidal activity of some common weed extracts against different plant pathogenic fungi. J Phytopathol 144:157–61. https://doi.org/10.1111/j.1439-0434.1996.tb01507.x
Rahman M, Islam T, Schwegel R, Louws F (2019) Simultaneous detection of Colletotrichum acutatum and C. gloeosporioides from quiescently infected strawberry foliage by real-time PCR based on high resolution melt curve analysis. Am J Plant Sci 10:382–401. https://doi.org/10.4236/ajps.2019.103028
Rambaut A (2010) FigTree v1.3.1. Institute of Evolutionary Biology, University of Edinburgh, Edinburgh. http://tree.bio.ed.ac.uk/software/figtree/
Rojo-Báez I, Álvarez-Rodríguez B, García-Estrda RS, León-Félix J, Sañudo Baraje A, Allende-Mollar R (2017) Current status of Colletotrichum spp. in Mexico: Taxonomy, characterization, pathogenesis and control. Mexican J Phytopathol 35(3):549–570
Siddiqui S, Naheed S, Ahmad S, Haider I (1987) A novel withanolide from Datura metel. Phytochemistry 9:2641–2643
Tequida-Meneses M, Cortez-Rocha M, Rosas-Burgos EC, López-Sandoval S, Corrales-Maldonado C (2002) Effect of alcoholic extracts of wild plants on the inhibition of growth of Aspergillus flavus, Aspergillus niger, Penicillium chrysogenum, Penicillium expansum, Fusarium moniliforme and Fusarium poae moulds. Rev Iberoam Micol 19(2):84–88
White T, Bruns T, Lee S, Taylor J, Innis M, Gelfand D, Sninsky J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis DG MA, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications, vol 18. Academic Press, Cambridge, pp 315–322
Acknowledgements
The authors thank Juan Manuel Tovar Pedraza and Juan Luis Pérez-Mora for providing the Colletotrichum gloeosporioides isolate used in this study, and for their advice during the development of the experiments. We thank Dr. Brandon Loveall from improvence editing services for English proofreading of the manuscript.
Funding
This research received external funding from the Instituto Politécnico Nacional (SIP20211643). XEVC received a master’s fellowship from CONACyT (757974).
Author information
Authors and Affiliations
Contributions
X.E.V.C., J.C.M.A. and G.A.M.R. were involved in the conceptualization and design of the project, and drafted the manuscript; R.F.G. and S.P.D.C. revised and critically edited the manuscript; K.Y.L.M. performed the phylogenetic analysis to identify the weeds from which extracts were obtained; and C.R.I.S. and C.R.U. performed the statistical analysis and data interpretation. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Verdugo-Contreras, X.E., Martínez-Álvarez, J.C., Díaz-Camacho, S.P. et al. Antifungal activity of weed aqueous extracts on Persian lime anthracnose caused by Colletotrichum gloeosporioides. J Plant Dis Prot 130, 293–300 (2023). https://doi.org/10.1007/s41348-022-00671-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-022-00671-y