Abstract
The overuse of broad-spectrum insecticides against Plutella xylostella (L.) has brought several environmental and health issues, and an urgent need for more sustainable pest management, such as using botanical pesticides with selective target effects. The present study examined the efficacy of methanolic extracts of the leaves of rosemary (Rosmarinus officinalis L.), peppermint (Mentha piperita L.), sage (Salvia officinalis L.) and common fumitory (Fumaria officinalis L.), and the seeds of fennel (Foeniculum vulgare Mill) as larvicide and oviposition deterrent on P. xylostella. Bioassays showed higher toxicity (LC50 and LC90) of rosemary (76.51 and 3355.65 mg L−1) and peppermint (78.89 and 2502.95 mg L−1) extracts compared with sage (322.47 and 28,147.82 mg L−1), fennel (777.91 and 86,106.06 mg L−1) and common fumitory (1701.05 and 357,590.80 mg L−1) extracts. Tests using sublethal doses (LC25) revealed that the highest oviposition inhibition was provoked by peppermint (73.8%) and rosemary (72.75%) extracts compared with fennel (65.89%) and common fumitory (62.97%) extracts. The second best oviposition deterrent was sage extract (with an oviposition inhibition of 71.17%). There was no detrimental effects of the plant extracts on the survival of a larval parasitoid, Cotesia vestalis (Haliday). GC–MS analysis revealed that the major components of rosemary extract were 1,3,5-benzetriol 3tms derivative, verbenone and anethole. While menthol, trans-anethole and isomenthone were most dominant compounds in peppermint extract. The present study demonstrated that rosemary and peppermint extracts have great potential to be used as effective and safe insecticides and oviposition deterrents against P. xylostella. The cause and effects of these findings in relation with the plant extract components and from a pest management viewpoint were discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The overuse of synthetic chemical insecticides, which have brought harmful impacts on ecosystem and beneficial species, has encouraged scientists to concentrate on discovery of new and more ecologically safe insecticides (Isman 2006). Through many years of evolution, plants have been adapted to produce diverse secondary metabolites to defend themselves from herbivorous insects (Koul and Walia 2009). In recent decades, there has been a growing interest in research concerning the possible use of plant extracts, especially those from medicinal plants, as an alternative to synthetic insecticides and possibly a more sustainable pest management strategy (Awmack and Leather 2002; Koul 2008). Compared with chemical insecticides, botanicals have short persistence time in environment, less toxicity to mammals and natural enemies, and lower production costs (Isman 2006). It is also less likely that insects can develop resistance against botanical insecticides because of their complex formulations, resulting in multiple modes of action (Huang et al. 2008; Kodjo et al. 2011). Studies on antifeeding or toxic effects of botanical insecticides have shown that different aspects of insect physiology and behavior can be influenced by these compounds (Charleston et al. 2005a, b; Shikano et al. 2010).
The diamondback moth, Plutella xylostella (L.) (Lepidoptera, Plutellidae), is an oligophagous species that specifically feed on the members of family Brassicaceae, making huge amount of economic loss in crucifers worldwide. This insect is one of the most resistant arthropods to insecticides, as it has developed resistance to 95 insecticidal compounds (Arthropod Pesticide Resistance Database 2019). In addition, P. xylostella is the first species whose populations have shown resistance to Bacillus thuringiensis (Bt) Berliner (Bacillales, Bacillaceae) in the field (Furlong et al. 2013). This situation is due to overuse of broad-spectrum insecticides particularly in tropical and subtropical regions of the world. The emergence of insecticide resistance along with the incompatibility of chemical insecticides with biocontrol agents (Furlong et al. 2013) has led researchers to look for alternative products such as botanical insecticides (Charleston et al. 2005a). A recent review has indicated that organic extracts of medicinal plants not only are toxic to P. xylostella larvae, but also provokes oviposition repellency in P. xylostella adults (Karimzadeh and Rabiei 2020).
In Iran, the most cultivated Brassicaceae crops are vegetables (such as cabbages, cauliflower, broccoli, Brussels sprouts, turnip and radishes) and rapeseed/canola (as a source of edible oil). Other crucifers, however, are cultivated as condiment source (e.g., mustards), medicinal plants (e.g., flixweed) or ornamental species (e.g., stocks and sweet alyssum) (Ahmadi et al. 2020). Iran is ranked 5th (after China, India, U.S. and Turkey) among vegetable producing countries with an annual production of 20 million tons. Crucifer vegetables are the fifth (after tomato, potato, onion and eggplant) most cultivated vegetables in Iran, with a cultivation area of more than 18,000 ha (FAOSTAT 2021). Apart from crucifer vegetables, canola cultivation areas have been sharply increased and reached to more than 214,000 ha to reduce Iran’s dependence on the imported vegetable oil (Ahmadi et al. 2020). From 2000 onwards, P. xylostella has shown major outbreaks in cruciferous vegetables in central and northern Iran (Afiunizadeh et al. 2011). Plutella xylostella has also been reported in canola from different parts of Iran (Keyhanian et al. 2005), but it has only become a serious pest of canola in northern (Asghari et al. 2009) and central (personal observations, but unpublished, by Javad Karimzadeh, 2017) Iran. Early field studies have shown that larval and pupal parasitoids are actively engaged in natural parasitism of P. xylostella at common cabbage and cauliflower fields in central Iran (Afiunizadeh and Karimzadeh 2010, 2015; Afiunizadeh et al. 2011; Kazemzadeh et al. 2014); these hymenopteran parasitoids include the braconids Cotesia vestalis (Haliday), Bracon hebetor Say and Dolichogenidea appellator (Telenga), the ichneumonids Diadegma semiclausum (Hellen), Diadromus subtilicornis (Gravenhorst) and Diadromus collaris (Gravenhorst), and the eulophid Oomyzus sokolowskii (Kurdjumov).
Further studies have shown that C. vestalis and D. semiclausum are the most influential parasitoids involved in natural control of P. xylostella in fields of cruciferous vegetables and canola of Iran (Hasanshahi et al. 2014a; Pourian et al. 2014; Afiunizadeh and Karimzadeh 2015; Tajmiri et al. 2017). These two species are highly specific endoparasitoids of P. xylostella (Karimzadeh and Wright 2008; Furlong et al. 2013), and have been imported to many parts of world as the classical biocontrol agents (Sarfraz et al. 2005). Both the species have been reported from different parts of Iran (Bagheri et al. 2004; Golizadeh et al. 2008a,b; Afiunizadeh and Karimzadeh 2010; Ghahari et al. 2011, 2012a,b; Ghahari and Schwarz 2012; Barahoei et al. 2013; Karimzadeh and Broad 2013; Hasanshahi et al. 2014a,b,c; Pourian et al. 2014; Gadallah et al. 2015; Sarafi et al. 2015; Ghahari 2016; Mohammadi-Khoramabadi et al. 2016; Tajmiri et al. 2017). However, C. vestalis is more geographically distributed and more abundant during growing seasons in Iran (Pourian et al. 2014; Afiunizadeh and Karimzadeh 2015). It has also been documented that compared with D. semiclausum, C. vestalis populations are more frequently occurred in hot days of summer in Iran Pourian et al. 2014; Afiunizadeh and Karimzadeh 2015).
