Abstract
Essential oils are a real ore rich in bioactive compounds characterized by a wide spectrum of various biological activities. In this context, our research focused on the study of the chemical composition of Mentha pulegium and Myrtus communis essential oils growing in Northeast Algeria as well as the exploration of their antifungal activities in vitro and in vivo against Botrytis cinerea responsible for moulding on strawberries. GC–MS analysis indicated that M. pulegium essential oil was an isomenthone chemotype (55.59%) while M. communis essential oil was characterized as a eucalyptol chemotype (36.82%). M. pulegium essential oil expressed the best antifungal activity either with poisonous medium method (MIC = MFC:2.66 µl/ml) or with volatile activity method (MIC:30 µl) compared to M. communis essential oil, (MIC:5.33 µl/ml, MFC:10.66 µl/ml) which expressed no volatile activity. Both crude oils completely inhibited the germination of B. cinerea spores and resulted in up to 88% morphological changes in conidia. In vivo tests have revealed the effectiveness of M. pulegium essential oil in completely suppressing grey mould from strawberries previously inoculated with conidia of B. cinerea by direct contact or exposure to vapours. M. pulegium essential oil display weak phytotoxicity towards fumigated strawberries at low temperatures (T < 16 °C). This low phytotoxicity was confirmed by the preservation of some physical parameters of strawberries stored at 7 °C such as colour and weight loss.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Agricultural products are often subject to biotic and abiotic damage during production, conservation and processing. Post-harvest losses are mainly due to oxidative processes or attacks by destructive agents mainly insects and moulds. In developing countries, pre- and post-harvest fungal diseases cause 12% crop loss (El Reza et al. 2010) and up to 50% fruit loss during storage and transport (De Cicco et al. 2008). On the other hand, about 35% of crops are lost annually due to pests (Bounechada and Arab. 2011). In addition, fungal and insect contamination decreases the post-harvest storage life and declines the marketability of the fruits (Tripathi et al. 2007). Botrytis cinerea, the causa agent of grey mould disease, is multidrug resistant to anti-botrytis fungicides. It is a polyphagous pathogen attacking more than 200 plant species (Jarvis 1980) including greenhouse grown crops (Rosslenbroich and Stuebler 2000), as well as cold-stored fruits (Williamson et al. 2007). In particular, strawberries are very sensitive to the grey rot agent during cultivation, storage and marketing.
To control postharvest fungi, a variety of synthetic chemicals have been applied. However, overreliance on synthetic pesticides is disheartened because of their harmful effects on health and the environment, in addition to the development of resistance among strains of pests and pathogens. Moreover, pesticides are characterized by significant drawbacks including toxicity to non-target organisms (Soylu et al. 2010), carcinogenic and teratogenic potential, and persistence in the food chain (Lingk 1991). Furthermore, increasing public awareness of human health, food safety and environment preservation has led consumers to ban products treated with chemical agents (Teixeira et al. 2012). Against this background, the scientific community was compelled to seek new sustainable and healthy alternatives by exploring new natural molecules from plants to create a new generation of botanical pesticides for pests and disease management. Since ancient times, essential oils have been used in natural pharmaceutical preparations and in food conservation (Prabuseenivasan et al. 2006). The use of essential oils has recently become an attractive technique applied in post-harvest disease control (Znini et al. 2013); it is associated with the fungicidal and insecticidal activities of their vapour phase allowing them to be used as bio-fumigants for the management of rot in stored products (Tripathi et al. 2007) and as a relevant alternative to extend the shelf life and preservation of the overall quality of the fruit after harvest (Aloui et al. 2014). Due to their very rich and complex composition, resistance of organisms to essential oils is rarely established (Siroli et al. 2015). It is difficult to relate the biological activity of essential oils to a single component, but it seems to be the result of additive and synergistic action of their components (Bagamboula et al. 2004).
The interest of the present work was to characterize the chemical composition of Mentha pulegium L (Lamiaceae) and Myrtus communis L (Myrtaceae) essential oils from oriental Numidia (Algeria), then to assess their antifungal potential in vitro and in vivo against Botrytis cinerea on strawberries.
Material and methods
Plant material
Aerial parts of each species were sampled from two different sites in the Annaba region located in the Northeast of Algeria (Oriental Numidia) where they grow spontaneously in the wild state. For M. pulegium, samples were harvested during the flowering period (July, 2018) from Zâamcha locality (12 km south of Annaba: 36° 47′ N, 7° 45′ E), whereas M. communis samples were collected during the setting period (October, 2017) from the Edough Massif (13 km west of Annaba: 36° 55′ N, 7° 36′ E). The plant material was air-dried in the shade at room temperature (20–25 °C) for 7 days and then kept in glass boxes and stored in a dry place.
Essential oils extraction and GC/MS analysis
Dried leaves of each plant (100 g) were submitted to hydrodistillation for 90 min using a Clevenger-type apparatus. The obtained essential oils were stored in opaque airtight flasks and kept at 3–4 °C. Essential oil yields of each plant species were calculated according to Carrée (1953). The identification of the volatile compounds of essential oils was carried out using an Agilent 7890A gas chromatograph combined to an Agilent 5972C mass spectrometer with electron impact ionization (70 eV). The mass spectrometer was equipped with an HP-5 MS capillary column (19091S-433), length 30 m, diameter 250 μm and film thicknesses 2.5 μm (5% phenyl methyl silicone, 95% dimethylpolysiloxane; Hewlett-Packard, CA, USA). Column temperature was automated to rise from 50 to 250 °C at a rate of 7 °C/ min. The essential oils components were identified by comparing their retention indices (RI) relative to n-alkanes with those of authentic compounds published in the literature or available in our laboratory. Additionally, the identification was confirmed by matching their mass spectra with those recorded in the Wiley Registry 9th Edition/NIST 2011 Edition mass spectral library. The composition of the essential oils was given as a relative percentage of the total peak area.
Antifungal bioassay
Fungal isolation and molecular identification
A virulent strain of B. cinerea was isolated from a symptomatic strawberry showing grey mould symptoms. It was purified and identified on the basis of morphological criteria according to Jarvis (1980). The purified strain was maintained on potato dextrose agar (PDA) medium at 25 ± 2 °C for routine use and stored at − 80 °C on 30% glycerol for long term storage.
