Abstract
Bacterial wilt disease in tomato (Solanum lycopersicum) is a serious threat in agriculture that significantly reduces the production. The aim of the present investigation was to search for an effective biocontrol measure against bacterial wilt disease in tomato. The causative agent (bacterial wilt disease) isolated from infested tomato plants was identified as Ralstonia solanacearum BR-001. Ralstonia solanacearum is considered as one of the most dangerous plant pathogens globally due to its wide host range. The disease progression caused by R. solanacearum is very fast and difficult to control. Several strategies like mineral oil, lytic phages, and antimicrobial metabolites produced by different virulent bacterial strains and medicinal plants have been reported to control the infection/ wilt disease caused by R. solanacearum. However, it is difficult to control the progression once it enters the xylem tissue. Furthermore, R. solanacearum produces two different types of DNases which help the bacteria to escape from the plant defense system. We also have isolated 43 bacterial candidates from the rhizospheres of few unaffected tomato plants from the same field. Interestingly, one out of 43 candidates exhibiting efficacy against R. solanacearum BR-001 in-vivo was identified as Bradyrhizobium japonicum BRC 2485. But no isolate was found to control disease progression effectively during in-vitro condition. To understand the biocontrol potential of B. japonicum BRC 2485, an in-vivo comparative study was conducted with one Bradyrhizobium type strain (MTCC 120) and one Bradyrhizobium reference strain (MCC 2940). The experimental evidence suggests that the priming of tomato plants with B. japonicum BRC 2485 limits the multiplication of R. solanacearum BR-001 therein. The Bradyrhizobium strains were tested for the production of siderophores, ethylene, and abscisic acid (ABA). All the experimental Bradyrhizobium strains were found to be negative for siderophore production and positive for ethylene production. However, only B. japonicum BRC 2485 was found to produce ABA, which plays a major role in triggering induced systemic resistance (ISR) in plants. To the best of authors’ knowledge, this is the first report of a strain of B. japonicum with activity against bacterial wilt disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In order to meet the global demands of food for the ever-expanding human population, crop production needs to be doubled by 2050 (UN General Assembly 2009). However, pests and pathogens are creating serious problems to the crop production along with global climatic changes. Plant pathogens alone have been shown to reduce crop yield about 14% (Velasquez et al. 2018). To control bacterial plant pathogens (BPPs), the demand of biological control agents (BCAs) is increasing rapidly under the integrated crop management (ICM) practice (Chattopadhyay et al. 2017). Tomato is one of the major horticultural crops in the world. During 2016–2017, the tomato production was estimated to be around 19.7 million tones. In India, it is grown over an area of 0.497 million hectare with a production of 17.35 million tons (FAO/WHO 2006).
The bacterial wilt symptoms in tomato are characterized by initial wilting of upper leaves followed by complete wilting of the plants. Bacterial wilt management in tomato is difficult because the causative agent grows endophytically, survives in deeper layers of soil, and disseminates through water. Yuliar et al. have extensively reviewed the management of bacterial wilt disease with physical, chemical, biological, and cultural methods (Yuliar and Toyota 2015). The ideal candidate as BCA against bacterial wilt should have the following characteristics: (1) self-sustaining, (2) long-term disease suppression, (3) reduced input of nonrenewable resources, (4) safe for environment, and (5) economically cheap (Kulkarni et al. 2017).
Several bacterial BCAs have been reported to control bacterial wilt such as Acinetobacter sp. (Xue et al. 2009), Bacillus thuringiensis (Zhou et al. 2008), Burkholderia spp. (Nion and Toyota 2008), etc. In this present report, the BCA potential of bacterial isolates from the rhizosphere of healthy tomato plants in a wilt infected field has been investigated against the bacterial pathogen isolated from the infected tomato plants. The plant pathogen was identified to be a strain of Ralstonia solanacearum. R. solanacearum is considered as one of the top ranked plant pathogens that causes bacterial wilt disease in more than 200 plant species worldwide (Nion and Toyota 2015). It is also classified as ‘quarantine organisms’, ‘bioterrorism’, and ‘double usage agents’ by different regulatory authorities in USA and Europe (Cellier et al. 2007). The most potent BCA was identified as a strain of Bradyrhizobium japonicum. B. japonicum strains are well known for their biological nitrogen fixation property. Bradyrhizobium has been reported as an endophyte in the roots of the non-Fabaceae plant species like Arabidopsis thaliana, cotton, sweet corn, and tomato (de Matos et al. 2017; Msaddak et al. 2017). It has been shown to provide protection against a wide range of fungal pathogens like Macrophomina phaseolina, Rhizoctonia solani, and Fusarium spp. (Htwe et al. 2018). However, the potential of Bradyrhizobium as BCA against bacterial wilt was not clear till date. Thus, an attempt has also been made to elucidate the biocontrol mechanism of the B. japonicum isolate.
