Abstract
Fusarium oxysporum f. sp. capsici (Foc) induces wilt disease in chilli and affects its yield. Implementing microorganisms and plant extracts for plant disease management has recently gained momentum as chemical measures pose a serious threat to the environment. Field experiments were conducted over two consecutive years to evaluate the effectiveness of Trichoderma harzianum (either as seed treatment or as soil application) and neem (Azadirachta indica; as seed treatment with leaf extract) alone or in combination, in managing chilli wilt. Untreated plots served as control. Chilli plants were grown in field plots of 2 × 2 m2 size, and experiments were organised in complete randomised block design with three replications. In the absence of Foc, T. harzianum (sa) + A. indica (st) increased growth and yield of chilli. In Foc-inoculated plots, both, T. harzianum and A. indica, showed a reduction in disease severity. Disease severity and chlorophyll content were negatively correlated (P ≤ 0.05). During both years, T. harzianum (sa) + A. indica (st) caused the strongest reduction in wilt severity and the highest increase in chlorophyll content, number of fruit/plant, fresh yield/plant, fresh and dry weight as well as plant length. Soil population of T. harzianum increased significantly during crop growth and was higher in plots inoculated with the pathogen. Highest concentrations (4.52 × 104 and 4.87 × 104 cfu/g soil) were found in plot soil where T. harzianum was applied in the soil, whilst maximum increase of Foc (3.02 × 104 and 3.05 × 104 cfu/g soil) was observed in plots that lacked any treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chilli (Capsicum annum L.), a crop of the family Solanaceae, is one of the most important vegetable and is primarily grown for its pungency, colour and spicy taste. Chillies are also a valuable source of vitamins A and C (Rahman et al. 2011) and are used in beverages, cosmetics and medicines. Chilli is a native plant of South America and was introduced in the late fifteenth century to India by Portuguese traders (Krishna 2003). It has become an important commercial crop in India, and the country is therefore regarded as the secondary hub of its diversity (IBPGR 1983). Presently, India leads the world in production, consumption and export of chilli, contributing nearly 25% of the total chilli exports. It produces 1.49 million tonnes of chillies from 0.77 million hectare land and 1.92 MT/ha of productivity (Anonymous 2014). In the recent years, chilli production has struck down due to numerous restraints like incessant monoculture, improper cultivation methods adopted by the farmers, unavailability of healthy seeds, adverse climate, poor soil conditions, pests and various fungal, bacterial and viral diseases (Abdel-Monaim 2012). Amongst all these constraints, wilt disease of chilli caused by Fusarium oxysporum Schlecht. emend. Synd. and Hans. f. sp. capsici Riv. is one of the most devastating disease in chilli growing regions worldwide (Abd-Allah et al. 2011) with 50–80% yield losses in case of high disease incidence (Madhavi et al. 2006).
The wilt fungus can attack the plant from seedling up to harvesting stage, infecting mainly the plant root system, and thereby, inhibiting water and nutrient transport (Miller et al. 1996) and subsequently disrupting the physiological processes necessary for adequate production and quality (Morid et al. 2012). Typical disease symptoms of Fusarium wilt of chilli includes yellowing of leaves, stunted growth and decaying and gradually the entire plant turns brown and recessed (Alegbejo et al. 2006). Infected plants also exhibit discrete morphological appearance as compared to abiotic stress caused due to the deterioration of vascular system by pathogenic obstruction (El-Kazzaz et al. 2008). The pathogen is soil-borne and systemic in nature and can survive in the absence of host by forming chlamydospores (resting spores) in the soil. Chlamydospores remain viable for many years and can also withstand high temperatures making the pathogen thermophilic and hard to diagnose and manage (Astrom and Gerhardson 1988).
Owing to high disease severity, farmers are bound to use chemicals, but they are expensive and have adverse effects on the environment (Hossain et al. 2013). Therefore, finding alternative measures of disease management such as biocontrol agents and botanicals is recommended. The major goal of implementing biological control methods is to decrease the density and activity of the pathogen (Singh et al. 2017). Besides, these methods of disease management are safe, economical and non-hazardous to living organisms (Elshahawy et al. 2017) including humans. Deployment of biocontrol agents, particularly Trichoderma spp., has been proven to be highly efficient in managing diseases in different crops (Monte and Llobell 2003; Reena et al. 2013) as they have a high adaptability, are fast growing and have a broad antibiotic spectrum. They also possess an inhibitory effect against major soil-borne pathogens including Fusarium spp., Phytophthora spp. and Pythium spp. (Mukherjee et al. 2012; El-Nagdi and Abd-El-Khair 2014; Woo et al. 2014). Trichoderma spp. activate induced resistance in plants and stimulate their growth through rhizospheric competition, antibiotic and enzyme production or mycoparasitism (Harman et al. 2004; Howell 2003). Mycoparasitic Trichoderma species grow chemotropically towards its host and through lectin mediated processes they get attached to the host hyphae and coil around them. They are penetrating the cells of the pathogen by means of cell wall degrading enzymes like chitinases and β-1, 3 glucanases (Harman 2000). Trichoderma spp. increase defence-related enzymes in plants and thus reduce incidence of damping off, root rots, wilts and other soil-borne diseases (Abd-El-Khair et al. 2019).