Internal Transcribed Spacer (ITS) regions of fungal ribosomal DNA were amplified using primer pair (White et al 1990) ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATAT GC-3′) according to Kalai et al. (2010). PCR amplification products were then sequenced and the resulting sequence was checked and processed for sequence similarities in DNA databases by The BLAST program (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Poisoned food technique
The toxicity of M. pulegium and M. communis essential oils against B. cinerea was studied using the poisoned food technique (Mohana and Raveesha 2007). Eight increasing concentrations (0.16, 0.33, 0.66, 1.33, 2.66, 5.33, 10.66 and 21.33 µl/ml) of each essential oil were tested by being incorporated into 15 ml of sterile PDA medium cooled to 40 °C and mixed well before solidification. Subsequently, an 8 mm diameter agar plug was aseptically removed from the edge of seven-day-old B. cinerea cultures and placed in the centre of each Petri dish. The control was conducted under the same conditions but without any supplementation. Petri dishes were incubated for 5 days in dark at 25 ± 2 °C. Mycelial growth (cm) was measured daily. Three replicates were performed for each essential oil and concentration. Growth inhibition was calculated according to the formula of Cakir et al. (2005), in percentage inhibition of radial growth of the treated samples compared to the control.
where C Mean radial mycelial growth of pathogen alone (control), T Mean radial mycelial growth of treated samples.
The lowest concentration of essential oils without fungal growth was considered a minimum inhibitory concentration (MIC).
Effect of essential oil vapour exposure
The toxicity of M. pulegium and M. communis essential oils against B. cinerea was also performed using the volatile activity technique as described by Neri et al. (2006) slightly modified. For this assay, 8 mm of mycelial disc was inoculated in PDA dishes and exposed to volatiles compounds. Sterile squares of Whatman filter paper N °1 were glued with sterile adhesive tape to the inner surface of the lids of the petri dishes. Subsequently, the sterile squares were supplemented by 0, 25, 30 or 35 µl of pure essential oils. Petri dishes were sealed with Parafilm, inverted and incubated for 5 days in the dark at 25 ± 2 °C. Three replicates were carried out for each concentration and each oil. Mycelial growth diameters were noted daily and data were expressed as per cent inhibition of radial mycelial growth according to Plaza et al. (2004). Minimum inhibitory concentration (MIC) was assigned to the lowest concentration able to inhibit totally fungal growth.
Minimum fungitoxic concentration (MFC)
To determine the fungitoxic concentration of M. pulegium and M. communis essential oils against B. cinerea, fungal discs showing no visual fungal growth when exposed or contacted with a volatile compound were transferred and reinoculated into fresh PDA medium. Fungal growth was observed after 7 days of incubation at 25 ± 2 °C.
Spore germination assay
A conidial suspension of B. cinerea was prepared by scraping off a ten-day-old culture and resuspending the resulting mycelium in sterile glucose solution 5%. Conidial suspension was aseptically filtrated and adjusted to 105 spores/ml by hemocytometer (Malassez). In vitro assays were performed using concave micro-culture slides by mixing 40 µl of either crude or MFC (diluted in 5% DMSO) of essential oil with 40 µl of conidial suspension (105 cells/ml). Control was prepared by mixing 40 µl of sterile glucose solution 5% with 40 µl of conidial suspension (105 cells/ml). Slides were incubated in a wet and dark chamber at 25 ± 2 °C for 48 h then observed with an optical microscope (Leica) at 1000 magnification. Each treatment was conducted in quadruplicate. The percentage of conidial germination was evaluated using four regions per slide and corresponding to at least 300 conidia.
Microscopic observation
Mycelium was obtained by mixing on a slide 40 µl of sterile glucose solution 5% with 40 µl of conidial suspension (105 cells/ml). Four slides were incubated in a wet chamber at 25 °C in the dark and allowed to grow till a well-developed mycelium was obtained. The effect of essential oils on the mycelium structure was evaluated by adding 40 µl of crude essential oils to the mycelium (two replicates). Incubation was performed under the same conditions as previously described and observation of the mycelia morphology was monitored daily using an optical microscope. Control was run by mixing sterile glucose solution 5% instead of essential oil with the mycelium. Conidia were obtained from the previous spore germination test.
In vivo application
Selection of the most suitable essential oil to undertake in vivo testing was based on the higher yield and lower MFC. Therefore, firm and freshly harvested strawberries (cultivar camaroza) were first superficially sterilized by soaking in 2% sodium hyperchlorite solution for 5 min, rinsed in sterile distilled water and then dried on sterile Whatman filter paper before starting the tests. Fruits were then inoculated on the surface with 8 mm mycelium discs diameter obtained from 10-day-old culture of B. cinerea at the rate of one disc per strawberry. Treatment of inoculated fruits with essential oil was assessed either by direct contact or by fumigation immediately after inoculation assessed in plastic boxes covered with moistened sterile filter paper. Thus, 30 µl of pure essential oil was applied directly to the fruits for the first test, whereas in the second test, 400 µl/l air (in vitro MIC) was deposited in sterile Whatman filter paper glued inside the lids of the box. All boxes were sealed with parafilm to ensure airtightness and were incubated at 7 °C for 13 days. Treatments and controls were replicated three times at the rate of three strawberries by box and one box per repeat. The incidence of contamination was assessed using a rot index. The severity of strawberry disease in each replicate was assessed according to the empirical scales of Zhao et al. (2011) slightly modified: 0: healthy fruit, 1: rotten area less than 12% of the total fruit surface; 2: rot surface between 12 and 25% of the fruit surface, 3: rot surface between 25 and 40% of the fruit surface, 4: symptoms extend over an area ranging from 50 to 75% of the fruit surface; 5: rotten area greater than 75% of the fruit surface. The disease index was calculated by the following formula:
where N1 to N5 is the respective number of fruits on each scale and NT is the total number of fruits examined. The evaluation of decay inhibition (DI) was assessed according to De Corato et al. (2010) as follows:
where Nc is the average of the number of infected fruits in the untreated sets and N0 is the average of the number of infected fruits in the oil-treated sets.
Phytotoxicity assay of M. pulegium essential oil vapour
Healthy, firm and uniform strawberries were selected to assess the optimal conditions for safe application of essential oils vapour to the fruit by testing different storage temperatures (7, 12, 16, 20, 25 °C). Symptoms of phytotoxicity were evaluated by storing strawberries treated with the MIC of M. pulegium essential oil vapour at different temperatures according to a phytotoxicity index: 0: no change, 1: colour change, 2: superficial softening, 3: important softening, 4: modification of the pulp. The injury index was calculated by the following formula:
where N1 to N5 is the respective number of fruits of each index and NT is the total number of fruit examined. Fruits were stored in airtight boxes containing four strawberries and each box was considered as a replicate. In this experience, three replicates were assessed.