Materials and methods
Isolation of causative agent responsible for bacterial wilt in tomato
Field survey was conducted to determine the prevalence of bacterial wilt in tomato field plants from Tarapith (latitude: 24.114; longitude: 87.796), Birbhum district of West Bengal, India. Tomato plants showing typical wilt symptoms were taken, and a tentative diagnosis of R. solanacearum was made as a result of oozing from cut stems placed in water. Isolation was done on tetrazolium chloride (TZC) agar medium following a standard protocol (Kelman 1954).
Plant materials and growth conditions
Four-week-old tomato plants (Solanum lycopersicum cv. Pusa Ruby) were used for the experimental purpose. Seeds were surface-sterilized and germinated in aseptic conditions. Seedlings were grown in double autoclaved soil (loamy soil) under culture room conditions (temperature: 32 ± 2 °C, relative humidity: ≥ 75%, photoperiod 17 h, light intensity: 7200 ± 200 lx).
Pathogenicity tests
To check virulence, in-vivo pathogenicity tests were conducted in triplicate batches of 30 seedlings. The root tips of four-week-old tomato plants were excised to make entry points for bacteria. Excise root tips were dipped into a suspension of 2 × 107 CFUmL−1 for 24 h and observed until complete wilting of unprotected plants. Disease symptoms were scored according to Feng et al. (2012) following a 0–4 scale (0, no wilting; 1, 0–25%; 2, 25–50%; 3, 50–75%; 4, 75–100% of wilted leaves).
Isolation of rhizospheric bacteria
Soil samples were collected from rhizosphere of the live tomato plants from the same field from where the pathogen was isolated. One gram of soil was dissolved in 10 mL of sterile double distilled water, and serial dilution was done accordingly. 100 µL sample from each dilution was taken and plated on nutrient agar medium (HiMedia, India) plates (pH 7.2), followed by 24-h incubation period at 30 °C. Colonies were selected on the basis of distinctive morphology. Pure cultures were obtained through repeated streaking method. The cultures were stored at 4 °C for further use.
Pathogen density determination
Bacterial internal growth curves were performed according to the protocol described by Deslandes et al. (1998). Briefly, plants inoculated with the bacterial strain were weighed, sterilized with 250 mL of 70% ethanol for 3 min, rinsed three times in sterile water, and ground in a mortar after addition of sterile water (2.0 mL per g of fresh weight). Various dilutions of the ground material were then performed with sterile water and the bacteria spread on petri plates containing bacteria specific medium (TZC agar medium for R. solanacearum) and grown at 30 °C. For each time point, triplicate assays were performed for each bacterial strain.
Screening for BCA
For screening of BCA, four-week-old tomato plants were root-inoculated with the rhizospheric isolates using a suspension of 5 × 108 CFUmL−1 in triplicate batches of 30 seedlings. Twenty-four hours after the first inoculation, plants were challenged by the virulent isolate at a concentration of 2 × 107 CFU mL−1. The bacterial internal growth curves were performed according to Deslandes et al. (1998), whereas the disease symptoms were scored according to Feng et al. (2012).
Identification of the pathogen and BCA
Selected isolates (pathogen and BCA) were characterized through biochemical tests in accordance with Bergey's Manual (Smith et al. 1974). The species level identification of the isolated strain was done using 16S rRNA sequence analysis following the methods of Banerjee et al. (2018). The sequence was then edited and submitted to GenBank to obtain the NCBI accession number. The phylogenetic tree was constructed using the neighbor-joining method and validated with the bootstrap analysis (10,000 replicates) using Mega 6.0 software.