Compounds from plant origins like plant extracts from different plant parts and other secondary metabolites possessing antifungal properties are used against numerous plant diseases (Haikal 2007; Joseph et al. 2008) as they are effective, less noxious and more environmentally safe (Lee et al. 2007). Also, they are known to induce systemic resistance in plants by accumulating pathogenesis-related proteins (PR-proteins) (Kagale et al. 2004). Neem (Azadirachta indica) extracts contain alkaloids, glycosides, flavonoids and saponins (Pandey et al. 2014) which provide a broad spectrum of antimicrobial properties and thus have been promoted for its use in managing plant pathogens. Neem is capable of controlling several plant diseases, like Fusarium wilt by causing metabolic changes in plants, like phenol accumulation, induction of antioxidant defensive enzymes and phenol biosynthesis enzymes (Guleria and Kumar 2006; Aboellil 2007). Tetranortriterpenoids, such as gedunin (Sadre et al. 1983) and azadirachtin, found in neem extract have antifungal properties, resulting in a reduction of Fusarium wilt (Hanaa et al. 2011). Thus keeping in view the importance of biological methods of disease management, the present study was carried out to test the effectiveness of T. harzianum and leaf extract of A. indica in managing the Fusarium wilt disease of chilli.
Materials and methods
Experimental location
Experiments were carried out for 2 years, i.e. in 2018 and 2019, in the fields of Department of Plant Protection, Faculty of Agricultural Sciences, Aligarh Muslim University, Aligarh.
Soil sterilisation
Soil collected from the fields of the Department of Plant Protection was sandy clayey loam (64.8% sand, 17.9% clay and 17.3% silts) having pH 7.6 and 45% water holding capacity. Its organic carbon content was 0.017%, and organic matter content was 1.8%, respectively. Soil was amended with compost in the ratio of 3:1 (Rizvi et al. 2015a), steam-sterilised for 1 h at 15 kg/m2 pressure at 121 °C and then filled in pots (25 cm diameter and height; 2 kg soil/pot).
Sowing and maintenance of the chilli plants
Chilli seeds (C. annuum var. Pusa Jwala) were surfaced-sterilised in 0.01% mercuric chloride (HgCl2) solution for one minute and then gently rinsed thrice with double distilled water (DDW). Twenty seeds/pot were sown in the above-mentioned pots, and the plants were regularly watered to maintain sufficient moisture. Twenty-day-old seedlings were then transplanted in the field plots.
Isolation, identification and mass culturing of the wilt fungus
Fusarium oxysporum f. sp. capsici (Foc) was isolated from chilli plants exhibiting typical wilt symptoms in the fields. The plants were thoroughly washed under running tap water, and infected parts were cut into 4–5 mm size pieces, sterilised in 0.01% HgCl2 solution for one minute and gently washed 2–3 times with DDW before being aseptically transferred to the Petri plates containing sterilised potato dextrose agar (PDA). The inoculated plates were incubated in an incubator at 27 ± 2 °C. The fungal colonies developed after 2–3 days. The fungus was purified by isolation technique (Riker and Riker 1936), and pure cultures were sub-cultured on PDA slants for further studies. On the basis of cultural and morphological characters like white- and purple-coloured mycelia and presence of microconidia on short conidiophore (Soesanto et al. 2011), as examined under a compound microscope, it was identified as Fusarium oxysporum f. sp. capsici. The fungal isolate was also compared with the standard culture of F. oxysporum f. sp. capsici procured from the Division of Mycology and Plant Pathology, Indian Agricultural Research Institute (IARI), New Delhi, India.
The pathogen was mass multiplied on sorghum grains. Sorghum seeds were soaked in 5% sucrose and 0.003% chloramphenicol solution overnight (Whitehead 1957). The soaked seeds were then transferred to 500 ml conical flasks and autoclaved twice at 15 kg/m2 pressure at 121 °C for 15 min. The flasks were then inoculated with the mycelial disc (5 mm diameter) of pure culture of F. oxysporum f. sp. capsici and incubated for 8–10 days in an incubator having 27 ± 2 °C temperature. During incubation, the flasks were manually shaken on a daily basis for a few minutes to avoid clumping and to get early growth with uniform colonisation of seeds.
Pathogenicity test of the wilt fungus
A pathogenicity test was carried out in pots of the size 25 × 25 cm2 (diameter and height) filled with steam-sterilised soil mixed with compost in 3:1 ratio. The fungal inoculum was added to these pots (at 4 g/kg soil) and mixed thoroughly for uniform distribution. Surface sterilised seeds of chilli were sown in these pots (5seeds/pot), and five replicates were maintained. The pots were regularly watered with sterilised water to maintain sufficient moisture needed by the plants. Developing symptoms were observed 30 days after sowing, and the pathogen was re-isolated from roots and/or stem of infected plants, and thus, Koch’s postulates were verified (Ignjatov et al. 2012).
Procurement, maintenance and mass culturing of T. harzianum
A pure culture of T. harzianum was provided by the Division of Mycology and Plant Pathology, IARI. The culture was maintained on PDA, subcultured every 15 days and mass-multiplied in the same way as the pathogen.