Effect of M. pulegium essential oil vapour on physical parameters of stored strawberries
-
a.
Surface colour change measurement
Fruit surface colour was measured on three batches of strawberries considering 10 fruits/batch. Measurements were performed on M. pulegium essential oil vapour treated fruits and untreated control fruits before and after 6 days of storage at 7 °C. The CIE L*, a*, and b* parameters were provided by a chromameter (CR 400, Minolta) at the two opposite sides of the fruit. a* values indicate green or red colour from negative to positive values. b* values designate yellow colour: the higher this parameter, the more the colour becomes yellow. H or hue degree was also calculated according to the formula: h° = arctangent [b*/a*], considering 0° = red purple, 90° = yellow, 180° = bluish green and 270° = blue. Chroma or intensity or colour saturation is finally computed according to the formula C* = [a*2 + b*2]1/2).
-
b.
Weight loss measurement
Weight loss was evaluated by measuring the weight of three batches of each strawberry treated and untreated with M. pulegium essential oil considering 10 fruits/batch. Weight loss was calculated before and after 6 days of storage at 7 °C by the formula:
With NSW: weight of not stored fruits; SW: weight of stored fruits.
Statistical analysis
Results were expressed as mean ± SD. Data were compared by one-way analysis of variance (ANOVA) followed by Duncan’s multiple range tests. All analyses were performed using SPSS22.0. A P value < 0.05 was considered significant.
Results
Essential oils chemical composition
The oil yield of M. pulegium and M. communis was 2.14 and 0.63%, respectively (Table 1). Statistical analysis showed a significant difference in oil yield between the two species (F1,4 = 950.47, P < 0.001). The identified components, the percentage (%) and retention index (RI) of two essential oils are listed in Table 1. A total of 17 volatile constituents amounting 99.98% of the total oil of M. pulegium have been detected. The chemical composition of M. pulegium oil was strongly dominated by oxygenated monoterpenes (85.59%) represented especially by isomenthone (55.59%) followed by p-menthone (17.66%), piperitone (5.15%) and isopiperitenol (2.11%). The hydrocarbon monoterpenes class was weakly represented (14.39%), while the sesquiterpenes class was totally absent in the essential oil. GC–MS analysis of M. communis essential oil revealed the presence of 20 main components representing 95.13% of the total composition of the oil. The essential oil of M. communis exhibited a high percentage of oxygenated monoterpenes (58.92%) and hydrocarbon monoterpenes (35.25%), unlike to a very weak presence of the sesquiterpenoids class (1.38%). Eucalyptol and α-pinene were the main active compounds accounting for 36.82 and 29.08%, respectively, in common myrtle oil.
Molecular identification of Botrytis cinerea isolate
The sequence obtained (Accession number: OM530236) matched 100% with Botrytis cinerea ATCC 11,542 sequence deposited in Genbank (Accession number KU729081).
Antifungal bioassay
Poisoned food technique
The minimum inhibitory concentration of M. pulegium and M. communis essential oils not allowing the growth of B. cinerea was determined at 2.66 µl/ml and 5.33 µl/ml, respectively, in comparison with a negative control showing 100% growth (Fig. 1). At 1.33 µl/ml, the inhibition of mycelial growth reached 87.66%, for M. pulegium and 19.26% for M. communis. Below this concentration, no significant antifungal effect was registered for the two essential oils, showing that inhibition percentage increased with increasing concentrations of the oils with a dose–response way.
Inhibition of Botrytis cinerea mycelial growth by Mentha pulegium and Myrtus communis essential oils by poisonous medium method at 7 days incubation. a inhibition index; b Mycelial growth inhibition; a: Mentha pulegium; b: Myrtus communis. Different letters are significantly different according to Duncan test at P ≤ 0.01)
Re-incubation of fully inhibited mycelium discs at doses equal to or greater than the MIC on fresh culture medium (PDA) determined that the minimum fungitoxic concentration (MFC) corresponded to the MIC for M. pulegium and 10.66 µl/ml for M. communis.
Effect of essential oil vapour exposure
Statistical analysis allowed to classify M. pulegium essential oil as the most effective against B. cinerea when it is applied by volatile activity method. In fact, M. communis oil showed a very weak volatile effect in inhibiting B. cinerea mycelial growth even at the highest concentration of 35 µl not exceeding 3.67% (Fig. 2). Whereas M. pulegium allows fungal inhibition varying from 77.48 to 100% at 25 µl and 30 µl, respectively (Fig. 2), knowing that, 30 µl of M. pulegium essential oil is the minimum inhibitory concentration towards B. cinerea. Mycelial discs completely inhibited by M. pulegium essential oil (30 and 35 µl) displayed normal fungal growth when deposited in culture medium not treated with essential oil. This experiment demonstrated that the essential oil vapours of M. pulegium exhibit a fungistatic effect against B. cinerea.
Spore germination assay
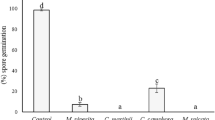
The observation of the effect of M. pulegium and M. communis essential oils on B. cinerea conidia showed that the two essential oils totally inhibited fungal germination compared to untreated control (Fig. 3and Table 2). Crude essential oil treated conidia also exhibited 88.68% and 87.28% of morphological modifications (Macrovacuolization, swelling and crumbling) related, respectively, to M. pulegium and M. communis assay (Fig. 3 and Table 2). MFC treated conidia showed a low percentage of germination reaching, respectively, 13.78% and 8.32% for the essential oils of M. pulegium and M. communis compared to the control (92.45%) (Table 2). However, ungerminated conidia treated with minimal fungitoxic concentrations of both oils showed no structural modifications.
Micrograph of morphological modifications of Botrytis cinerea conidia and mycelium treated with Mentha pulegium and Myrtus communis essential oils. a untreated conidia before germination; b untreated positive control of germinated conidia; c conidia treated with M. communis oil showing macrovacuolization of the cytoplasm and pore formation; d conidia treated with M. communis oil broken up releasing its intracellular content; e conidia treated with M. pulegium oil showing macrovacuolization; f Conidia treated with M. pulegium oil showing cell wall deformations; g conidia treated with M. pulegium oil broken up; h normal untreated mycelium; i mycelium treated with M. communis showing macrovacuolization and membrane deformation; j Mycelium treated with M. pulegium oil showing macrovacuolization
Microscopic observation of essential oil treated mycelium
Microscopic observation of the mycelium treated with essential oils exhibited serious structural alterations compared to the control (Fig. 3h, i and j). In fact, the treated hyphae appeared thinner and were characterized by an irregular surface, the absence of septation and the presence of macrovacuolization (Fig. 3i and j) when the untreated control hyphae showed a typical septate structure with a regular cell wall and microvacuoles (Fig. 3h).