Reference strains and growth conditions
One type strain B. japonicum MTCC 120 (Singha et al. 2015) and one reference strain Bradyrhizobium MCC 2940 (Ojha, 2016) were used in this study for comparative analysis. Both strains were grown in 250-mL flasks containing 100 mL of yeast extract mannitol (YEM) medium at 30 °C in a shaker incubator until exponential growth phase [OD at 600 nm nearly 1, equivalent to 1.25 × 109 colony-forming units (CFU) mL−1 in YEM-agar, respectively]. Selective mediums were used for testing bacterial phytopathogens used in this study, viz. Hofers alkaline medium (HiMedia, M717) for A. tumefaciens MTCC 609 (Karwasara et al. 2011); boric acid peptone (BAP) agar (HiMedia, PHM001) for P. syringae MTCC 1604 (Sharma et al. 2008); and tryptone sucrose tetrazolium (TST) agar medium (HiMedia, M1217) for X. campestris NCIM 2961 (Palaniraj et al. 2011).
Antimicrobial assay
In order to check the BCA potential, each of the Bradyrhizobium strains was grown in yeast extract manitol (YEM) broth as shake flask (240 rpm) culture up to 1 × 108 CFU mL−1(OD at 600 nm nearly 1). This fermented broth was then examined for antimicrobial activity against selected bacterial phytopathogens in nutrient agar (NA) medium by the cup plate method (Shetty et al. 2014). The zone of inhibition was measured by using a calibrated scale. To study the antimicrobial activity, solvent extraction followed by disc diffusion assay was also done following the method of Banerjee et al. (2018).
Competitive assay
BCA potential of the Bradyrhizobium strain might be the consequences of a direct physical competition for space within the xylem vessels. In order to check this hypothesis, R. solanacearum BR-001 and selected Bradyrhizobium strains were simultaneously co-inoculated in 1:1 ratio. Plant internal bacterial growth curves were performed according to the protocol described by Deslandes et al. (1998). Control plants were inoculated with R. solanacearum BR-001 only.
Measurement of siderophore, ethylene and ABA
Siderophore production was determined according to the protocol of Louden et al. by using the blue agar CAS (chrome azurol S) assay (Louden et al. 2011). In brief, plates were sown with 1 μl of YEM pure bacterial culture in halfway points of a Petri dish containing agar CAS medium, incubated at 30 °C and observed daily for the orange color formation around each colony for up to 7 days. Ethylene production was detected according to the protocol described by Strzelczyk et al. (1994). Briefly, air samples (500 μl) were taken from air-tight flasks after 7 days of inoculation in YEM. Air samples were then analyzed by gas chromatography (GC) with a flame-ionizing detector (FID). ABA production was estimated according to the protocol of Ali et al. (2013). Briefly, centrifuged broth of the 7 days old YEM culture were partitioned four times with the same volume of acetic acid–saturated ethyl acetate (1%, v/v). Then acidic ethyl acetate was evaporated at 36 °C, and the dried samples were diluted in 100 μL of acetic acid/methanol/water (1:30:70) and analyzed by High Performance Liquid Chromatography (HPLC) with an ultraviolet (UV) detector. A stainless-steel column (250 × 4.6 mm I.D.) packed with LiChrosphere RP-18e (5 μm) was applied for the separation. A mixture of 0.5% acetic acid (40 mL) and acetonitrile (60 mL) was used as mobile phase at a flow rate of 0.3 mL min−1. Samples were analyzed using UV wavelength at 254 nm.
Exogenous ABA application
To confirm the role of ABA produced by the BCA in biocontrol, ABA was exogenously applied to the tomato plant and challenged with the pathogen (Hu and Bidochka 2021). Disease index and bacterial internal colonization was assessed. Briefly, three doses of ABA (0.02 μgmL−1, 0.2 μg mL−1, and 2.0 μg mL−1) were prepared in 25% aqueous ethanol. Roots of four leaf stage plants were treated with 10 mL of ABA solution per plant with these three concentrations separately. Aqueous ethanol (25%) was used as the control. After 24 h of ABA treatment, plants were challenged by the isolated virulent candidate at a concentration of 2 × 107 CFU mL−1.
Statistical analysis
Each experiment was performed at least 3 times unless stated otherwise. Standard deviation for each treatment was determined. Statistical significance was measured by using the data of the pathogenicity tests by a two-way analysis of variance (ANOVA). Means were separated using Duncan’s multiple range test (DMRT; P = 0.05) using SPSS software.