Previous studies have indicated that C. vestalis females attack and parasitize all feeding larvae (e.g., 2nd, 3rd and 4th instars) of P. xylostella but they prefer to parasitize the 2nd and 3rd host larval instars (Karimzadeh and Wright 2008; Alizadeh et al. 2011). Field studies in cruciferous vegetables of central Iran have revealed that P. xylostella populations can be suppressed under economic threshold using a combination of C. vestalis release and Bt applications (Karimzadeh and Besharatnejad 2017b). To maintain a sustainable management of P. xylostella in field parasitoid populations must be conserved as far as possible through ecological pest management strategies (Daniarzadeh et al. 2014; Karimzadeh and Besharatnejad 2017a, b, 2019). Finally, when chemical control is necessary it is important to use selective insecticides that are relatively harmless to parasitoids (e.g., such as biopesticides or botanicals) (Charleston et al. 2005b; Liu et al. 2006; Rezaei et al. 2014).
Phytochemicals and their bioactive features are widely varied between plant species. Among phytochemicals, three groups (e.g., terpenes, phenolics and alkaloids) have shown the most insecticidal activity (Boulogne et al. 2012). Here, five medicinal plants (including Rosmarinus officinalis L., Mentha piperita L., Salvia officinalis L., Foeniculum vulgare Mill. and Fumaria officinalis L.) were chosen from the plant families that are rich in secondary metabolites. Previous studies have shown toxicity and deterrence of these extracts on other insects and mites (e.g., Kim et al. 2003a, b; Bouayad et al. 2013). The present study objectives were (1) to determine bioactive compounds and their amounts in methanolic extracts of the mentioned medicinal plants using gas chromatography and mass spectroscopy (GC–MS), (2) to examine larvicidal and oviposition deterrent activities of the plant extracts on P. xylostella, and (3) to investigate sublethal effects of the plant extracts on adult survival rate of C. vestalis.
Material and methods
Plant and insect rearing protocol
Brassica rapa L. var. pekinensis (Chinese cabbage) cv. Hero (Takii Seed, Kyoto, Japan) was grown under glasshouse conditions (25 ± 5 °C; L:D 16:8 h) without the application of any pesticide. Field populations of P. xylostella and C. vestalis were originally collected from the common cabbage fields of Mobarakeh County (32° 20ʹ 47ʺ N, 51° 30ʹ 16ʺ E, 1673 m; with a cold and dry climate; Isfahan province, central Iran) in July 2018. Cultures of P. xylostella were maintained on 6-week-old Chinese cabbage in ventilated oviposition cages (40 × 40 × 40 cm). Cultures of C. vestalis were, in turn, maintained on P. xylostella larvae in similar cages. Adult insects were fed on honey solution (30%), and cultures were maintained under a standard constant environment (25 ± 2 °C; 70 ± 5% RH; a photoperiod of L:D 16:8 h) as described previously (Karimzadeh et al. 2004, 2013).
Plant extracts preparation
The leaves of rosemary (Rosmarinus officinalis L.), peppermint (Mentha piperita L.), sage (Salvia officinalis L.) (Lamiaceae) and common fumitory (Fumaria officinalis L. (Papaveraceae)), and the seeds of fennel (Foeniculum vulgare Mill (Apiaceae)) were collected from a medicinal plant field in Najafabad County (32° 37ʹ 59ʺ N, 51° 21ʹ 59ʺ E, 1648 m; a semi-desert climate and calcareous soil; Isfahan province, central Iran) in June 2018. The collected plants were identified and the voucher specimens (16,063, 16,064, 16,065, 16,061 and 16,062) were deposited at the Herbarium of Isfahan Research and Education Center for Agriculture and Natural Resources (Isfahan, Iran). The plant materials were washed, shadow dried at room temperature, and then finely powdered using a blender. The samples (50 g of powdered plant materials) were extracted with 250 ml of MeOH (97%) in a shaker (Labcon SPL 15-MP, SelecTech, Johannesburg, South Africa) at 120 rpm for 24 h, filtered twice with a Whatman filter paper (No. 4, Munktell Grade 389) and evaporated to dryness using a rotary evaporator under reduced pressure at 40 °C. The concentrated extracts were then filtered through sodium sulfate to remove the moisture. The dishes containing crude extracts were covered with aluminum foil and stored at 4 ºC (Karimzadeh and Rabiei 2020).
Chemical analysis
GC–MS was used to determine composition of the plant extracts. The analysis of the extract was performed using GC–MS system with GC-7890A/MS-5975C model (Agilent Technologies, Santa Clara, CA, USA) equipped with HP-5MS column (60.00 m × 0.25 mm, 0.25 μm film thickness). Spectroscopic detection by GC–MS was involved an electron ionization system that utilized high energy electrons (70 eV). The carrier gas was pure helium (99.99%) at a flow rate of 1.3 ml/min, and a split ratio of 1:30. The initial temperature was set at 40 °C and held for 3 min. The temperature gradually increased to 295 °C with a rate of 10 °C/min. The total running time of GC–MS was 38 min. About 1 μl of the methanol extract was injected into the GC–MS. Kovats indices were calculated for all volatile constituents using a homologous series of n-alkanes C9–C29 on DB-5MS column. The major components of extracts were identified by conjunction with standards, confirmed with Kovats indices using the Wiley and National Institute of Standards and Technology. The relative quantity of the chemical compounds present in each of the extracts was expressed as percentage based on the peak area produced in chromatogram (Zoubiri et al. 2014).
Dose–response bioassays
The leaf dipping bioassay was used to evaluate toxicity of the raw extracts of five above-mentioned medicinal plants against P. xylostella larvae. To perform bioassay, the procedure of Karimzadeh and Rabiei (2020) was used with some modifications. To determine the concentrations of main experiment for each extract, a preliminary test was performed using 30 P. xylostella 3rd instar larvae for each one of the dilutions of 0 (control), 30, 100, 300, 1000, 3000, 10,000 mg L−1. To prepare a stock solution, the extracts were diluted in distilled water containing Tween 80 (0.05%) as emulsifier. The main test was done with five concentrations (which showed 10–90% mortality) with logarithmic intervals between 4 and 3706, 6–2918, 15–9558, 20–13,289 and 24–18,780 mg L−1 for R. officinalis, M. piperita, S. officinalis, F. vulgare and F. officinalis, respectively. To perform the main test, the leaf discs (5 cm diameter) of the 4-week-old Chinese cabbage were immersed in the test solutions for 30 s, and then kept on a corrugated sheet of aluminum foil with the adaxial leaf surface uppermost for 2 h at room temperature to dry up. Control leaf discs were immersed in distilled water containing Tween 80 (0.05%). The leaf discs were then transferred to individual plastic Petri dishes (6 cm diameter) containing a moistened filter paper. Ten early 3rd instar larvae of P. xylostella were then placed on each leaf disc. The leaf discs were then replaced every 48 h with fresh, untreated ones. All the concentrations as well as the control were replicated five times, and mortality was recorded after 96 h. All the tests were performed under the standard constant environment (25 ± 2 °C; 70 ± 5% RH; a photoperiod of L:D 16:8 h). In all the experiments, the institutional and national guidelines for the care and use of laboratory animals (Institutional Animal Care and Use Committee Guidebook) were followed.