In vivo assay
Symptoms evaluation on inoculated and treated strawberries showed complete inhibition of B. cinerea decay by M. pulegium essential oil both by direct contact and by vapour exposure (Fig. 4). Untreated control strawberries showed 100% symptom development corresponding to a maximum disease index (Table 3 and Fig. 4).
Protective effect of Mentha pulegium essential oil against Botrytis cinerea on strawberries by direct contact (A) and vapor phase (B) treatment after 13 days of inoculation.1: Control strawberries non treated with essential oil and inoculated with Botrytis cinerea; 2: strawberries treated with essential oil and inoculated with Botrytis cinerea
Phytotoxicity test
Strawberries exposed to oil vapours displayed a very good appearance aspect at low temperatures (7, 12 and 16 °C). Statistical analysis showed a significant effect of storage temperature on the phytotoxicity of essential oils. Indeed, the increase in temperatures induced an increase in damage to the strawberries (Fig. 5) with injury indices varying from 0.08 to 1 for temperatures ranged from7 to 25 °C.
Effect of M. pulegium essential oil fumigation on strawberries surface colour during storage
Evaluation of M. pulegium essential oil fumigation on stored strawberries revealed that there was overall no significant change in the surface colour of strawberries compared to untreated control strawberries. However, there was a significant preservative effect on certain fruit colour parameters (Table 4). Indeed, H° and b* decreased in the stored fruits treated or not treated compared to the fruits not preserved (Table 4). a* also showed a significant difference between strawberries stored at 7 °C and pre-stored fruits highlighting a storage effect. Otherwise, chroma (c) was not affected by storage but fruits treated with essential oils showed a slight decrease in colour intensity (− 8%) compared to untreated strawberries before and after storage. Knowing that the treatment with essential oils did not affect any colour parameter before storage.
Effect of M. pulegium essential oil fumigation on strawberries weight during storage
Statistical analysis revealed a significant difference between the weight loss of strawberries treated or not with M. pulegium essential oils. Indeed less than 2% of weight loss was recorded in fruits treated with essential oils and preserved, i.e. 1420 times less than untreated and preserved fruits showing a weight loss of 22.90% (Table 5).
Discussion
In this study, the antifungal activities of essential oils (EO) of Mentha pulegium and Myrtus communis were evaluated on the mycelial growth and the sporulation rate of B. cinerea on synthetic medium and on the development of grey mould in strawberries. The results showed that the two oils completely stopped the mycelial growth of the fungus in the solid medium. In particular, essential oil of M. pulegium. was the most effective against B. cinerea with a lower MIC (2.66 µl/ml) and allowing a fungitoxic effect (by Poisoned food technique and exposure to essential oil vapours)). However, M. communis essential oil expressed higher MIC (5.33 µl/ml) and MFC (10.66 µl/ml) by the Poisoned food technique and showing a very weak volatile antagonist effect against mycelial growth of B. cinerea. This difference in the antifungal potential of the essential oils of the two plants can be attributed to their chemical compositions. This is because M. pulegium oil is dominated by a ketone while M. communis oil is mainly constituted of terpene oxide. Previous works (El Arch et al. 2003; Saeed et al. 2012) have shown that ketones are more active against microbial agents than terpene oxides. Although, according to Koba et al. (2004), the inhibitory power of essential oils towards a microbial strain is classified as excellent inhibitory power for: MIC < 50 µl/ml. Thus, on the basis of their MIC, the oils of M. pulegium and M. communis originating from Algeria are considered to be natural antifungal compounds potent against B. cinerea. These results corroborate previous works which demonstrated the good antifungal effect of these two essential oils against dermatophyte strains (M. furfur, M. sympodialis, Malassezia sp (Barac et al. 2017)) and non-dermatophyte strains (Alternaria alternata, Penicillium expansum, Botrytis cinerea, Fusarium culmorum, Fusarium oxysporum, Aspergillus flavus, Aspergillus niger and Trichoderma sp (Uwineza et al. 2018); Rhizopus sp, Aspergillus sp. and Penicillium sp (Amalich et al. 2016)). This antifungal potency of essential oils is certainly attributed to their chemical composition rich in oxygenated compounds (Soltani and Kellouche. 2004; Bourkhiss et al. 2007; Amarti et al. 2010) representing 85.59% in M. pulegium and 58.92% in M. communis.
In addition to the quantitative dissimilarities, there are mainly qualitative differences, between the essential oils of M. pulegium and M. communis. In fact, M. communis essential oil mainly consists of eucalyptol (36.82%) and α-pinene (29.08%) whereas in M. pulegium essential oil the major compound is isomenthone (55.59%) followed by p-menthone (17.66%).
These results corroborate previous works on the chemical composition of M. communis essential oil (Aouadi et al. 2020), whereas they contradict the results of Beghidja et al. (2007), Zekri et al. (2013), Ouakouak et al. (2015); Abdelli et al. (2016) stipulating that Algerian pennyroyal oils are characterized by the predominance of pulegone. Likewise, Tunisian (Snoussi et al. 2008; Hajlaoui et al. 2009), Portuguese (Rodrigues et al. 2013), Moroccan (Farah et al. 2001; Chebli, et al. 2003; Ouraini et al. 2005; Ouraini et al. 2007), Brazilian (Silva et al. 2015), Uruguayan (Lorenzo et al. 2002), Egyptian (El Ghorab 2006) and Yugoslavian (Teixeira et al. 2012) M. pulegium essential oils are reported to be predominantly pulegone. Nevertheless, Spanish (De Gavina and Ochoa. 1974), Uruguayan (Grosso and Moyna. 1985), Cuban (Pino et al. 1996) and Greek (Kokkini et al. 2004) pennyroyal oils are characterized by the prevalence of isomenthone which is in concordance with our findings. Although, another chemotype rich in pipéritone/pipéritenone has also been reported in Morocco (Derwich et al. 2010) and in Greece (Kokkini et al. 2004).
Indeed, the study of essential oils from different populations of M. pulegium allowed to classify three chemotypes: pulegone-type, piperitenone/piperitone-type and isomenthone neoisomenthol-type (Lawrence 1978).