Results
Identification of bacterial wilt causing agent
Morphological studies revealed that the isolated bacterial strain (BR-001) from the wilt infected tomato plants was Gram-negative, rod-shaped, non-capsulated, and non-spore forming (Table S1). The isolate grown on SMSA medium (Engelbrecht-Wiggans 1994) was highly fluidal, white-colored (with a light pink center) and round shape; typical characteristics of R. solanacearum. In pathogenicity tests, the isolate produced typical wilt symptoms on tomato within 5–6 weeks after inoculation using root injury (Table 1). The 16S rRNA sequence analysis of the isolate (GenBank Accession No. MH718793) showed 99% similarity with R. solanacearum strain UY031 with a max score 2601 (E value 0.0). The distinct phylogenetic position of the strain BR-001 along with R. solanacearum UY031 is presented in Fig. 1. Thus, based on 16S rRNA sequence, the isolate was identified as R. solanacearum BR-001.
Bootstrap consensus neighbor-joining tree based on 16S rRNA gene sequences presenting relationship of the pathogenic isolate R. solanacearum BR-001 and rhizospheric isolate B. japonicum BRC 2485 (marked with circles) with other bacterial strains. R. solanacearum BR-001 is showing maximum similarity with R. solanacearum UY031, whereas B. japonicum BRC 2485 is showing maximum similarity with B. japonicum HMS-02. Bacillus subtilis RN40 was used as an outgroup. Bootstrap values are presented at the nodes (1000 replications). The scale bar represents 2 substitutions per 1000 bases
Identification of biocontrol agents from the rhizospheric isolates
All together 43 isolates were obtained from the rhizospheric soil of the unaffected plants. None of these isolates was found to exhibit inhibition zones against R. solanacearum in in-vitro NA cup plate method. To check the potential of these strains in-vivo, four-week-old healthy tomato plants were first root inoculated with a rhizospheric isolates, followed by challenge with R. solanacearum BR-001 after 24 h of inoculation. Out of 43 rhizospheric isolates, BRC 2485 exhibited potential biocontrol activity (Table 2). The colony of BRC 2485 grown on YEM agar medium appeared as circular, small (≥ 1 mm in diameter), white, convex, and granular in texture within 5–7 days of incubation. Based on biochemical characterization (Table S2) and 16S rRNA sequence analysis (GenBank Accession No. MK377407), the rhizospheric isolate BRC 2485 was identified as B. japonicum. The distinct phylogenetic position of the strain BRC 2485 along with B. japonicum HMS-02 is also presented in Fig. 1.
Efficacy of B. japonicum BRC 2485 against R. solanacearum
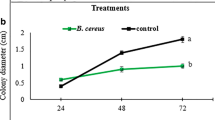
To check the efficacy of BRC 2485 against bacterial wilt, a disease progression curve was plotted against time (Fig. 2). From the plot it is clear that BRC 2485 can control bacterial wilt of tomato effectively when challenged with R. solanacearum. In control set of experiments, R. solanacearum was found to induce heavy wilt (3.8 ± 0.2) at 9th day of inoculation. But tomato plants treated with BRC 2485 show a significant decrease (1.8 ± 0.25) in disease progression within the stipulated time. B. japonicum BRC 2485 did not show any in vitro antimicrobial activity against the experimental plant pathogen R. solanacearum BR-001 (Table S3), as well as other tested bacterial pathogens (viz. A. tumefaciens, P. syringae, and X. campestris (Table S3).
Disease symptom development curves: Inoculated B. japonicum BRC 2485 protects tomato plants from a subsequent challenge with R. solanacearum BR-001. Plants were scored daily using a scale between 0 and 4. Means and standard errors (SE) were calculated from a total of 30 plants per assay (from three independent experiments)
A comparative analysis of biocontrol of B. japonicum BRC 2485 with reference strains
Four-week-old healthy tomato plants were first inoculated with B. japonicum BRC 2485 and two other Bradyrhizobium reference strains (MTCC 120 and MCC 2940) in three different sets of experiments, followed by challenge with R. solanacearum BR-001. In each experimental set, non-primed tomato plants inoculated with B. japonicum BRC 2485 have been taken as a control. Among the three strains used for priming, B. japonicum BRC 2485 was recorded to induce maximum protection (1.34 ± 0.05), followed by MTCC 120 (3.75 ± 0.25) and MCC 2940 (4.2 ± 0.35) against infection caused by R. solanacearum BR-001 (Table 1).