Preparation of plant extract of A. indica
Hundred gram leaves of A. indica were thoroughly washed with double distilled water and dried at room temperature. Thereafter, they were surface-sterilised in 0.01% HgCl2 solution for one minute and with DDW. The material was then crushed with mortar and pestle by adding 100 ml of double distilled water. The crushed material was filtered through double layer of muslin cloth and the filtrate obtained was centrifuged at 5000 rpm for 15 min. After this, the filtrate was further placed on Whatman filter paper no. 1 (Shetty et al. 1989; Achimu and Schlosser 1992) and the collected plant extract was assumed to be 100% standard solution. The extract was heated to 40 °C for 5 min to avoid contamination (Jaganathan and Narasimhan 1988).
Application of T. harzianum, F. oxysporum f. sp. capsici and A. indica extract
T. harzianum was applied at 4 g/kg both in seed treatment of chilli and in soil application in field plots prior to sowing. For seed treatment with T. harzianum, 50 g of chilli seeds was coated with 20 mg of T. harzianum to obtain the treatment value of 4 g/kg seed, respectively. Jaggery solution (unrefined cane sugar; 5%) was applied to seeds as sticker for proper adhering of the biocontrol agent. Seed coating was done by thoroughly shaking 20 mg of fresh T. harzianum mycelium, 5 ml of jaggery solution and 50 g of chilli seeds in 100 ml flask, in order to get uniform coating of the seeds with the biocontrol agent.
In field experiments, the biocontrol agent was applied to the top layer of the soil (up to 8–10 cm depth). Average weight of the top soil from five plots of 2 × 2 m2area was estimated as 281 kg and accordingly T. harzianum suspension containing 1124 g of colonised sorghum seeds roughly ground and mixed in eight litres tap water was sprinkled in a plot. The next day, the soil was turned upside down with the help of spade. This was done to achieve a uniform distribution of the biocontrol agent in the plot soil, and the inoculation was done three days before the crop transplanting. The wilt pathogen was also applied in the field in the same manner 10 days prior to transplanting. To apply A. indica as seed treatment, chilli seeds were dipped in a 20% leaf extract (2 ml standard solution of the leaf extract mixed in 8 ml DDW) for half an hour prior to sowing.
Experimental design
During the 2 years of chilli cultivation, 20-day-old seedlings were transplanted in field in the second week of January. Each plot has 3 rows, and in each row 10 seedlings were transplanted, i.e. 30 plants/plot. No fertilizer was applied in the field throughout the cropping season. However, the field was regularly watered at a ten-day interval till the plants reached flowering stage. Once the plants started flowering, field was irrigated only twice: once at the fruit setting stage and second after the first harvesting was done. Experiments include F. oxysporum f. sp. capsici (Foc), T. (TH), A. indica (AI), seed treatment (st) and soil application (sa) in the following combinations (each in 3 replicates): (1) Control, (2) Foc, (3) TH (st), (4) TH (sa), (5) AI (st), (6) TH (st) + AI (st), (7) TH (sa) + AI (st); (8) TH (st) + Foc, (9) TH (sa) + Foc, (10) AI (st) + Foc, (11)TH (st) + AI (st) + Foc, (12) TH (sa) + AI (st) + Foc.
Soil populations of F. oxysporum f. sp. capsici and T. harzianum
Soil population of the pathogen and the biocontrol agent was recorded monthly from January to April. Therefore, the soil near the plant roots was dug 5–8 cm deep using hand digging trowel in the early hours of the day during the second week of the month. Extreme care was taken that roots do not get damage. Fifty gram soil was collected from the rhizosphere of 5 plants randomly selected from each 3 plots of a particular treatment and mixed to get a composite sample. It was sieved using a coarse sieve to separate the bigger soil particles and other debris present in the samples. The suspension was prepared in a conical flask by adding one g of the sieved soil to 9 ml DDW and was stirred for 10 min over a magnetic stirrer. One ml of this suspension was then added to a test tube having 9 ml DDW, and the process was repeated to get 10−4 dilution. Soil populations of both, the pathogen as well as the biocontrol agent, were determined in terms of colony forming units (cfu/g soil) by applying a dilution plate method. Suspensions having 10−4 dilution were spread on Petri-plates containing PDA and incubated at 27 ± 2 °C for 72 h. The colonies growing on PDA were then counted using a colony counter. Three replicate plates for each treatment were analysed.
Estimation of chlorophyll content
Chlorophyll content was recorded at the time of first harvest. Therefore, 5 plants/plot were randomly selected. The estimation of the chlorophyll content in chilli leaves was done according to the method of Hiscox and Israelstam (1979). Hundred mg of leaves from 3 month old plants was placed in a test tube having 7 ml dimethyl sulphoxide (DMSO) and left for 1 h to extract all the chlorophyll. The extract was transferred to another test tube, and the net volume was increased to 10 ml by adding DMSO. The samples were immediately assayed by transferring 3 ml chlorophyll extract into a cuvette. Optical density (OD) was measured with a spectrophotometer (SHIMADZU UV-2450) at 645 and 663 nm against the absolute DMSO, and total chlorophyll was calculated using Arnon’s Eq. (1949):
where A = absorbance of light at a particular wavelength, W = weight of the leaf tissue used, and V = final volume of the extract.