According to Rodrigues et al. (2013), the chemical composition is closely related to the phenological stage; thus, the relative amount of pulegone in M. pulegium essential oil increases until the vegetative phase then decreases while anticipating the pre-flowering phase. Towards full flowering, the relative amount of pulegone increased further. These changes are followed by changes in the relative amounts of isomenthone and menthone, as pulegone decreased isomenthone and menthone tended to increase.
Chemical composition also depends on seasonal variations, geographic areas, climatic and edaphic conditions (Müller-Riebau et al. 1997) generating variable bioactivity profiles within the same species (Gon Çalves et al. 2007).
However, most essential oils rich in alcohols and/or ketones have a stronger antimicrobial activity than those which have high hydrocarbon contents (Charai et al. 1996; Koroch et al. 2007). Indeed, pulegone have been widely reported to be responsible for the antifungal activity of essential oil (Muller-Riebau et al. 1995; Samber et al. 2014; Boni et al. 2016) but is also known to be hepatotoxic and is not suitable for aromatherapy or post-harvest or stored food processing. Moreover, 1,8-cineole, piperitenone (Dorman and Deans. 2000; El Arch et al. 2003; Satrani 2010), menthone (Hmiri et al. 2011) and isomenthone (Gon Çalves et al. 2007) have also been reported to exhibit an antifungal effect.
Biological activity could also be the result of the synergistic effect of minor compounds (Bouzouita et al. 2008; Saban et al. 2008). The work of Chebli et al. (2003) and Vilela et al. (2009) has shown that major compounds inhibit mycelial growth, but at higher concentrations when the essential oils are in their entirety; thus, the activity of essential oil is the result of its major compounds and also of the synergistic effect of minor compounds (Chebli et al. 2003; Ouraini et al. 2007).
The mechanism of action of terpenes is not fully understood, but it is likely that these lipophilic compounds soluble in aqueous media cause significant damage to the cell walls of microorganisms (Griffin et al. 1999) through loss of membrane integrity (Cowan 1999; Hajlaoui et al. 2009) and inducing deleterious effects on mitochondrial membranes leading to inhibition of mitochondrial energy metabolism, resulting in disturbances in a wide range of physiological and biochemical processes in the cell (Yoshimura et al. 2010). It is also suggested that the essential oil compound can act as an H + carrier or a depleting adenosine triphosphate pool (Farag et al. 1989; Adams et al. 1996; Ultee et al. 2002).
In this context, microscopic observations have corroborated the loss of membrane integrity of B. cinerea conidia treated with crude essential oil exhibiting nearly 90% morphological modifications (macrovacuolization, swelling and crumbling). The two crude essential oils studied abolished the germination process probably resulting from disturbances of physiological and biochemical processes induced by essential oils. Whereas, treated conidia with the minimum fungitoxic concentration (MFC) exhibited a germination inhibition of nearly 80% and no membrane deformation which corroborating the dose-response activity of the two oils. In fact, the activity of essential oils has been investigated by many authors who implied that antimicrobial activity results from the interaction of the active compound at low concentrations, with the enzymatic systems of microbial cells (Omidbeygi et al. 2007; Russo et al. 2013), whereas at higher concentrations, they cause protein denaturation (Russo et al. 2013).
The ability of M. pulegium essential oil to inhibit the growth of B. cinerea was also confirmed by direct application of the oil to the fruit surface or by fumigation. Accordingly, both methods of application were efficient when in vitro MIC was tested. Indeed no rot symptoms were observed on the fruits treated and inoculated with essential oil compared to the untreated and inoculated control fruits showing severe symptoms of grey rot. The in vivo tests were carried out under storage condition (7 °C) and indicated that at low temperature, M. pulegium essential oil did not affect the quality of the fruits. Nevertheless, knowing that several essential oils could be phytotoxic (Kalai-Grami et al. 2019; Tsao and Zhou. 2000), the optimal conditions for a safe application of the essential oil of M. pulegium were evaluated. Results revealed a significant effect of storage temperature on the phytotoxicity of essential oils. Indeed, when the temperature exceeds 16 °C, the quality of the strawberries is drastically affected. Similarly, Tsao and Zhou (2000) found that the phytotoxicity of monoterpenoids is also related to temperature and becomes negligible at 2 °C against post-harvest pathogens.
Therefore, phytotoxicity testing is necessary before considering an essential oil as an alternative disease control method. However, these drawbacks can be overcome by the formulation (Plotto et al. 2003), the association with other compounds and the application temperature (Tsao and Zhou. 2000). Besides, the level of phytotoxicity depends on the species, variety and phonological stage treated by the essential oil (Vidal et al. 2018). Therefore, M. pulegium oil could be non-phytotoxic when applied to other fruits or other stored products even at higher temperature.
The low phytotoxicity of this essential oil at 7 °C was also confirmed by studying the effect of M. pulegium essential oil vapour on the physical parameters of stored strawberries. Indeed, no significant colour change was recorded in the stored treated strawberries compared to the untreated and preserved strawberries although a small decrease in the chroma value was observed. These results are in accordance with Ulukanli and Tulin (2015) and Aitboulahsen et al. (2018) who noted a slight decrease in the chroma value in treated stored fruits.
Similarly, no significant weight loss was registered in the treated and stored strawberries compared to the stored control. In addition, the M. pulegium essential oil even had a protective effect on the treated fruits by reducing the weight loss by 22.90% compared to the control being up to the strawberry acceptance limit (6%) (Aitboulahsen et al. 2018). The reduction in weight loss by treatment with essential oils has also been reported by many authors (Martinez-Romero et al. 2005; Serrano 2005) which implies that it could probably be attributed to the creation of a protective layer on the surfaces of fruit openings preventing water loss by minimizing metabolic activity, respiration and transpiration during post-harvest fruit storage (Shafiee et al. 2010).
Conclusion
Overall, it can be concluded that the essential oil of M. pulegium displays interesting contact and fumigant toxicity against B. cinerea. Accordingly, M. pulegium essential oil could be applied as a potential botanical pesticide and can be considered as a safer and cleaner alternative to preserve the fruits from fungal attacks.