Oviposition deterrent activity
The sublethal concentrations (LC25; Table 3) were used to examine the effects of the extracts on oviposition deterrence of P. xylostella females. Each extract was spread on the internal surface of an 85-ml plastic cup. Control cups were treated with distilled water containing Tween 80 (0.05%). The treated cups were air dried at room temperature for 2 h, and covered with a piece of netting cloth to prevent the test insects from escaping. A pair (male and female) of newly emerged P. xylostella adults was introduced to each cup and fed with honey solution (20%). Each treatment was replicated five times and kept under the standard constant environment (25 ± 2 °C; 70 ± 5% RH; L:D 16:8 h). The number of laid (fecundity) and hatched (fertility) eggs was recorded daily for 7 days. Oviposition deterrence (OD) was calculated using the following equation (Chaubey 2008):
where Nt and Nc denote the number of eggs in treatment and control, respectively (Rafiei-Karahroodi et al. 2011; Karimzadeh and Rabiei 2020).
Sublethal effects on C. vestalis survival
To examine the contact effect of plant extracts, the sublethal concentrations (LC25) of each extract was spread on the internal surface of a 35-ml test tube. Control tubes were treated with distilled water containing Tween 80 (0.05%). The treated tubes were air dried at room temperature for 2 h, and covered with a piece of netting cloth to prevent the test insects from escaping. Five 1–2-day-old adults of C. vestalis (randomly selected from the laboratory colony) were released to each tube and fed with honey solution (30%). Each treatment was replicated six times and kept under the standard constant environment (25 ± 2 °C; 70 ± 5% RH; L:D 16:8 h). The insects were kept in the treated tubes for 96 h, and then mortality was recorded (Rezaei et al. 2014).
Statistical analyses
Generalized linear models (GLMs) with Poisson and binomial families were applied to the data (Crawley 2013). In particular, count data with categorical explanatory variables (i.e., fecundity) were analyzed using log-linear analysis of deviance (log-linear models, Poisson errors) (Karimzadeh and Wright 2008; Saadat et al. 2014). Proportional data with categorical explanatory variables (i.e., percentage survival) were analyzed using logistic analysis of deviance (binomial errors) (Karimzadeh and Besharatnejad 2019). In case of over dispersion, the model was refitted using quasi-Poisson/quasi-binomial rather than Poisson/binomial errors. To achieve the minimal adequate model, non-significant terms were removed through model simplification, in which, the original and simplified models were compared by a χ2 test or F test (in case of overdispersion). The statistical significance of minimal adequate model was then expressed as a standard normal deviate (z value) or t value (in case of overdispersion) (Karimzadeh et al. 2013). The dose–response data (proportional data with continuous explanatory variables) were analyzed using logistic regression (binomial errors), where regression lines were fitted to dose-mortality data on a log-logit scale, and the estimated lethal concentrations (LC) and associated 95% confidence intervals (CI) were then calculated from the estimated linear regression parameters (Jafary et al. 2016). Because of non-parallel regression lines, toxicity ratio test (lethal dose ratios) was used to compare LCs, instead of overlapping CIs, based on the statistical power of ratio test and its better type I error rates (Robertson et al. 2017). Non-binomial percentage data (i.e., oviposition deterrence percentage) were analyzed using one-way ANOVA after arcsine transformation. Pairwise comparisons were performed using Tukey’s Honestly Significant Difference (Karimzadeh and Rabiei 2020). All statistical analyses were completed in R 3.6.0 (R Development Core Team).
Results
Chemical analysis
GC–MS analysis revealed that terpene, terpenoid and phenolic compounds were the main compounds of the methanolic extract of tested plants (Table 1). The major components of rosemary extract were 1,3,5-benzetriol, 3tms derivative (12.78%), verbenone (7.79%) and anethole (4.62%). The three most important components of peppermint extract were menthol (31.23%), trans-anethole (14.53%) and isomenthone (12.17%). But these were anethole (11.98%), isomenthone (8.75%) and levomenthol (8.74%) for sage extract. While trans-anethole (66.22% and 17.64%), isomenthone (4.52% and 13.57%) and fenchone (4.70% and 5.57%) were the most dominant compounds detected in fennel and common fumitory extracts.
Dose–response bioassays
Logistic regression showed a significant (z-value = 9.008, 8.981, 8.313, 7.968 and 7.463; P < 0.001) linear relationship between the extract concentrations (as logarithm) and P. xylostella larval mortality (as logit) for all the extracts (Table 2). Based on the lethal concentrations (Table 3), the lethal dose ratios and their confidence intervals (Table 4), there was no significant difference between LC10s of different extracts (ranged between 1.74 and 8.09 mg L−1). Higher lethal concentrations however showed significant differences between extracts. LC25 of rosemary extract (11.55 mg L−1) was significantly smaller than sage (34.51 mg L−1), fennel (73.94 mg L−1) and common fumitory (117.32 mg L−1) extracts. LC25 of the second best toxicant (peppermint extract; 14.00 mg L−1) was significantly smaller than fennel and common fumitory extracts. LC25 of the third best toxicant (sage extract) was significantly smaller than common fumitory extract only. Other LC25 pair comparisons were not significantly different. Based on the median lethal concentrations, the LC50s of rosemary (76.51 mg L−1) and peppermint (78.89 mg L−1) extracts were significantly smaller than sage extract (322.47 mg L−1), which in turn, was significantly smaller than fennel (777.91 mg L−1) and common fumitory (1701.05 mg L−1) extracts. Other LC50 pair comparisons were not significantly different. Similarly, the LC90s of rosemary (3355.65 mg L−1) and peppermint (2502.95 mg L−1) extracts were significantly smaller than sage (28,147.82 mg L−1), fennel (86,106.06 mg L−1) and common fumitory (357,590.80 mg L−1) extracts. In addition, LC90s of sage extract was significantly smaller than common fumitory extract. Other LC90 pair comparisons were not significantly different. More detailed explanations of different extract toxicities can be observed using the dose–response curves (Fig. 1). It is clear that in whole range of doses rosemary and peppermint extracts are more toxic than other extracts. In addition, rosemary extract was the most toxic for the doses equal or lower than LC50. For the doses higher than LC50, however, peppermint extract was the most toxic plant extract.