Plant growth parameters
For each treatment fruit/plant, yield/plant (g), plant fresh weight (g), plant dry weight (g), plant length (cm) and wilt severity were analysed. Severity of the wilt disease was observed on two-and-a-half-month-old plants (i.e. at fruit bearing stage) and was expressed using the formula:
It was then scored on 0–5 scale where, 0 = no wilt, 1 = 1–20%, 2 = 21–40%, 3 = 41–60%, 4 = 61–80% and 5 = 81–100%.
From each plot, 5 plants were randomly selected to record plant yield, fresh and dry weight of plants and plant length. Plant yield was summed up by adding three individual harvests each at three, three and a half and four months of plant age, while data for fresh and dry weight of plants and plant length were noted when the plants stopped flowering and bearing fruit, i.e. after the final third harvesting. For obtaining dry weight (including plant roots), plants were first sun-dried for three days and then placed in an oven for an hour at 80 °C to remove all the water content.
Statistical analysis
The data obtained were statistically analysed by the method of Panse and Sukhatme (1985). Critical difference (CD) was calculated at 1% probability level for laboratory experiments, whereas field trials were analysed at 5% probability level. To test for significant differences between treatments Turkey’s test was also used. All the statistical tests were performed using Minitab 11.0 software.
Results
Effect of T. harzianum and A. indica on wilt severity
One month after transplanting, characteristic symptoms of Fusarium wilt were observed on plants grown in plots inoculated with Foc with a diseases severity of 3.4 in 2018 and 3.7 in 2019, respectively. Initially, plants exhibited yellowing of leaves which later turned chlorotic and necrotic. Steadily the plants wilted, and approximately 8% of the total plants died. Application of T. harzianum and A. indica, either alone or in combination, significantly reduced the disease severity. In plots with soil application of T. harzianum, the wilt severity score was 2.2; in plots where seed treatment with T. harzianum was followed disease severity recorded was 2.5 and when A. indica was used for seed treatment, disease severity was 2.7 in the year 2018. In 2019, the scores were 2.7 when T. harzianum was mixed into soil, 2.8 when T. harzianum was used as seed treatment and 3.1 with A. indica extract. The lowest disease severity of 1.4 in 2018 and 1.7 in 2019 was observed in plants that received T. harzianum (sa) + A. indica (st) treatment when compared to other treatments (Table 1).
Effect of T. harzianum and A. indica on chlorophyll content
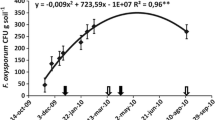
The wilt pathogen significantly reduced the chlorophyll content in chilli plants. In 2018, untreated plants grown in pathogen-inoculated plots contained the lowest amount of chlorophyll (1.056 mg/g), whereas plants from pathogen-inoculated plots treated with T. harzianum (sa) + A. indica (st) showed maximum chlorophyll content of 3.985 mg/g, (Table 1). In the following cropping year, the chlorophyll content was 0.996 mg/g in F. oxysporum f. sp. capsici-inoculated plots, while T. harzianum (sa) + A. indica (st) enhanced this value to 3.952 mg/g even in the presence of the pathogen. In both years, the highest chlorophyll content (4.219 mg/g and 4.286 mg/g) was recorded in T. harzianum (sa) + A. indica (st)-treated plants in field plots, not inoculated with the pathogen. Correlation analysis between chlorophyll content and wilt severity showed a negative relationship, where chlorophyll content was inversely proportional to wilt severity, i.e. greater the percentage of disease severity, higher the reduction in the plant’s chlorophyll content (Fig. 1).
Effect of T. harzianum and A. indica on plant yield parameters
Foc significantly reduced fruit/plant and fruit yield in both seasons. In the absence of Foc, all the treatments showed enhancement in fruit number and fruit yield. The best results were observed when T. harzianum (sa) + A. indica (st) was applied, giving maximum number of fruits (48.45 and 45.28) and yield (159.64 g and 154.11 g) (Table 2).
In Foc-inoculated plots, once again application of T. harzianum (sa) + A. indica (st) treatment showed best yield results with 42.97 and 40.31 fruits/plant and 145.97 g and 145.02 g of plant yield and was followed by T. harzianum (st) + A. indica (st) treatment (Table 2). T. harzianum (st) was shown to be the least effective treatment, with a minimum increase in fruit/plant (20.13 and 23.01 fruit) and yield/plant (102.57 g and 98.68 g) during both years.
Effect of T. harzianum and A. indica on plant weight and plant length
A significant decrease in fresh and dry weight and length of the plants was seen when they were grown in plots inoculated with Foc against non-inoculated control during the 2 cropping years (Fig. 2). In plots lacking Foc inoculation, application of T. harzianum (either as seed treatment or mixed in the soil) in combination with leaf extract of A. indica substantially increased the plant weight (both fresh and dry) along with the plant length during both the years except for T. harzianum (st) + A. indica (st) which in 2019 exhibited non-significant increase in plant fresh weight (84 g). In 2018, combined use of T. harzianum (sa) + A. indica (st) in pathogen-free soil elevated fresh weight up to 124 g, dry weight up to 41 g and plant length up to 89 cm, whereas in 2019 these figures were 118 g, 38 g and 85 cm. Seed treatment with leaf extract of A. indica did not increase fresh and dry weight compared to the control. Besides, it was also observed that seed treatment with A. indica did not show any improvement in plant length (61 and 62 cm) when compared with the control (62 and 68 cm) during both years.