References
Abdelli M, Moghrani H, Aboun A, Maachi R (2016) Algerian Mentha pulegium L. leaves essential oil: chemical composition, antimicrobial, insecticidal, and antioxidant activities. Ind Crops Prod 96:197–205
Adams S, Kunz B, Weidenborner M (1996) Mycelial deformations of Cladosporium herbarum due to the application of eugenol and carvacrol. J Essent Oil Res 8:535–540
Aitboulahsen M, Zantar S, Laglaoui A, Chairi H, Arakrak A, Bakkali M, Hassani Zerrouk M (2018) Gelatin-Based edible coating combined with Mentha pulegium essential oil as bioactive packaging for strawberries. J Food Qual 2018:8408915
Aloui H, Khwaldia K, Licciardello F, Mazzaglia A, Muratore G, Hamdi M, Restuccia C (2014) Efficacy of the combined application of chitosan and Locust Bean Gum with different citrus essential oils to control postharvest spoilage caused by Aspergillus flavus in dates. Int J Food Microbiol 170:21–28
Amalich S, Zerkani H, Cherrat A, Soro NK, Bourakhouadar M, Mahjoubi M, El Hilali F, Zair T (2016) Study on Mentha pulegium L. from M’rirt (Morocco): antibacterial and antifungal activities of a pulegone-rich essential oil. J Chem Pharm Res 8(5):363–370
Amarti F, Satrani B, Ghanmi M, Farah A, Aafi A, Aarab L, El Ajjouri M, Chaouch A (2010) Composition chimique et activité antimicrobienne des huiles essentielles de Thymus algeriensis Boiss & Reut. Et Thymus ciliatus (Desf) Benth du Maroc. Biotechnol Agron Soc Environ 14(1):141–148
Aouadi G, Haouel S, Soltani A, Ben Abada M, Boushih E, Elkahoui S, Taibi F, Mediouni Ben Jemâa J, Bennadja S (2020) Screening for insecticidal efficacy of two Algerian essential oils with special concern to their impact on biological parameters of Ephestia kuehniella (Lepidoptera: Pyralidae). J Plant Dis Prot. https://doi.org/10.1007/s41348-020-00340-y
Bagamboula C, Uyttendaele M, Debevere J (2004) Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonnei and S. flexneri. Food Microbiol 21(1):33–42
Barac A, Donadu M, Usai D, Spiric VT, Mazzarello V, Zanetti S, Nikolic E, Stevanovic G, Popvic N, Rubino S (2017) Antifungal activity of Myrtus communis against Malassezia sp. Isolated from the skin of patients with Pityriasis versicolor. Infection 46:253–257
Beghidja N, Bouslimani N, Benayache F, Benayache S, Chalchat JC (2007) Composition of the oils from Mentha pulegium growing in different areas of the east of Algeria. Khim Prir Soedin 4:394–395
Boni G, Feiria S, Santana P, Anibal P, Buso-Ramos M, Barbosa J, Oliveira T, Hofling F (2016) Antifungal and cytotoxic activity of purified biocomponents as carvone, menthone, menthofuran and pulegone from Mentha spp. Afr J Plant Sci 10(10):203–210
Bounechada M, Arab R (2011) Effet insecticide des plantes Melia azedarach L. et Peganum harmala L. sur Tribulium castaneum Herbst (Coleoptera : Tenebrionidae). Agromomie 1
Bourkhiss M, Hnach M, Boukhriss B, Chaouch OM, A, (2007) Composition chimique et propriétés antimicrobienne de l’huile essentielle extraite des feuilles de Tetraclinis articulate (Vahl) du Maroc. Afr Sci 3:232–242
Bouzouita N, Kachouri F, Ben Halima M, Chaabouni MM (2008) Composition chimique et activités antioxydante, antimicrobienne et insecticide de l’huile essentielle de Juniperus phoenicea. J Soc Chim Tunisie 10:119–125
Cakir A, Kordali S, Kilic H, Kaya E (2005) Antifungal properties of essential oil and crude extracts of Hypericum linarioides Bosse. Biochem Syst Ecol 33(3):245–256
Carrée P (1953) Précis de technologie et de chimie industrielle. Tome II. Ed. Balliére J. B. et fils
Charai M, Mossadak M, Faid M (1996) Research on the antimicrobial activities of two aromatic plants: Origanum majorana and Origanum compactum. J Essent Oils Res 8:657–664
Chebli B, Achouri M, Idrissi Hassani LM, Hmamouchi M (2003) Chemical composition and antifungal activity of essential oils of seven Moroccan Labiatae against Botrytis cinerea Pers: Fr. J Ethnopharmacol 89:165–169
Cowan MM (1999) Plant products as antimicrobial agent. Clin Microbiol Revue 12:564–582
De Cicco V, Bertolini P, Salerno MG (2008) Patologia postraccolta dei prodotti vegetali. Ed. Piccin. Italy
De Corato U, Maccioni O, Trupo M, Di Sanzo G (2010) Use of essential oil of Laurus nobilis obtained by means of a supercritical carbon dioxide technique against post-harvest spoilage fungi. Crop Prot 29:142–147
De Gavina M, Ochoa JT (1974) Aceite esencial de Mentha pulegium L. poleo. In: contrbucion al studio de los aceites essenciales espagnoles II. Aceites esentiales de la provencia de Guadalajara. Minist. Agric. Instituto Nacional de Investigaciones Agrarias, Madrid 235–254
Derwich E, Benziane Z, Boukir A (2010) Antibacterial activity and chemical composition of the leaf essential oil of Mentha rotundifolia from Morocco. Elec J Environ Agric Food Chem 9(1):19–28
Dorman HJD, Deans SG (2000) Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol 88(2):308–316
El Arch M, Satrani B, Farah A, Bennani L, Boriky D, Fechtal M, Blaghen M, Talbi M (2003) Composition chimique et activité antimicrobienne et insecticide de l’huile essentielle de Mentha rotundifolia du Maroc. Acta Bot Gall 150(3):267–274
El Ghorab AH (2006) The chemical composition of the Mentha pulegium L. essential oil from Egypt and its antioxidant activity. J Essent Oil Bear Plants 9(2):183–195
El Reza S, Rahman A, Ahmed Y, Kang SC (2010) Inhibition of plant pathogens in vitro and in vivo with essential oil and organic extracts of Cestrum nocturnum L. Pestic Biochem Phys 96:86–92
Farag RS, Daw ZY, Abo-Raya SH (1989) Influence of some spice essential oils and Aspergillus parasiticus growth and production of aflatoxins in synthetic medium. J Food Sci 54:74–76
Farah A, Fechtal M, Afifi A (2001) Valorization des huiles essentielles de la menthe pouliot (Mentha pulegium). Ann Rech for Maroc 34:76–86