Dose–response curves (of fitted logistic regression) of Plutella xylostella larvae subjected to different plant extracts. Solid (big filled circle), dash-dot (empty square), long-dashed (filled square), dotted (empty circle) and dashed (small filled circle) lines (points) represent rosemary, peppermint, sage, fennel and common fumitory extracts, respectively
Oviposition deterrent activity
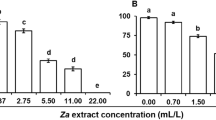
Log-linear models showed that the sublethal concentrations (LC25) of extracts had significant (z5 = – 14.56, P < 0.001) effects on fecundity of P. xylostella females. The mean number of laid eggs on control cup (116.0 ± 1.7) was significantly greater than other cups treated with extracts (Fig. 2). In addition, comparisons between the extracts showed that the mean number of laid eggs on the cup treated with common fumitory extract (43.0 ± 1.9) was significantly greater than rosemary (31.6 ± 2.0) and peppermint (31.2 ± 1.5) extracts. While fennel (39.6 ± 1.8 eggs/cup) and sage (33.4 ± 1.8 eggs/cup) extracts were intermediate of these two groups for their effects on P. xylostella fecundity. When egg survival was analyzed, logistic analysis of deviance showed no significant (t5 = 0.569, P = 0.574) difference between treatments. Mean percentage survival of P. xylostella eggs varied between 78.1 and 88.6 for different treatments. When the data were analyzed using one-way ANOVA, oviposition deterrence of P. xylostella females was significantly (F4,20 = 9.219, P < 0.001) different between treatments. Oviposition deterrent effects of peppermint (73.8 ± 1.34%) and rosemary (72.75 ± 1.66%) extracts were significantly greater than fennel (65.89 ± 1.30%) and common fumitory (62.97 ± 1.31%) extracts (Fig. 3). In addition, oviposition deterrence of sage extract (71.17 ± 1.64%) was only significantly greater than common fumitory extract.
The effects of sublethal concentrations (LC25) of different plant extracts on the fecundity of Plutella xylostella adults. CO, CF, FE, SA, RO and PE denote control, common fumitory, fennel, sage, rosemary and peppermint extracts, respectively. Columns marked with different letters are significantly different means (P < 0.05, Tukey’s HSD)
Oviposition deterrence of Plutella xylostella adults by sublethal concentrations (LC25) of different plant extracts. PE, RO, SA, FE and CF denote peppermint, rosemary, sage, fennel and common fumitory extracts, respectively. Columns marked with different letters are significantly different means (P < 0.05, Tukey’s HSD)
Sublethal effects on C. vestalis survival
The logistic analysis of deviance showed that there was no significant (z5 = – 0.006, P = 0.995) differences between the treatments (including the plant extracts and control) for their sublethal effects on C. vestalis survival. The mean survival of C. vestalis adults ranged from 70 to 90% for different extract, while it was 100% for control.
Discussion
Here, we indicated that plant extracts may be used as effective botanical pesticides and deterrents against the diamondback moth. Based on LC50s and LC90s, it was indicated that rosemary and peppermint leaf extracts were highly toxic against P. xylostella larvae. The toxicity of sage leaf extract was also considerable. Based on the fecundity influenced by LC25s it was also shown that all the extracts inhibited oviposition behavior of P. xylostella females. In addition, rosemary and peppermint leaf extracts exhibited the highest oviposition deterrent effect on the diamondback moth. Oviposition deterrence of sage leaf extract was considerable (> 70%) as well. Karimzadeh and Rabiei (2020) have summarized the results of previous studies on larvicidal, antifeedant and oviposition deterrent effects of plant extracts against P. xylostella. In this regard, many plant extracts have shown larvicidal effects on P. xylostella. The present study, however, is the first report of toxicity of rosemary, peppermint and sage against P. xylostella larvae.
The toxicity of botanical pesticides can be due to nervous and hormonal system disruption (e.g., azadirachtin, nicotine, essential oils) or mitochondrial function interferences (e.g., rotenone) (Isman 2006). For instance, the extract of Ricinus communis L. (Euphorbiaceae) seed contains a lectin (i.e., ricin) that caused cell death in P. xylostella larvae through inactivation of ribosomal RNA and inhibition of protein synthesis (Kodjo et al. 2011). In addition, the toxicity of plant extracts could be due to their antifeedant activity against insects. For example, the extracts of Azadirachta indica A.Juss., Melia volkensii Gürke (Meliaceae) and Origanum vulgare L. (Lamiaceae) have shown antifeedant effects on P. xylostella larvae, which inhibit mouthpart chemoreceptors or interfere with signal transduction process in central nervous system (Akhtar and Isman 2004; Charleston et al. 2005a).
In addition to lethal effects, plant extracts may have sublethal effects on biological characteristics of target insects, for example, by changing oviposition behavior. Few studies have shown detrimental effects of plant extracts on P. xylostella oviposition (see Karimzadeh and Rabiei (2020) for a review). It is well known that female herbivorous insects select their oviposition site using plant chemical cues (Charleston et al. 2005a; Furlong et al. 2013). The deterrent/repellant compounds, therefore, may decrease the potential fecundity or fertility of insect herbivores as a result of egg load reduction or low-quality egg production, respectively (Awmack and Leather 2002). The present study documented the potential of all the tested plant extracts for reducing fecundity of P. xylostella females. With a completely different procedure, Dover (1985) also indicated oviposition deterrent and antifeedant activities of rosemary and sage extracts on P. xylostella. The major and most common sensory modality involved in moth oviposition is contact-chemoreception, which may plays a role in the oviposition of P. xylostella females (Ramaswamy 1988; Basukriadi and Wilkins 2014). However, different oviposition deterrence of plant extracts in the present study may also be due to existence of different volatile compounds in the extracts.
Here, the highest toxicity and oviposition deterrence of rosemary and peppermint leaf extracts might be mainly because of the components that exist only in such extracts. In this regard, the higher toxicity of rosemary extract might be related to the existence of 1,3,5-benzetriol 3tms derivative (12.78%), verbenone (7.79%), bornyl acetate (2.04%), camphor (1.57%) and α-pinene (0.22%). No information is available on toxicity of 1,3,5-benzetriol 3tms derivative. Verbenone is naturally found in a variety of plants, and can also be prepared synthetically by oxidation of α-pinene. This terpene has shown toxic effects on insects and mites (e.g., Lee et al. 1997; Kanda et al. 2017). In addition, verbenone and its analogs are insect pheromones, which play an important role in control of bark beetles. For example, the low concentrations of verbenone synergized the attraction of aggregation pheromones of Dendroctonus valens LeConte (Coleoptera, Curculionidae) but its high concentrations inhibited the beetle host location (Rappaport et al. 2001; Zhang et al. 2006). Verbenone also could interrupt the attraction of bark beetles to their aggregation pheromones (Paine and Hanlon 1991; Miller et al. 1995). Moreover, verbenone has shown attracting effects on Sitophilus zeamais (Motschulsky) (Coleoptera, Curculionidae) (Pizzolitto et al. 2015) and repellent activity against Tribolium castaneum (Herbst) (Coleoptera, Tenebrionidae) and Liposcelis bostrychophila Badonnel (Psocoptera, Liposcelididae) (Guo et al. 2017).