Effect of various treatments on different growth parameters (fresh weight, dry weight and plant length) of chilli plants in 2018 (a) and 2019 (b). Error bars indicate ± standard deviation. Means marked with different letters are significantly different from each other according to Tukey’s test at P ≤ 0.05 (Foc = Fusarium oxysporum f. sp. capsici, TH = T. harzianum, AI = A. indica, st = seed treatment, sa = soil application)
In the presence of Foc, concomitant use of T. harzianum and A. indica increased plant fresh and dry weight and length which was observed to be higher than the control. Plots having T. harzianum (sa) + A. indica (st) exhibited significant increase in plant fresh (102 and 98 g) and dry weight (36 and 32 g) during 2 years of trial but showed no increase in plant length (75 cm) in 2018, while significant improvement with 70 cm length was recorded in 2019. Amongst all the treatments, A. indica (st) showed the least significant increase in plant fresh weight (37 and 39 g). During the first year, it did not affect dry weight (13 g) and plant length (48 cm) while in 2019 elevation in dry weight (12 g) and plant length (47 cm) were found to be significant (Fig. 2).
Effect of T. harzianum and A. indica on soil population of biocontrol agent and pathogen
The biocontrol agent as well as the pathogen showed manifold increase in their population throughout the crop growth. It was observed that the soil population increased to a peak in the third month (i.e. March) of crop growing when the crop was in the reproductive stage. In March, utmost significant increment in the soil population of T. harzianum (4.52 × 104 cfu/g soil in the first year and 4.87 × 104 cfu/g soil during second year) was recorded from the rhizospheric soil of chilli plants growing in the plots inoculated with Foc, where it was applied as soil treatment. This population significantly declined to 4.25 × 104 cfu/g soil in April 2018 and to 4.78 × 104 cfu/g soil in April 2019. The second highest increase in soil population of biocontrol agent was counted in plots having T. harzianum (st) in pathogen-inoculated soil (P ≤ 0.05). The lowest increase in T. harzianum population was recorded in the plots lacking Foc and having concomitant use of T. harzianum (st) + A. indica (st) as this treatment helped the population of the respective biocontrol agent reach the highest count up to 3.12 × 104and 3.04 × 104 cfu/g soil during the 2 years of field trials (Fig. 3). In both years, concomitant use of T. harzianum (sa) + A. indica (st) in Foc-inoculated plots showed a significant increase in biocontrol agent population in the month of April, whereas combined application of T. harzianum (st) + A. indica (st) in pathogen-infested plots caused a non-significant increase in T. harzianum population in April 2018 and a substantial increase in the pathogen population in April during the second year of crop cultivation. Besides, in 2019 T. harzianum population also increased non-significantly from March to April in the absence of Foc in plots having either soil application or seed treatment with T. harzianum. Interestingly, when comparing for the population built-up of the biocontrol agent between soil application and seed treatment, soil application of T. harzianum was found to be the better option.
Effect of various treatments on soil population of a T. harzianum and b F. oxysporum f. sp. capsici during the two years of crop growth. Error bars indicate ± standard deviation. Means marked with different letters are significantly different from each other according to Tukey’s test at P ≤ 0.05 (Foc = Fusarium oxysporum f. sp. capsici, TH = T. harzianum, AI = A. indica, st = seed treatment, sa = soil application)
Increase in the population built-up of biocontrol agent caused negative effect on the population count of the pathogen. Besides, A. indica extract also significantly inhibited the pathogen reproduction (P ≤ 0.05) by limiting its colonisation to 3.86 × 104 cfu/g and 4.02 × 104 cfu/g during the 2 years. The highest growth in Foc soil population was observed when no treatment was applied (Fig. 3) as the pathogen growth flourished to a maximum 4.28 × 104 cfu/g in 2018 and in 2019 attained cfu count 4.57 × 104 per g soil. In the month of April, this value significantly decreased to 3.66 × 104 cfu/g and 4.32 × 104 cfu/g during both the years. Concomitant application of T. harzianum (sa) + A. indica (st) caused maximum significant hindrance in pathogen growth and restricted its population to 3.02 × 104 cfu/g in 2018 and 3.05 × 104 cfu/g in 2019, respectively, which in April further deteriorated to 2.33 × 104 cfu/g and 2.47 × 104 cfu/g during the two consecutive years.
Discussion
Fusarium wilt has emerged as an important disease of chilli responsible for low yield in many chilli growing regions in India (Mishra et al. 2018). The present study has revealed significant decline in growth and yield of C. annuum var. Pusa Jwala due to the devastating effect of the wilt fungus, F. oxysporum f. sp. capsici. Biological control measures to manage plant diseases offer encouraging and cost-effective alternatives to chemical use (Khan et al. 2011). Researchers have shown the efficacy of Trichoderma spp. (Khan et al. 2017) and A. indica extract (Sarawaneeyaruk et al. 2015) in the biocontrol of plant diseases.