Gon Çalves MJ, Vicente AM, Cavaleiro C, Salgueiro L (2007) Composition and antifungal activity of the essential oil of Mentha cervina from Portugal. Nat Prod Res 21(10):867–871
Griffin SG, Wyllie SG, Markham JL, Leach DN (1999) The role of structure and molecular properties of terpenoids in determining their antimicrobial activity. Flavour Fragr J 14:322–332
Grosso M, Moyna P (1985) Composicon quimica de las esencias de Mentha del Uruguay. An Real Acad Farm 51:333–338
Hajlaoui H, Trabelsi N, Noumi E, Snoussi M, Fallah H, Ksouri R, Bakhrouf A (2009) Biological activities of the essential oils and menthol extract of two cultivated mint species (Mentha longifolia and Mentha pulegium) used in the Tunisian folkloric medicine. World J Microbiol Biotechnol 25:2227–2238
Hmiri S, Amrani N, Rahouti M (2011) Détermination in vitro de l’activité antifongique des vapeurs d’eugénol et d’huiles essentielles de Mentha pulegium L. et de Tanacetum annuum L. vis-à-vis de champignons responsables de la pourriture des pommes en post-récolte. Acta Botan Gall 158(4):609–616
Jarvis W (1980) Epidemiology. In: Coley-Smith JR, Verhoeff K, Jarvis WR (eds) The biology of Botrytis. Academic Press, London
Kalai Grami L, Bahri BA, Chemekh M, Hammami M, Limam F, Hajlaoui MR (2019) In vitro antifungal activities of essential oils of three Lamiaceae plant species against Plenodomus tracheiphilus (Syn. Phoma tracheiphila). Tunis J Plant Protec 14:11–32
Kalai Grami L, Mnari Hattab M, Hajlaoui MR (2010) Molecular diagnostic to assess the progression of Phoma tracheiphila in Citrus aurantium seedlings and analysis of genetic diversity of isolates recovered from different Citrus species in Tunisia. J Plant Path 92:629–636
Koba K, Sanda K, Raynaud CYYA, Millet J, Chaumont JP (2004) Annales de Médecine Vétérinaire 148: 202–206
Kokkini S, Hanlidou E, Karousou R, Lanaras T (2004) Clinal variation of Mentha pulegium essential oils along the climatic gradient of Greece. J Essent Oil Res 16(6):588–593
Koroch A, Juliani HR, Zygadlo JA (2007) Bioactivity of essential oils and their components. In: Berger RG (ed) Flavours and fragrances chemistry, bioprocessing and sustainability. Springer, Berlin, pp 87–111
Lawrence, M (1978) A study of the monoterpene interrelationship in the genus Mentha with reference to the origin of pulegone and menthofuran PhD thesis, State University, Groningen, Netherlands
Lingk W (1991) Health risk evaluation of pesticide contaminations in drinking water. Gesunde Pflangen 43:21–25
Lorenzo D, Paz D, Dellacassa E, Davies P, Canigueral S (2002) Essential oils of Mentha pulegium and Mentha rotundifolia from Uruguay. Braz Arch Biol Technol 45(2):519–524
Martinez Romero D, Castillo S, Valverde JM, Guillen F, Valero D, Serrano M (2005) The use of natural aromatic essential oils helps to maintain postharvest quality of crimson table grapes. Acta Hortic 682:1723–1729
Mohana DC, Raveesha KA (2007) Anti-fungal evaluation of some plant extracts against some plant pathogenic field and storage fungi. J Agric Sci Technol 4:119–137
Muller Riebau F, Berger B, Yegen O (1995) Chemical composition and fungitoxic properties to phytopathogenic fungi of essential oils of selected aromatic plants growing wild in Turkey. J Agric Food Chem Ed Davis 43(8):2262–2266
Müller Riebau FJ, Berger BM, Yegen O, Cakir C (1997) Seasonal variations in the chemical compositions of essential oils of selected aromatic plants growing wild in Turkey. J Agric Food Chem 45(12):4821–4825
Neri F, Mari M, Brigati S (2006) Control of Penicillium expansum by plant volatile compounds. Plant Path 55:100–105
Omidbeygi M, Mohsen BA, Hamidi A, Naghdibadi H (2007) Antifungal activity of thyme, summer savory and clove essential oils against Aspergillus flavus in liquid medium and tomato paste. Food Control 18(12):1518–1523
Ouakouak H, Chohra M, Denane M (2015) Chemical composition, antioxidant activities of the essential oil of Mentha pulegium L, South East of Algeria. Inter Lett Nat Sci 39:49–55
Ouraini D, Agoumi A, Ismaili Aloui M, Alaoui K, Cherrah Y, Amrani M, Belabbas MA (2005) Etude de l’activité des huiles essentielles de plantes aromatiques à propriétés antifongiques sur les différentes étapes du développement des dermatophytes. Phytothérrapie Et Pharmacognosie 4:147–157
Ouraini D, Agoumi A, Ismaili Aloui M, Alaoui K, Cherrah Y, Alaoui MA, Belabbaas MA (2007) Activité antifongique de l’acide oléique des huiles essentielles de Thymus saturejoides L. et Mentha pulegium L, comparée aux antifongiques dans les dermatoses mycosiques. Phytothérapie 1:6–14
Pino JA, Rosado A, Fluentes V (1996) Chemical composition of the essential oil of Mentha pulegium L. from Cuba. J Essent Oil Res 8:295–296
Plaza P, Torres R, Usall J, Lamarca N, Viňas I (2004) Evaluation of potential of commercial post-harvest application of essential oils to control citrus decay. J Hortic Sci Biotechnol 79(6):935–940
Plotto A, Roberts DD, Roberts RG (2003) Evaluation of plant essential oils as natural postharvest disease control of tomato (Lycopersicon esculentum). Issues ans Adventices in Postharvest Hort. Ed. R.K. Prange Acta Hort. 628, ISHS. 737–745
Prabuseenivasan S, Jayakumar M, Ignacimuthu S (2006) In vitro antibacterial activity of some plant essential oils Evid. Based. Complementary Altern Med 6(39)
Rodrigues MBC, Favaro LCL, Pallu APS, Ferreira A, Sebastianes FS, Rodrigues MJC, Sposito MB, De Araujo WL, Pizzirani AA (2013) Agrobacterium-mediated transformation of Guignardia citricarpa : an efficient tool to gene transfer and random mutagenesis. Fungal Biol 117:556–568
Rosslenbroich HJ, Stuebler D (2000) Botrytis cinerea-history of chemical control and novel fungicides for its management. Crop Prot 19(8–10):557–561
Russo M, Suraci F, Postorino S, Demetrio S, Roccotelli A, Agosteo GE (2013) Essential oil chemical composition and antifungal effects on Sclerotium cepivorum of Thymus capitatus wild populations from Calabria, southern Italy. Braz J Pharmacognosy 23:239–248