Priming of B. japonicum BRC 2485 limits multiplication of R. solanacearum BR-001
To determine whether the reduced disease symptoms are correlated with lower population of R. solanacearum BR-001 in plants primed with B. japonicum BRC 2485, endophytic bacterial growth was measured from tissue homogenates after 0, 3, 8, and 0 days of the administration of the pathogen (Table 3). Control plants were inoculated either with R. solanacearum BR-001 (positive control) or with B. japonicum BRC 2485 (negative control). When tomato plants primed with B. japonicum BRC 2485 were challenged with R. solanacearumBR-001, the BR-001 population remained low (4.10 ± 0.55Log CFU g FW−1) in comparison to positive control (12.25 ± 2.25 Log CFU g FW−1) after 8 days of challenge (Table 3). Even, co-inoculated (1:1) plants also showed difference in R. solanacearum BR-001 population (4.50 ± 0.55 Log CFU g FW−1) after 8 days of co-inoculation (Table 3). After 10 days of challenge all the plants of the positive control died due to disease progression. Therefore, to demonstrate the effect of B. japonicum BRC 2485 on R. solanacearum BR-001 in-vivo, endophytic populations of both strains were plotted after 8 days of challenged period (Fig. 3). There is a huge difference in the population count of positive (inoculated only with R. solanacearum BR-001) and negative (inoculated only with B. japonicum BRC 2485) control (Fig. 3a). However, the endophytic population of B. japonicum BRC 2485 does not vary much after challenge with R. solanacearum BR-001 even in comparison with negative control. But, the endophytic population of R. solanacearum BR-001 remains drastically low in plants primed with B. japonicum BRC 2485 (Fig. 3a). The trend remains the same even when both strains were co-inoculated in a ratio of 1:1. The endophytic population of R. solanacearum BR-001 varies from 45 to 48% in case of immunized and co-inoculated plants, respectively (Fig. 3b).
Effect of B. japonicum BRC 2485 on multiplication of R. solanacearum inside tomato plants: a Bar diagram showing different multiplication pattern of R. solanacearum and B. japonicum in control, in B. japonicum BRC 2485 inoculated plant, and in co-inoculated (1:1) plants; b pie chart sowing competitive growth of R. solanacearum and B. japonicum in B. japonicum BRC 2485 treated plants; c pie chart sowing competitive growth of R. solanacearum and B. japonicum in co-inoculated (1:1) plants
Production of siderophore, ethylene, and ABA by experimental Bradyrhizobium strains
Siderophores secreted by pathogens regulate the production of many virulence factors (e.g., exotoxin, endoprotease, pyoverdine, etc.), which are major contributors to the ability of the pathogen to disease establishment. B. japonicum BRC 2485 and the reference Bradyrhizobium strains were unable to produce siderophores. But all these strains were able to produce ethylene. The production of ethylene in liquid cultures was confirmed by GC–MS analysis (Fig. 4). The ethylene production was recorded highest in MTCC 120 (8.1 ng mL−1 h−1), followed by B. japonicum BRC 2485 (3.5 ng mL−1 h−1) and MCC 2940 (2.0 ng mL−1 h−1) on 8th day of inoculation (Table 4). ABA production was measured through a HPLC–UV detection system. Only B. japonicum BRC 2485 was found to have the unique property of ABA production (Fig. 5). The ABA concentration (0.019 μg mL−1 h−1) was measured on 8th day of inoculation (Table 4).
Effect of exogenous ABA on wilt control
To check the effect of exogenous ABA against bacterial wilt, disease index and internal colonization of the pathogen were studied against three different concentrations (Table 5). From the table it is clear that exogenous ABA (2.0 μgmL−1) effectively reduces the disease index and internal colonization of the virulent strain. However, a lower concentration (0.02 μg mL−1) of exogenous ABA could not effectively control the disease progression.
Discussion
Bradyrhizobium is a well-known bacterial genus for biological nitrogen fixation. It is already known that Bradyrhizobium strains can colonize in root nodules of Fabaceae family plants including soybean, kidney bean, and cowpea (de Matos et al. 2017; Pena-Cabriales and Alexander 1983). B. japonicum was also reported to be endophytic in the roots of non-modulating plants like sugarcane, sweet corn, and cotton (de Matos et al. 2017; McInroy and Kloepper 1995). Different Bradyrhizobium strains are known to control disease caused by fungal phytopathogens including Macrophomina phaseolina, Rhizoctonia solani, and Fusarium spp. (Ehteshamul-Haque and Ghaffar 1993). Rhizobial protection against Fusarium wilt was previously reported on chickpea (Arfaoui et al. 2006). Only a few reports are available about the antagonistic activity of Bradyrhizobium strains against bacterial phytopathogens (Gross and Vidaver 1978; Kohlmeier et al. 2015). In the present investigation, for the first time a B. japonicum strain was reported to control bacterial wilt disease effectively.