In the present study, T. harzianum resulted in increased plant growth and yield when applied in the soil or used as seed treatment in field plots lacking the wilt pathogen. It was in line with the findings of Windham et al. (1986) who reported T. harzianum promote plant growth even in the absence of the pathogen. Gajera et al. (2013) stated Trichoderma spp. helps control plant diseases by producing phytohormones that have plant growth promoting effect as one of the mechanisms. Hoyos-Carvajal et al. (2009) and Macías-Rodríguez et al. (2018) also found that Trichoderma spp. increased plant growth in crops such as tomato, cacao, cucumber and beans. This may be attributed to the fact that Trichoderma spp. colonise the plant roots and act as endophytic symbionts by secreting chemical stimulants beneficial for plants. These biocontrol and bio-stimulant properties have plant growth-promoting effects along with increased plant yield (Harman et al. 2012) and are known to reduce abiotic stress in plants and increase their nutrient uptake (Saba et al. 2012) by solubilising the soil phosphorus and producing siderophores (Li et al. 2018).
In our study, application of T. harzianum in Foc-inoculated plots exposed to different treatments persistently improved the overall growth and development of the plants in contrast to the untreated control. However, lower wilt severity with higher chlorophyll content, number of fruit and yield per plant and other growth aspects were seen in plots where T. harzianum was mixed in the soil rather than its seed treatment. The increase in plant’s chlorophyll content may be due to reduced blockage in the vascular system by the pathogen. Shahid and Khan (2016) reported that infection of M. phaseolina caused reduction in leaf pigments in mung bean. T. harzianum also increased other growth traits which may be due to decreased activity of the pathogen as it is known for its versatile action as plant growth promoter and antagonist of various soil-borne fungal pathogens such as Fusarium spp. Besides, Trichoderma spp. protects the plant against the pathogen infection immediately after the seed germinates (Sharma et al. 2014). The observations are in agreement with the findings of Bhat et al. (2016) who reported an increase in growth attributes, plant yield and a reduced disease incidence when T. harzianum was used in the fields. Godwin-Egein and Arinzae (2001) also found T. harzianum inhibits Fusarium spp. through competition, lysis and hyperparasitism and thereby restricts its mycelial growth. In our study, a rapid increase in the soil population of biocontrol agent was seen in both pathogen-free and inoculated soil over the initial population. This increase was due to availability of the host fungus in the inoculated soil on which the biocontrol agents multiply and was significantly greater in the pathogen-inoculated soil than in soil lacking Foc. In this regard, Khan and Anwer (2011) noticed antibiotics like trichodermin, harzianolide, viridian, trichodermol and gliotoxin produced by different Trichoderma spp. supress the disease by deteriorating germination and production of the pathogen spores. Amira et al. (2017) and López et al. (2019) observed increased sporulation of Trichoderma spp. around hyphae of Fusarium solani and other phytopathogens which caused a decrease in the pathogen population.
In our field trials, it was observed that extract of A. indica significantly accelerated plant length and yield against the Foc-inoculated soil. It could be due to azadirachtin in neem extract, which might enhance plant growth by delaying transformation of ammonium nitrogen into nitrate nitrogen (Akhtar 1999) and improves crop yield by providing essential nutrients to the plants (Lokanadhan et al. 2012). In pathogen-inoculated soil, the use of A. indica leaf extract promoted growth of chilli plants by exhibiting a deleterious impact on pathogen colonisation and thus caused yield enhancement. Srivastava and Yadav (2008) and Rizvi et al. (2015b) also suggested that the use of neem leaf extract obstructs development of F. oxysporum. This may be due to their better defence action against the wilt fungus as leaf extract of neem enhances protein and phenol contents, activities of peroxidase, chitinase, phenylalanine ammonia-lyase, polyphenol oxidase, β-1, 3-glucanase and other enzymes in the roots of treated plant (Gawande et al. 2015). Neem oil reduces the production of fusaric acid and thereby the pathogenic ability of the pathogen (Geraldo et al. 2010). Moreover, applying neem cake in crop fields can cause a reduction in the soil population of different soil-borne pathogens including Fusarium spp. (Champawat and Sharma 2003; Yelmame et al. 2010; Elnasikh et al. 2011) and promotes plant growth by helping in population built-up of several antagonistic microbes (Altintas and Bal 2008). Neem extract inhibited spore germination of several Fusarium spp. infecting different crops (Agbenin and Marley 2006; Haikal 2007).
In our study, integrated use of T. harzianum and A. indica leaf extract showed the highest control of the wilt problem in chilli plants apart from increasing the various growth attributes and crop yield and the same was reported by Raghu et al. (2018) who found T. harzianum and neem cake as the best measure for disease management. Rajani and Parakhia (2009) too found combined application of neem cake and T. harzianum effectively helped in managing a root rot disease of castor bean. Choudhary and Ashraf (2019) noticed these two as the best measure to control dry root rot of mung bean and found their combined application resulted in a maximum decrease in the soil population of the pathogen. Besides, their joint application showed enhanced chlorophyll content. Rizvi et al. (2015b) also found both T. harzianum and neem-based pesticides when used in combination elevate nutrient uptake in plants which in turn increase physiological parameters like photosynthetic activity and translocation of water, nutrients and other metabolic products and increase plants chlorophyll contents. In the present study, we tested only one strain of T. harzianum and A. indica leaf extract in managing the wilt disease of chilli. However, further studies may be carried out to explore local strains of T. harzianum isolated from the crop fields for their ability to suppress the F. oxysporum f. sp. capsici.