Bornyl acetate is a monoterpene ester, which is known as a sex pheromone in various species of insects, such as cockroaches, beetles and flies. Plants, however, produce a structurally different version (i.e., an enantiomer) of this molecule. The bornyl acetate found in natural essential oils does not attract insects. This terpene has exhibited toxicity and repellent effects on insects (Park et al. 2003; Feng et al. 2019). Camphor is a terpenoid that is naturally found in plants such as camphor laurel, kapur tree, rosemary, camphorweed and camphor basil, and can also be synthetically produced from turpentine oil. Camphor have shown toxicity and repellent effects on many insects (e.g., Pizzolitto et al. 2015; Zhang et al. 2015; Chen et al. 2018). Besides repellent activity at high concentrations, camphor was attractive for T. castaneum and Lasioderma serricorne (F.) (Coleoptera, Anobiidae) at low concentrations (Chen et al. 2018). Alfa-pinene is found in the oils of many species of coniferous trees, notably the pine. It is also found in the essential oil of rosemary and Satureja myrtifolia (Boiss. & Hohen.) Greuter & Burdet. This terpene has shown toxicity, antifeeding, growth inhibitory and repellent effects on coleopteran insects (Huang et al. 1998; Ojimelukwe and Adler 2000; Zhang et al. 2015).
The higher toxicity of peppermint extract, however, may be related to the content of menthol (31.23%), α-thujone (5.20%), trans-caryophyllene (3.49%) and germacrene D (2.11%). No information is available on toxicity of trans-caryophyllene. Menthol, also called peppermint camphor, is a terpene alcohol that is obtained from peppermint oil or is produced synthetically by hydrogenation of thymol. The menthol molecule can exist as one of two enantiomers. The naturally occurring material is the levorotatory form (i.e., (-)-menthol or l-menthol). Synthetic menthol is racemic, consisting of equal amounts of l-menthol and d-menthol (( +)-menthol) (Wade 2018). Menthol has shown toxicity and antifeedant activity against insects and mites (e.g., Lee et al. 1997, 2001; Ojimelukwe and Adler 2000; Rajkumar et al. 2019).
Thujone is a monoterpene ketone that is naturally found in several plants, for instance Salvia officinalis L., Salvia sclarea L., Tanacetum vulgare L., Artemisia absinthium L. and Thuja occidentalis L. (Pelkonen et al. 2013). Thujone occurs naturally in two diastereomeric forms: ( −)-α-thujone and ( +)-β-thujone. In addition, two other forms are possible as ( +)-α-thujone and ( −)-β-thujone, which were found in nature as well as in S. officinalis. While ketones generally are not toxic, thujone is the most toxic ketone (Roslan 2014). Alfa-thujone has shown toxicity, repellent and deterrent effects on insects (e.g., Szołyga et al. 2014; Pizzolitto et al. 2015). Germacrenes are a class of volatile organic hydrocarbons, specifically sesquiterpenes, which are produced in a number of plant species for their antimicrobial and insecticidal properties. However, germacrenes also function as insect pheromones. Germacrene D has shown attractive properties for some insect species (e.g., Mozuraitis et al. 2002).
It must be considered that the greater amounts of plant extract components do not necessarily result in higher toxicity. For example, Yu et al. (2013) indicated that minor compounds in rosemary essential oil (i.e., limonene and pinene) had more insecticidal activity against southern house mosquito compared with major compound (i.e., eucalyptol and camphor). In addition, the toxicity of an extract or essential oil may be attributed to the interactions between two or more components. For instance, the minor constituents may play a synergistic role in toxicity of plant extracts. Therefore, botanical pesticides can be used in sustainable pest management mainly due to such interactions and different modes of actions and target sites in insects (Kumar et al. 2011).
The safety of botanical pesticides for natural enemies has been a major concern in different studies. For instance, no harmful effect of azadirachtin on predatory mites, such as Neoseiulus cucumeris (Oudemans), Neoseiulus californicus (McGregor) and Phytoseiulus macropilis (Banks), has been reported (Stara et al. 2011; Bernardi et al. 2013). Charleston et al. (2005a, b, 2006) indicated that application of Melia azedarach L. and A. indica (Meliaceae) extracts had no significant effects on the survival of C. vestalis and D. collaris, the parasitoids of the diamondback moth. These extracts, furthermore, increased the parasitism of P. xylostella larvae by C. vestalis. Other studies, however, have indicated the adverse effects of plant extracts on beneficial insects (Al-mazra'awi and Ateyyat 2009; Tunca et al. 2014). To improve pest management efficiency, thus, it is important to get accurate information about direct and indirect effects of botanical pesticides on natural enemies. Larval endoparasitoids are the most effective biocontrol agents of P. xylostella (Furlong et al. 2013; Afiunizadeh and Karimzadeh 2015), and it is necessary to study the effects of botanical pesticides on these beneficial insects. The present study showed no detrimental effect of sublethal doses of the tested plant extracts on C. vestalis adult survival. Here, it was demonstrated that rosemary and peppermint leaf extracts have great potential to be used as botanical insecticides and oviposition inhibitors against the diamondback moth. Future studies are necessary to examine the stability and persistence of these findings in field.
Availability of data and material
Data are available from the corresponding author upon request.
Code availability
R Codes used for statistical analyses are available from the corresponding author upon request.