In general, there are three possible suppression mechanisms which are commonly used by different BCAs against phytopathogens: (1) production of antimicrobials and siderophores, (2) competition, and (3) induced systemic resistance (ISR). Few Bradyrhizobium strains are known to produce bacteriocin (Kohlmeier et al. 2015). On the other hand, few Bradyrhizobium strains have been reported to produce siderophores to overcome iron starvation (Plessner et al. 1993). However, in the present investigation, B. japonicum BRC 2485 along with reference Bradyrhizobium strains does not show any antimicrobial activity. All the strains were also negative for siderophore production.
Previously, it was reported that BCA and pathogenic strains compete to invade xylem vessels in tomato (Etchebar et al. 1998; Lace and Ott 2018). Bacterial wilt disease progression in tomato can be easily correlated with colonization of the phytopathogen. In the present investigation, priming effect of B. japonicum BRC 2485 limits the multiplication of R. solanacearum BR-001 within the host plant. The present experimental evidence suggests that the B. japonicum BRC 2485 provides good competition to R. solanacearum BR-001 for colonization within the plant host.
The mechanism of ISR within the host plant may differ from one species to another. For example, ISR against R. solanacearum by many plants growth-promoting rhizobacteria (PGPR) is mediated through the jasmonic acid and ethylene dependent signaling pathways (Hyakumachi et al. 2013). But ISR by Bacillus thuringiensis against R. solanacearum is mediated through the salicylic acid-responsive defense-related genes (Heil and Bostock 2002). On the other hand, ISR against R. solanacearum in Arabidopsis thaliana was achieved by the ΔhrpB mutant of R. solanacearum through ABA-responsive defense-related genes (Feng et al. 2012). In the present investigation, the rhizospheric isolate, B. japonicum BRC 2485 was found to have the unique property to produce ABA. Boiero et al. also have reported ABA synthesis by B. japonicum USDA110 (Boiero et al. 2007). Therefore, there may be a probability that the ABA produced by B. japonicum BRC 2485 may trigger ISR of tomato plants. In the present investigation, exogenous ABA (2.0 μg mL−1) effectively reduced the disease index and internal colonization of the virulent strain. These results may be correlated with the findings of Hu and Bidochka (2021). They have reported ABA as key player in differential responses to endophytic colonization by Metarhizium and pathogenic colonization by Fusarium. However, ABA production by B. japonicum BRC 2485 in vitro was found to be as low as 0.019 ± 0.002 μg mL−1(0.02 μg mL−1 approx.). Application of exogenous ABA of the same concentration failed to demonstrate the same effect produced by B. japonicum BRC 2485. ABA-mediated immune response of tomato plants is still not fully elucidated, and the present investigation could not provide any insight into the plant immunity aspect.
Conclusion
In this present study, biological control of bacterial wilt using an ABA-producing B. japonicum BRC 2485 has been demonstrated. The observed biocontrol activity in tomato plants may be explained by competition or ABA production or both by the Bradyrhizobium strain. However, its effectiveness should be investigated at different agro-climatic conditions. Further investigation on ISR against R. solanacearum in tomato plants through ABA-responsive defense-related genes will be important. We strongly believe that this information will be helpful to develop new-generation biocontrol agents.
References
Ali HM, Siddiqui MH, Al-Whaibi MH, Basalah MO, Sakran AM, El-Zaidy M (2013) Effect of proline and abscisic acid on the growth and physiological performance of faba bean under water stress. Pak J Bot 45:933–940
Arfaoui A, Sifi B, Boudabous A, Hadrami EI, Chérif M (2006) Identification of Rhizobium isolates possessing antagonistic activity against Fusarium oxysporum f. sp. ciceris, the causal agent of Fusarium wilt of chickpea. J Plant Pathol 88:67–75
Banerjee G, Gorthi S, Chattopadhyay P (2018) Beneficial effects of bio-controlling agent Bacillus cereus IB311 on the agricultural crop production and its biomass optimization through response surface methodology. An Acad Bras Ciênc 90:2149–2159
Boiero L, Perrig D, Masciarelli O, Penna C, Cassán F, Luna V (2007) Phytohormone production by three strains of Bradyrhizobium japonicum and possible physiological and technological implications. Appl Microbiol Biotechnol 74:874–880
Cellier G, Arribat S, Chiroleu F, Prior P, Robene I (2007) A diagnostic microarray for the multiplex characterization of strains of the Ralstonia solanacearum species complex. In: 15th Congress of the Mediterranean Phytopathological Union: Plant health sustaining Mediterranean ecosystems Abstracts book. MPU, Universidad de Córdoba. Cordoba: MPU-Universidad de Córdoba, Résumé, p. 76.