References
Abd-Allah EF, Hashem A, Al-Huqail A (2011) Biologically-based strategies to reduce postharvest losses of tomato. Afr J Biotechnol 32:6040–6044
Abd-El-Khair H, Elshahawy IE, Haggag HEK (2019) Field application of Trichoderma spp. combined with thiophanate-methyl for controlling Fusarium solani and Fusarium oxysporum in dry bean. Bull Nat Res Cent 43:19. https://doi.org/10.1186/s42269-019-0062-5
Abdel-Monaim MF (2012) Induced systemic resistance in tomato plants against Fusarium wilt diseases. Int Res J Microbiol 3:14–23
Aboellil AH (2007) Trilogy, a product of neem (Azadirachta indica) induces resistance in cucumber against Podosphaera xanthi. Res J Microbiol 2:402–414. https://doi.org/10.3923/jm.2007.402.414
Achimu P, Schlosser E (1992) Effect of neem extracts (Azadirachta indica A. Juss.) against downy mildew (Plasmopara viticola) of grapevine. In: Kleeberg H (ed) Practice oriented results on use and production of neem-ingredients. Druck and graphic, Giessen, pp 99–107
Agbenin NO, Marley PS (2006) In vitro assay of some plant extracts against Fusarium oxysporum f. sp. lycopersici causal agent of tomato wilt. J Plant Protect Res 46:215–220
Akhtar M (1999) Biological control of plant-parasitic nematodes in pigeonpea filed crops using neem-based products and manorial treatments. Appl Soil Ecol 12:191–195. https://doi.org/10.1016/s0929-1393(99)00009-8
Alegbejo M, Lawal A, Chindo P, Banwo O (2006) Outbreak of basal stem rot and wilt disease of pepper in northern Nigeria. J Plant Protect Res 46:7–13
Altintas S, Bal U (2008) Effects of the commercial product based on Trichoderma harzianum on plant, bulb and yield characteristics of onion. Sci Hortic 116:219–222. https://doi.org/10.1016/j.scienta.2007.11.012
Amira MB, Lopez D, Mohamed AT, Khouaja A, Chaar H, Fumanal B, Gousset-Dupont A, Bonhomme L, Label P, Goupil P, Ribeiro S (2017) Beneficial effect of Trichoderma harzianum strain Ths97 in biocontrolling Fusarium solani causal agent of root rot disease in olive trees. Biol Control 110:70–78. https://doi.org/10.1016/j.biocontrol.2017.04.008
Anonymous (2014) Directorate of economics and statistics and national horticulture board, Ministry of agriculture, Government of India, New Delhi
Arnon DI (1949) Copper enzymes isolated chloroplasts, polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15. https://doi.org/10.1104/pp.24.1.1
Astrom B, Gerhardson B (1988) Differential reactions of wheat and pea genotypes to root inoculation with growth-affecting rhizosphere bacteria. Plant Soil 109:263–269. https://doi.org/10.1007/bf02202093
Bhat MN, Mesta R, Yenjerappa ST, Tatagar MH, Sardana HR, Singh D, Vennila S, SabirMobin, N (2016) Biological control of Fusarium wilt of chillies using Trichoderma spp. Indian J Hortic 73:74–77. https://doi.org/10.5958/0974-0112.2016.00021.9
Champawat RS, Sharma RS (2003) Integrated management of nursery diseases in brinjal, chilli, cabbage and onion. J Mycol Plant Pathol 33:290–291
Choudhary A, Ashraf S (2019) Utilizing the combined antifungal potential of Trichoderma spp. and organic amendments against dry root rot of mungbean. Egypt J Biol Pest Control. https://doi.org/10.1186/s41938-019-0187-8
El-Kazzaz MK, El-Fadly GB, Hassan MAA, El-Kot GAN (2008) Identification of some Fusarium spp. using molecular biology techniques. Egypt J Phytopathol 36:57–69
El-Nagdi WMA, Abd-El-Khair H (2014) Biological control of Meloidogyne incognita and Fusarium solani in dry common bean in the field. Arch Phytopathol Plant Protect 47:388–397. https://doi.org/10.1080/03235408.2013.809931
Elnasikh MH, Osman AG, Sherif AM (2011) Impact of neem seed cake on soil microflora and some soil properties. J Sci Technol 12:144–150
Elshahawy IE, Saied N, Abd-El-Kareem F, Morsy A (2017) Field application of sclerotial mycoparasites as biocontrol agents to Stromatinia cepivora, the cause of onion white rot. J Plant Pathol 99:391–401. https://doi.org/10.4454/jpp.v99i2.3888
Gajera H, Domadiya R, Patel S, Kapopara M, Golakiya B (2013) Molecular mechanism of Trichoderma as bio-control agents against phytopathogen system-a review. Curr Res Microbiol Biotechnol 1:133–142
Gawande AD, Gangopadhyay S, Rakhonde PN, Mane SS (2015) Evaluation of plant extracts for induction defence against Fusarium wilt in chickpea. Indian Phytopathol 68:264–269