References
Afiunizadeh M, Karimzadeh J (2010) Larval and pupal parasitoids of Plutella xylostella (Lep.: Plutellidae) in Isfahan province, Iran. Plant Prot J 2:79–97
Afiunizadeh M, Karimzadeh J, Shojai M (2011) Naturally-occurring parasitism of diamondback moth in central Iran. In: Management of the Diamondback moth and other crucifer insect pests- Proceedings of the 6th International Workshop, pp. 93–96. AVRDC, Tainan, Taiwan
Afiunizadeh M, Karimzadeh J (2015) Assessment of naturally-occurring parasitism of diamondback moth in field using recruitment method. Arch Phytopathol Plant Prot 48:43–49
Ahmadi K, Ebadzadeh H, Hatami F, Abdshah H, Kazemian A (2020) Agricultural Statistics of Iran- Growing Season 2018–2019. Ministry of Agriculture, Tehran, Iran
Akhtar Y, Isman MB (2004) Comparative growth inhibitory and antifeedant effects of plant extracts and pure allelochemicals on four phytophagous insect species. J Appl Entomol 128:32–38
Alizadeh M, Rassoulian GR, Karimzadeh J, Hosseini-Navhe V, Farazmand H (2011) Biological study of Plutella xylostella (L.) (Lep: Plutellidae) and it’s solitary endoparasitoid, Cotesia vestalis (haliday) (Hym. Braconidae) under laboratory conditions. Pak J Biol Sci 14:1090–1099
Almazra’awi MS, Ateyyat M (2009) Insecticidal and repellent activities of medicinal plant extracts against the sweet potato whitefly, Bemisia tabaci (Hom.: Aleyrodidae) and its parasitoid Eretmocerus mundus (Hym.: Aphelinidae). J Pest Sci 82:149–154
Arthropod Pesticide Resistance Database (2019) Michigan State University. https://www.pesticideresistance.org/. Accessed 22 August 2021
Asghari A, Fathi AA, Mohammadi S, Mohammad Doust H (2009) QTL analysis for diamondback moth resistance in canola (Brassica napus L.). Int J Plant Prod 3:29–34
Awmack CS, Leather SR (2002) Host plant quality and fecundity in herbivorous insects. Annu Rev Entomol 47:817–844
Bagheri M, Hatami B, Nematollahi M (2004) The first record of Diadegma semiclausum (Hellen), endoparasitoid of larvae of Plutella xylostella on Wallflower in greenhouses of Isfahan. In: Proceeding of the 16th Iranian Plant Protection Congress, p. 162
Barahoei H, Rakhshani E, Kasparyan DR, Schwarz M, Riedel M (2013) Contribution on the knowledge of Ichneumonidae (Hymenoptera) in the northern part of Sistan and Baluchestan province, Iran. Acta Zool Bulgar 65:131–135
Basukriadi A, Wilkins RM (2014) Oviposition deterrent activities of Pachyrhizus erosus seed extract and other natural products on Plutella xylostella (Lepidoptera: Plutellidae). J Insect Sci 14:1–6
Bernardi D, Botton M, Cunha US, Bernardi O, Malausa T, Garcia MS, Nava DE (2013) Effects of azadirachtin on Tetranychus urticae (Acari: Tetranychidae) and its compatibility with predatory mites (Acari: Phytoseiidae) on strawberry. Pest Manag Sci 69:75–80
Bouayad N, Rharrabe K, Ghailani NN, Jbilou R, Castañera P, Ortego F (2013) Insecticidal effects of Morocan plant extracts on development, energy reserves and enzymatic activities of Plodia interpunctella. Span J Agric Res 11:189–198
Boulogne I, Petit P, Ozier-Lafontaine H, Desfontaines L, Loranger-Merciris G (2012) Insecticidal and antifungal chemicals produced by plants: a review. Environ Chem Lett 10:325–347
Charleston DS, Kfir R, Dicke M (2005a) Behavior responses of diamondback moth Plutella xylostella (Lepidoptera: Plutellidae) to extracts derived from Melia azedarach and Azadirachta indica. Bull Entomol Res 95:457–469
Charleston DS, Kfir R, Dicke M, Vet LEM (2005b) Impact of botanical pesticides derived from Melia azedarach and Azadirachta indica on the biology of two parasitoid species of the diamondback moth. Biol Control 33:131–142
Charleston DS, Kfir R, Dicke M, Vet LEM (2006) Impact of botanical extracts derived from Melia azedarach and Azadirachta indica on populations of Plutella xylostella and its natural enemies: a field test of laboratory findings. Biol Control 39:105–114
Chaubey MK (2008) Fumigant toxicity of essential oils from some common spices against pulse beetle, Callosobruchus chinensis (Coleoptera: Bruchidae). J Oleo Sci 57:171–179
Chen ZY, Guo SS, Cao JQ, Pang X, Geng ZF, Wang Y, Zhang Z, Du SS (2018) Insecticidal and repellent activity of essential oil from Amomum villosum Lour. and its main compounds against two stored-product insects. Int J Food Prop 21:2265–2275
Crawley MJ (2013) The R book, 2nd edn. John Wiley & Sons, Chichester, UK
Daniarzadeh S, Karimzadeh J, Jalalizand A (2014) The strategy of trap cropping for reducing the populations of diamondback moth in common cabbage. Arch Phytopathol Plant Prot 47:1852–1859
Dover JW (1985) The responses of some Lepidoptera to labiate herb and white clover extracts. Entomol Exp Appl 39:177–182
FAOSTAT (2021) http://www.fao.org/faostat/en/#home. Accessed 25 August 2021
Feng YX, Wang Y, Chen ZY, Guo SS, You CX, Du SS (2019) Efficacy of bornyl acetate and camphene from Valeriana offcinalis essential oil against two storage insects. Environ Sci Poll Res 26:16157–16165
Furlong MJ, Wright DJ, Dosdall LM (2013) Diamondback moth ecology and management: problems, progress and prospects. Annu Rev Entomol 58:517–541
Gadallah NS, Ghahari H, Peris-Felipo FJ (2015) Catalogue of the Iranian Microgastrinae (Hymenoptera: Braconidae). Zootaxa 4043:1–69
Ghahari H (2016) A study on the fauna of Ichneumonidae (Hymenoptera) in the province of Tehran, Iran. Arq Entomol 16:125–132
Ghahari H, Schwarz M (2012) A study of the Ichneumonidae (Hymenoptera: Ichneumonoidea) from the Qazvin province, Iran. Linz Biol Beitr 44:855–862
Ghahari H, Fischer M, Papp J (2011) A study on the braconid wasps (Hymenoptera: Braconidae) from Isfahan province, Iran. Entomofauna 32:261–272
Ghahari H, Jussila FMR (2012a) Braconid and ichneumonid wasps (Hymenoptera, Ichneumonoidea) as the parasitoids of Plutella xylostella (L.)(Lepidoptera: Plutellidae) in Iran. Entomofauna 18:281–288
Ghahari H, Fischer M, Tobias VI (2012b) A study on the Braconidae (Hymenoptera: Ichneumonoidea) from Guilan province, Iran. Entomofauna 33:317–324
Golizadeh A, Kamali K, Fathipour Y, Abbasipour H, Jussila R (2008a) Report of the parasitoid wasp, Diadegma anurum (Hym.: Ichneumonidae), from Iran. J Entomol Soc Iran 27:15–16
Golizadeh A, Kamali K, Fathipour Y, Abbasipour H, Lozan A (2008b) Report of the parasitoid wasp, Cotesia plutellae (Hym.: Braconidae), from Iran. J Entomol Soc Iran 27:19–20