Chattopadhyay P, Banerjee G, Mukherjee S (2017) Recent trends of modern bacterial insecticides for pest control practice in integrated crop management system. 3 Biotech 7:60
de Matos GF, Zilli JE, de Araújo JLS, Parma MM, Melo IS, Radl V, Baldani JI, Rouws LFM (2017) Bradyrhizobium sacchari sp. nov., a legume modulating bacterium isolated from sugarcane roots. Arch Microbiol 199:1251–1258
Deslandes L, Pileur F, Liaubet L, Camut S, Can C, Williams K, Holub E, Beynon J, Arlat M, Marco Y (1998) Genetic characterization of RRS1, a recessive locus in Arabidopsis thaliana that confers resistance to the bacterial soilborne pathogen Ralstonia solanacearum. Mol Plant-Microbe Interact 11:659–667
Ehteshamul-Haque S, Ghaffar A (1993) Use of rhizobia in the control of root rot diseases of sunflower, okra, soybean and mungbean. J Phytopathol 138:157–163
Engelbrecht-Wiggans R (1994) Sequential auctions of stochastically equivalent objects. Econ Lett 44:87–90
Etchebar C, Trigalet-Demery D, van Gijsegem F, Vasse J, Trigalet A (1998) Xylem colonization by an HrpB mutant of Ralstonia solanacearum is a key factor for the efficient biological control of tomato bacterial wilt. Mol Plant-Microbe Interact 11:869–877
Feng DX, Tasset C, Hanemian M, Barlet X, Hu J, Trémousaygue D, Deslandes L, Marco Y (2012) Biological control of bacterial wilt in Arabidopsis thaliana involves abscisic acid signaling. New Phytol 194:1035–1045
Gross DC, Vidaver AK (1978) Bacteriocin-like substances produced by Rhizobium japonicum and other slow-growing rhizobia. Appl Environ Microbiol 36:936–943
Heil M, Bostock RM (2002) Induced systemic resistance (ISR) against pathogens in the context of induced plant defenses. Ann Bot 89:503–512
Htwe AZ, Moh SM, Moe K, Yamakawa T (2018) Effects of co-inoculation of Bradyrhizobium japonicum SAY3-7 and Streptomyces griseoflavus P4 on plant growth, nodulation, nitrogen fixation, nutrient uptake, and yield of soybean in a field condition. Soil Sci Plant Nutr 64:222–229
Hu S, Bidochka MJ (2021) Abscisic acid implicated in differential plant responses of Phaseolus vulgaris during endophytic colonization by Metarhizium and pathogenic colonization by Fusarium. Sci Rep 11:1–12
Hyakumachi M, Nishimura M, Arakawa T, Asano S, Yoshida S, Tsushima S, Takahashi H (2013) Bacillus thuringiensis suppresses bacterial wilt disease caused by Ralstonia solanacearum with systemic induction of defense-related gene expression in tomato. Microbes Environ 28:128–134
Joint FAO/WHO (2006) Expert Committee on Food Additives. Meeting and World Health Organization, 2006. Safety evaluation of certain food additives (No. 56). World Health Organization
Karwasara VS, Tomar P, Dixit VK (2011) Influence of fungal elicitation on glycyrrhizin production in transformed cell cultures of Abrus precatorius Linn. Pharmacogn Mag 7:307
Kelman A (1954) The relationship of pathogenicity of Pseudomonas solanacearum to colony appearance in a tetrazolium medium. Phytopathol 44:693–695
Kohlmeier MG, Yudistira H, Zhang KL, Fristensky B, Levin DB, Sparling R, Oresnik IJ (2015) Draft genome sequence of the bacteriocin-producing Bradyrhizobium japonicum strain FN1. Genome Announce 3:e00812-e815
Kulkarni M, Gorthi S, Banerjee G, Chattopadhyay P (2017) Production, characterization and optimization of actinomycin D from Streptomyces hydrogenans IB310, a (n antagonistic bacterium against phytopathogens. Biocatal Agric Biotechnol 10:69–74
Lace B, Ott T (2018) Commonalities and differences in controlling multipartite intracellular infections of legume roots by symbiotic microbes. Plant Cell Physiol 59:661–672
Louden BC, Haarmann D, Lynne AM (2011) Use of blue agar CAS assay for siderophore detection. J Microbiol Biol Edu 12:51
McInroy JA, Kloepper JW (1995) Survey of indigenous bacterial endophytes from cotton and sweet corn. Plant Soil 173:337–342
Msaddak A, Durán A, Rejili M, Mars M, Ruiz-Argüeso T, Imperial J, Palacios J, Rey L (2017) Members of Microvirga and Bradyrhizobium genera are native endosymbiotic bacteria modulating Lupinus luteus in Northern Tunisian soils. FEMS Microbiol Ecol. https://doi.org/10.1093/femsec/fix068
Nion YA, Toyota K (2008) Suppression of bacterial wilt and Fusarium wilt by a Burkholderia nodosa strain isolated from Kalimantan soils, Indonesia. Microbes Environ 23:134–141
Nion YA, Toyota K (2015) Recent trends in control methods for bacterial wilt diseases caused by Ralstonia solanacearum. Microbes Environ ME14144.