Saban AK, Cakir A, Ozer H, Cakmakci R, Kesdek M, Mete E (2008) Antifungal phytotoxic and insecticidal properties of essential oil isolated from Turkish Origanum acutidens and its three components, carvacrol, thymol and p-cymene. Bioresour Technol 99:8788–8795
Saeed MO, Ali W, Tail Al-Hulitan AM (2012) The effect of infestation by the confused flour beetle (Tribolium confusum Duy) on specifications of wheat flour Vol 2
Samber N, Varma A, Manzoor N (2014) Evaluation of Mentha piperita essential oil and its major constituents for antifungal activity in Candida spp. IJIRSET 3(2):9402–9411
Satrani B (2010) Valorisation des plantes aromatiques et médicinales du Maroc. Éditions Universitaires Européennes. ISBN : 978-613-51855-3, p 153
Serrano, (2005) The use of natural aromatic essential oils helps to maintain postharvest quality of Crimson table grapes. Acta Hortic 682:1723–1729
Shafiee M, Taghavi TS, Babalar M (2010) Addition of salicylic acid to nutrient solution combined with postharvest treatments (hot water, salicylic acid and calcium dipping) improved postharvest fruit quality of strawberry. Sci Hortic 124:40–44
Silva LF, Cardoso MG, Batista LR, Gomes MS, Rodrigues LMA, Rezende DACS, Teixeira ML, Carvalho MSS, Santiago JA, Nelson DL (2015) Chemical characterization, antibacterial and antioxidant activities of essential oils of Mentha viridis L. and Mentha pulegium L. (L). Am J Plant Sci 6:666–675
Siroli L, Patrignani F, Serrazanetti DI, Tappi S, Rocculi P, Gardini F, Lanciotti R (2015) Natural antimicrobials to prolong the sheil-life of minimally processed lamb’s lettuce. Postharvest Biol Technol 103:35–44
Snoussi M, Hajlaoui H, Noumi E, Usai D, Sechi LA, Zanetti S, Bakhrouf A (2008) In vitro and anti-Vibrio spp. Activity and chemical composition of some Tunisian aromatic plants. World J Microbiol Biotechnol 24:3071–3076
Soltani N, Kellouche A (2004) Activité biologique des poudres de cinq plantes et de l’huile essentielle d’une d’entre elles sur Collosobruchus maculatus (F.). Int J Trop Insect Sci 24(2):184–191
Soylu EM, Kurt Ş, Soylu S (2010) In vitro and in vivo antifungal activities of the essential oils of various plants against tomato grey mould disease agent Botrytis cinerea. Int J Food Microbiol 143(3):183–189
Teixeira B, Marques A, Ramos C, Batista I, Serrano C, Matos O, Neng NR, Nogueira JMF, Saraiva JA, Nunes ML (2012) European pennyroyal (Mentha pulegium) from Portugal : chemical composition of essential oil and antioxidant and antimicrobial properties of extracts and essential oil. Indus Crops Prod 36:81–87
Tripathi P, Dubey NK, Shkula AK (2007) Use of some essential oils as post-harvest botanical fungicides in the management of grey mould of grapes caused by Botrytis cinerea. World J Microbiol Biotechnol 24:39–46
Tsao R, Zhou T (2000) Antifungal activity of Monoterpenoids against postharvest pathogens Botrytis cinerea and Monilinia fructicola. J Essent Oil Res 12(1):113–121
Ultee A, Bennik MHJ, Moezelaar R (2002) The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl Environ Microbiol 68:1561–1568
Ulukanli Z, Oz AT (2015) The effect of oleum myrtle on the fruit quality of strawberries during MAP storage. J Food Sci Technol 52(5):2860–2868
Uwineza MS, El Yousfi B, Lamiri A (2018) Activités antifongiques in vitro des huiles essentielles de Mentha pulegium, Eugenia aromatica et Cerdrus atlantica sur Fusarium culmorum et Bipolaris sorokiniana. Rev Maroc Prot Plantes 12:19–32
Vidal R, Muchembled J, Deweer C, Tournant L, Corroyer N, Flammier S (2018) Evaluation de l’intérêt de l’utilisation d’huiles essentielles dans les stratégies de protection des cultures. Innov Agronomiques 63:191–210
Vilela GR, Almeida GS, Regitano D’Arce MAB, Moraes MHD, Brito JO, Da Silva MFGF, Silva SC, Piedade SMS, Calori-Domingues MA, Gloria EM (2009) Activity of essential oil and its major compound 1,8-cineole, from Eucalyptus globulus Labill, against the storage fungi Aspergillus flavus Link and Aspergillus parasiticus Speare. J Stored Prod Res 45:108–111
White TJ, Bruns T, Lee S, Taylor J (1990) Analysis of phylogenetic relationships by amplification and direct sequencing of ribosomal RNA genes. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) A guide to methods and applications. Academic press, New York, p 322
Williamson B, Tudzynski B, Tudzynski P, Van Kan JA (2007) Botrytis cinerea: the cause of grey mould disease. Mol Plant Path 8(5):561–580
Yoshimura H, Sawai Y, Tamotsu S, Sakai A (2010) 1,8-cineole inhibits both proliferation and elongation of BY-2 cultured tobacco cells. J Chem Ecol 1–9
Zekri N, Amalich S, Boughdad A, El Belghiti MA, Zair T (2013) Phytochemical study and insecticidal activity of Mentha pulegium L. oils from Morocco against Sitophilus oryzae. Mediterr J Chem 2(4):607–619
Zhao Y, Wang R, Tu K, Liu K (2011) Efficacy of postharvest spraying with Pichia guilliermondii on postharvest decay and quality of cherry tomato fruit during storage. Afr J Biotech 10(47):9613–9622
Znini M, Cristofari G, Majidi L, Paolini J, Desjobert JM, Costa J (2013) Essential oil and antifungal activity of Pulicaria mauritanicaCoss, against postharvest phytopthogenic fungi apples. LWT Food Sci Technol 54(2):564–569
Funding
No sources of funding or financial interests are provided by any authorities for the achievement of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflict of interest of any authority or persons in the field of our work in national and international levels.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aouadi, G., Grami, L.K., Taibi, F. et al. Assessment of the efficiency of Mentha pulegium essential oil to suppress contamination of stored fruits by Botrytis cinerea. J Plant Dis Prot 129, 881–893 (2022). https://doi.org/10.1007/s41348-022-00623-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-022-00623-6