Guo SS, Zhang WJ, Yang K, Liang JU, You CX, Wang CF, Li YP, Geng ZF, Deng ZW, Du SS (2017) Repellence of the main components from the essential oil of Glycosmis lucida Wall. ex Huang against two stored product insects. Nat Prod Res 31:1201–1204
Hasanshahi G, Abbasipour H, Askarianzadeh A, Karimi J, Jahan F, Rahimi A (2014a) Situation of the diamondback moth (DBM), Plutella xylostella and its parasitoids in the cauliflower fields of Tehran. Annu Rev Res Biol 3:473–486
Hasanshahi G, Abbasipour H, Jahan F, Askarianzadeh A, Karimi J, Rahimi AH (2014b) Natural parasitism of the diamondback moth, Plutella xylostella (L.)(Lep.: Plutellidae) by a larval parasitoid wasp, Diadegma anurum on different cauliflower cultivars. Arch Phytopathol Plant Prot 47:456–463
Hasanshahi G, Askarianzadeh A, Abbasipour H, Karimi J (2014c) Study on the seasonal parasitism rate of the diamondback moth, Plutella xylostella (L.)(Lep.: Plutellidae) parasitoids in the cauliflower fields in south of Tehran. Biocontrol Plant Prot 2:17–29
Huang Y, Hee SK, Ho SH (1998) Antifeedant and growth inhibitory effects of α-pinene on the stored-product insects, Tribolium castaneum (Herbst) and Sitophilus zeamais Motsch. Int Pest Cont 40:18–20
Huang Z, Zhou FC, Xu D, Afzal M, Bashir MH, Ali S, Freed S (2008) Antifeedant activities of secondary metabolites from Ajuga nipponensis against Plutella xylostella. Pak J Bot 40:1983–1992
Isman MB (2006) Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu Rev Entomol 51:45–66
Jafary M, Karimzadeh J, Farazmand H, Rezapanah M (2016) Plant-mediated vulnerability of an insect herbivore to Bacillus thuringiensis in a plant-herbivore-pathogen system. Biocontrol Sci Tech 26:104–115
Kanda D, Kaur S, Koul O (2017) A comparative study of monoterpenoids and phenylpropanoids from essential oils against stored grain insects: acute toxins or feeding deterrents. J Pest Sci 90:531–545
Karimzadeh J, Besharatnejad MH (2017a) Ecological management of the diamondback moth (Plutella xylostella (L.)) using trap cropping strategy. J Agroecol 6:212–224
Karimzadeh J, Besharatnejad MH (2017b) Integrated management of diamondback moth (Plutella xylostella (L.)) using Cotesia vestalis (Haliday) and Bacillus thuringiensis Berliner. J Plant Prot Res 40:81–96
Karimzadeh J, Besharatnejad MH (2019) Ecological control of Plutella xylostella (Lepidoptera, Plutellidae) using trap cropping and Bt applications. Arch Phytopathol Plant Prot 52:1326–1347
Karimzadeh J, Broad G (2013) Amendment to 'report of the parasitoid wasp, Diadegma anurum (Hym.: Ichneumonidae), from Iran’. J Entomol Soc Iran 33:91–92
Karimzadeh J, Rabiei A (2020) Larvicidal and oviposition deterrent effects of the jimsonweed (Datura stramonium L.) extracts on the diamondback moth, Plutella xylostella (L.). J Agric Sci Technol 22:1279–1293
Karimzadeh J, Wright DJ (2008) Bottom-up cascading effects in a tritrophic system: interactions between plant quality and host-parasitoid immune responses. Ecol Entomol 33:45–52
Karimzadeh J, Bonsall MB, Wright DJ (2004) Bottom-up and top-down effects in a tritrophic system: the population dynamics of Plutella xylostella (L.)- Cotesia plutellae (Kurdjumov) on different host plants. Ecol Entomol 29:285–293
Karimzadeh J, Hardie J, Wright DJ (2013) Plant resistance affects the olfactory response and parasitism success of Cotesia vestalis. J Insect Behav 26:35–50
Kazemzadeh Z, Shaw MR, Karimzadeh J (2014) A new record for Iran of Dolichogenidea appellator (Hym.: Braconidae: Microgastrinae), a larval endoparasitoid of diamondback moth, Plutella xylostella (Lep.: Plutellidae). J Entomol Soc Iran 33:81–82
Keyhanian A, Taghizadeh M, Taghadosi M, Khajehzadeh Y (2005) A faunistic study on insect pests and its natural enemies in canola fields at different regions of Iran. Res Constr 68:2–8
Kim SI, Park C, Ohh MH, Cho HC, Ahn YJ (2003a) Contact and fumigant activities of aromatic plants extracts and essential oils against Lasioderma serricorne (Coleoptera: Anobiidae). J Stored Prod Res 39:11–19
Kim SI, Roh JY, Kim DH, Lee HS, Ahn YJ (2003b) Insecticidal activities of aromatic plant extracts and essential oils against Sitophilus oryzae and Callosobruchus chinensis. J Stored Prod Res 39:293–303
Kodjo TA, Gbénonchi M, Sadate A, Komi A, Yaovi G, Dieudonne M, Komla S (2011) Bio-insecticidal effects of plant extracts and oil emulsions of Ricinus communis L. (Malpighiales: Euphorbiaceae) on the diamondback, Plutella xylostella L. (Lepidoptera: Plutellidae) under laboratory and semi-field conditions. J Appl Biosci 43:2899–2914
Koul O (2008) Phytochemicals and insect control: An antifeedant approach. Critical Rev Plant Sci 27:1–24
Koul O, Walia S (2009) Comparing impacts of plant extracts and pure allelochemicals and implications for pest control. CAB Rev: Perspect. Agric Vet Sci Nutr Nat Resour 4(49):1–30
Kumar P, Mishra S, Malik A, Satya S (2011) Insecticidal properties of Mentha species. Ind Crops Prod 34:802–817
Lee S, Tsao R, Peterson C, Coats JR (1997) Insecticidal activity of monoterpenoids to western corn root worm (Coleoptera: Chrysomelidae), two spotted spider mite (Acari: Tetranychidae) and Housefly (Diptera: Muscidae). J Econ Entomol 90:883–892
Lee SE, Lee BH, Choi WS, Park BS, Kim JG, Campbell BC (2001) Fumigant toxicity of volatile natural products from Korean spices and medicinal plants towards the rice weevil, Sitophilus oryzae (L). Pest Manag Sci 57:548–553
Liu SS, Li YH, Lou YG (2006) Non-host plant extracts reduce oviposition of Plutella xylostella (Lepidoptera: Plutellidae) and enhance parasitism by its parasitoid Cotesia plutellae (Hymenoptera: Braconidae). Bull Entomol Res 96:373–378
Miller DR, Borden JH, Lindgren BS (1995) Verbenone: dose-dependent interruption of pheromone-based attraction of three sympatric species of pine bark beetles (Coleoptera: Scolytidae). Environ Entomol 24:692–696
Mohammadi-Khoramabadi A, Hesami S, Shafiei S (2016) A contribution to the knowledge of the fauna of Ichneumonidae in Rafsanjan county of Kerman province, Iran. Entomofauna 37:453–468
Mozuraitis R, Stranden M, Ramirez MI, Borg-Karlson AK, Mustaparta H (2002) (-)-Germacrene D increases attraction and oviposition by the tobacco bud-worm moth Heliothis virescens. Chem Senses 27:505–509
Ojimelukwe PC, Adler C (2000) Toxicity and repellent effects of eugenol, thymol, linalool, menthol and other pure compounds on Dinoderus bifloveatus (Coleoptera: Bostrichidae). J Sustain Agric Environ 2:47–54