Ojha A (2016) Host range analysis and genotypic characterization of rhizobia associated with native lagumes of Meghalaya. Ph.D. Thisis. Supervisor Rao SR, Northeastern Hill University
Palaniraj A, Jayaraman V, Hariram SB (2011) Influence of nitrogen sources and agitation in xanthan gum production by Xanthomonas campestris. Int J Adv Biotechnol Res 2:305–309
Pena-Cabriales JJ, Alexander M (1983) Growth of Rhizobium in Unamended Soil. Soil Sci Soc Am J 47:81–84
Plessner O, Klapatch T, Guerinot ML (1993) Siderophore utilization by Bradyrhizobium japonicum. Appl Environ Microbiol 59:1688–1690
Sharma RK, Negi DS, Gibbons S, Otsuka H (2008) Chemical and antibacterial constituents of Skimmia anquetelia. Planta Med 74:175–177
Shetty PR, Buddana SK, Tatipamula VB, Naga YV, Ahmad J (2014) Production of polypeptide antibiotic from Streptomyces parvulus and its antibacterial activity. Braz J Microbiol 45:303–312
Singha B, Das P, Mazumder PB (2015) Morphological and biochemical characterization of rhizobia isolated from root nodule of Crotolaria junceae L. grown in Assam. Int J Sci Res 4:1928–1931
Smith NR, Hobbs G, Buchanan RE, Gibbons NE (1974) Bergey's manual of determinative bacteriology. 8th, pp. 370–373.
Strzelczyk E, Kampert M, Li CY (1994) Cytokinin-like substances and ethylene production by Azospirillum in media with different carbon sources. Microbiol Res 149:55–60
UN General Assembly (2009) Food Production Must Double by 2050 to Meet Demand From World's Growing Population. Press Release.
Velasquez AC, Castroverde CD, He SY (2018) Plant–pathogen warfare under changing climate conditions. Current Biol 28:R619–R634
Xue QY, Chen Y, Li SM, Chen LF, Ding GC, Guo DW, Guo JH (2009) Evaluation of the strains of Acinetobacter and Enterobacter as potential biocontrol agents against Ralstonia wilt of tomato. Biol Control 48:252–258
Yuliar NYA, Toyota K (2015) Recent trends in control methods for bacterial wilt diseases caused by Ralstonia solanacearum. Microbes Environ 30:1–11
Zhou J, Zhang H, Yang Y, Zhang Z, Zhang H, Hu X, Chen J, Wang XC, Huang R (2008) Abscisic acid regulates TSRF1-mediated resistance to Ralstonia solanacearum by modifying the expression of GCC box-containing genes in tobacco. Jexp Bot 59:645–652
Acknowledgements
We are very much thankful to the Department of Biotechnology, Gauhati University, for providing necessary support. This work was supported by Dr. D S Kothari Post-Doctoral Fellowship from University Grant Commission (UGC), Government of India (Award No. F.42/2006 (BSR)/BL/1415/0391 dated 01 July, 2015).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chattopadhyay, P., Banerjee, G. & Handique, P.J. Use of an abscisic acid-producing Bradyrhizobium japonicum isolate as biocontrol agent against bacterial wilt disease caused by Ralstonia solanacearum. J Plant Dis Prot 129, 869–879 (2022). https://doi.org/10.1007/s41348-022-00604-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-022-00604-9