Paine TD, Hanlon CC (1991) Response of Dendroctonus brevicomis and Ips paraconfusus (Coleoptera: Scolytidae) to combinations of synthetic pheromone attractants and inhibitors verbenone and ipsdienol. J Chem Ecol 17:2163–2176
Park C, Kim SI, Ahn YJ (2003) Insecticidal activity of asarones identified in Acorus gramineus rhizome against three coleopteran stored product insects. J Stored Prod Res 39:333–342
Pelkonen O, Abass K, Wiesner J (2013) Thujone and thujone-containing herbal medicinal and botanical products: toxicological assessment. Regul Toxicol Pharm 65:100–107
Pizzolitto RP, Herrera JM, Zaio YP, Dambolena JS, Zunino MP, Gallucci MN, Zygadlo JA (2015) Bioactivities of ketones terpenes: antifungal effect on F. verticillioides and repellents to control insect fungal vector, S. Zeamais. Microorganisms 3:851–865
Pourian HR, Talaei-Hassanloui R, Ashouri A, Lotfalizadeh H, Nozari J (2014) Abundance and parasitism rate of larval and pupal parasitoids of diamondback moth, Plutella xylostella L. (Lepidoptera: Plutellidae) in four regions of Iran. Iran J Plant Prot 45:265–278
Rafiei-Karahroodi Z, Moharramipour S, Farazmand H, Karimzadeh J (2011) Insecticidal effect of six native medicinal plants essential oil on Indian meal moth, Plodia interpunctella Hübner (Lep.: Pyralidae). Munis Entomol Zool 6:339–345
Rajkumar V, Gunasekaran C, Kanith I, Dharmaraj J, Chinnaraj P, Cheruvathur AP (2019) Toxicity, antifeedant and biochemical efficacy of Mentha piperita L. essential oil and their major constituents against stored grain pest. Pestic Biochem Physiol 156:138–144
Ramaswamy SB (1988) Host finding by moths: sensory modalities and behaviours. J Insect Physiol 34:235–249
Rappaport NG, Owen DR, Stein JD (2001) Interruption of semiochemical-mediated attraction of Dendroctonus valens (Coleoptera: Scolytidae) and selected nontarget insects by verbenone. Environ Entomol 30:837–841
Rezaei M, Karimzadeh J, Shakarami J (2014) Side effects of insecticides on the adult longevity of Cotesia vestalis, a larval parasitoid of the diamondback moth, Plutella xylostella. J Entomol Zool Stud 2:49–51
Robertson JL, Jones MM, Olguin E, Alberts B (2017) Bioassays with arthropods. Taylor and Francis, Boca Raton, FL
Roslan J (2014) Characterization of essential oil from Malaysian curry leaves. Dissertation, Universiti Malaysia Pahang
Saadat D, Seraj AA, Goldansaz SH, Karimzadeh J (2014) Environmental and maternal effects on host selection and parasitism success of Bracon hebetor. Biocontrol 59:297–306
Sarafi T, Barahoei H, Madjdzadeh SM, Askari M (2015) A contribution to the knowledge of the Ichneumonidae (Hym.: Ichneumonoidea) from Neyriz county of Fars province, Iran. J Crop Prot 4:643–654
Sarfraz M, Keddie AB, Dosdall LM (2005) Biological control of the diamondback moth, Plutella xylostella: a review. Biocontrol Sci Tech 15:763–789
Shikano I, Ericsson JD, Cory JS, Myers JH (2010) Indirect plant-mediated effects on insect immunity and disease resistance in a tritrophic system. Basic Appl Ecol 11:15–22
Stara J, Ourednickova J, Kocourek F (2011) Laboratory evaluation of the side effects of insecticides on Aphidius colemani (Hymenoptera: Aphidiidae), Aphidoletes aphidimyza (Diptera: Cecidomyiidae), and Neoseiulus cucumeris (Acari: Phytoseidae). J Pest Sci 84:25–31
Szołyga B, Gniłka R, Szczepanik M, Szumny A (2014) Chemical composition and insecticidal activity of Thuja occidentalis and Tanacetum vulgare essential oils against larvae of the lesser meal worm, Alphitobius diaperinus. Entomol Exp Appl 151:1–10
Tajmiri P, Fathi SAA, Golizadeh A, Nouri-Ganbalani G (2017) Strip-intercropping canola with annual alfalfa improves biological control of Plutella xylostella (L.) and crop yield. Int J Trop Insect Sci 37:208–216
Tunca H, Kilinçer N, Özkan C (2014) Toxicity and repellent effects of some botanical insecticides on the egg-larval parasitoid Chelonus oculator Panzer (Hymenoptera: Braconidae). Sci Res EssaYs 9:106–113
Wade LG (2018) Menthol. Encyclopædia Britannica. https://www.britannica.com/science/menthol. Accessed 13 July 2020
Yu J, Liu XY, Yang B, Wang J, Zhang FQ, Feng ZL, Wang CZ, Fan QS (2013) Larvicidal activity of essential extract of Rosmarinus officinalis against Culex quinquefasciatus. J Am Mosq Control Assoc 29:44–48
Zhang L, Sun J, Clarke SR (2006) Effects of verbenone dose and enantiomer on the interruption of response of the red turpentine beetle, Dendroctonus valens LeConte (Coleoptera: Scolytidae), to its kariomones. Environ Entomol 35:655-660.
Zhang WJ, Yang K, You CX, Wang Y, Wang CF, Wu Y, Geng ZF, Su Y, Du SS, Deng ZW (2015) Bioactivity of essential oil from Artemisia stolonifera (Maxim.) Komar. and its main compounds against two stored-product insects. J Oleo Sci 64:299–307
Zoubiri S, Baaliouamer A, Seba N, Chamouni N (2014) Chemical composition and larvicidal activity of Algerian Foeniculum vulgare seed essential oil. Arab J Chem 7:480–485
Acknowledgements
We would like to thank Azadeh Akhavan-Roofigar (Department of Natural Resources, Isfahan Research and Education Center for Agriculture and Natural Resources, AREEO, Isfahan, Iran), Helen Alipanah (Department of Insect Taxonomy, Iranian Research Institute of Plant Protection, AREEO, Tehran, Iran) and Mark R. Shaw (Department of Natural Sciences, National Museum of Scotland, Edinburgh, U.K.) for identification of the collected samples of medicinal plants, P. xylostella and C. vestalis, respectively.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MA performed the experiments and wrote the initial manuscript. JK designed the experiments, analyzed the data, and revised the final manuscript. SI and SM helped in designing the experiments and reviewed the results. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethics approval
In all the experiments, the institutional and national guidelines for the care and use of laboratory animals (Institutional Animal Care and Use Committee Guidebook) were followed.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Afiunizadeh, M., Karimzadeh, J., Imani, S. et al. Insecticidal and oviposition deterrent effects of five medicinal plant extracts on the diamondback moth. J Plant Dis Prot 129, 805–817 (2022). https://doi.org/10.1007/s41348-022-00592-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-022-00592-w