Geraldo MRF, Arroteia CC, Kemmelmeier C (2010) The effects of neem [Azadirachta indica A. Juss (meliaceae)] oil on Fusarium oxysporum f. sp. medicagenis and Fusarium subglutinans and the production of fusaric acid toxin. Adv Biosci Biotechnol 1:1–6. https://doi.org/10.4236/abb.2010.11001
Godwin-Egein MI, Arinzae AE (2001) Antagonism between Trichoderma harzianum Rifai and Fusarium oxysporum schlecht emend sny and hans. J Mycol Plant Pathol 31:22–30
Guleria S, Kumar A (2006) Azadirachta indica leaf extract induces resistance in sesame against Alternaria leaf spot disease. J Cell Mol Biol 5:81–86
Haikal NZ (2007) Improving biological control of Fusarium root-rot in cucumber (Cucumis sativus L.) by allelopathic plant extracts. Int J Agric Biol 9:459–461
Hanaa RF, Abdou ZA, Salama DA, Ibrahim MA, Sror HA (2011) Effect of neem and willow aqueous extracts on Fusarium wilt disease in tomato seedlings: induction of antioxidant defensive enzymes. Ann Agric Sci 56:1–7. https://doi.org/10.1016/j.aoas.2011.05.007
Harman GE (2000) Myths and dogmas of biocontrol: changes in perceptions derived from research on Trichoderma harzianum T-22. Plant Dis 84:377–393. https://doi.org/10.1094/pdis.2000.84.4.377
Harman GE, Herrera-Estrella AH, Horwitz BA, Lorito M (2012) Special issue: Trichoderma-from basic biology to biotechnology. Microbiology 158:1–2. https://doi.org/10.1099/mic.0.056424-0
Harman GE, Howell CR, Viterbo A, Chet I, Lorito M (2004) Trichoderma species-opportunistic, avirulent plant symbionts. Nat Rev Microbiol 2:43–56. https://doi.org/10.1038/nrmicro797
Hiscox JD, Israelstam GF (1979) A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57:1332–1334. https://doi.org/10.1139/b79-163
Hossain MM, Hossain N, Sultana F, Islam SMN, Islam MS, Bhuiyan MKA (2013) Integrated management of Fusarium wilt of Chickpea (Cicer arietinum L.) caused by Fusarium oxysporum f. sp. ciceri with microbial antagonist, botanical extract and fungicide. Afr J Biotechnol 12:4699–4706. https://doi.org/10.5897/AJB2013.12503
Howell C (2003) Mechanisms employed by Trichoderma species in the biological control of plant diseases: the history and evolution of current concepts. Plant Dis 87:4–10. https://doi.org/10.1094/pdis.2003.87.1.4
Hoyos-Carvajal L, Orduz S, Bissett J (2009) Growth stimulation in bean (Phaseolus vulgaris L.) by Trichoderma. Biol Control 51:409–416. https://doi.org/10.1016/j.biocontrol.2009.07.018
IBPGR (1983) Genetic resources of capsicum: a global plan of action. International board for plant genetic resources, Rome
Ignjatov M, Milosevic D, Nikolic Z, Gvozdanovic VJ, Jovicic D, Zdjelar G (2012) Fusarium oxysporum as causal agent of tomato wilt and fruit rot. Pestic Phytomed 27:25–31. https://doi.org/10.2298/pif1201025i
Jaganathan R, Narasimhan V (1988) Effect of plant extracts/ products on two fungal pathogens of finger millet. Indian J Mycol Plant Pathol 181:250–254
Joseph B, Dar MA, Kumar V (2008) Bioefficacy of plant extracts to control Fusarium solani f. sp. melongenae incitant of brinjal wilt. Global J Biotechnol Biochem 3:56–59
Kagale S, Marimuthu T, Thayumanavan B, Nandakumar R, Samiyappan R (2004) Antimicrobial activity and induction of systemic resistance in rice by leaf extract of Datura metel against Rhizoctonia solani and Xanthomonas oryzae pv. oryzae. Physiol Mol Plant Pathol 65:91–100. https://doi.org/10.1016/j.pmpp.2004.11.008
Khan MR, Anwer A (2011) Fungal bioinoculants for plant disease management. In: Paul M, Clinton M, Ahmad I (eds) Microbes and microbial technology. Springer, USA, pp 447–488
Khan MR, Anwer MA, Shahid S (2011) Management of grey mould of chickpea, Botrytis cinerea with bacterial and fungal biopesticides using different modes of inoculation and application. Biol Control 57:13–23. https://doi.org/10.1016/j.biocontrol.2011.01.004
Khan MR, Shahid S, Mohidin FA, Mustafa U (2017) Interaction of Fusarium oxysporum f. sp. gladioli and Meloidogyne incognita on gladiolus cultivars and its management through corm treatment with biopesticides and pesticides. Biol Control 115:95–104. https://doi.org/10.1016/j.biocontrol.2017.09.010
De Krishna A (2003) Capsicum: the genus Capsicum. Taylor and Francis, London and New York
Lee SO, Choi GJ, Jang KS, Lim HK, Cho KY, Kim JC (2007) Antifungal activity of five plant essential oils as fumigant against postharvest and soil-borne plant pathogenic fungi. Plant Pathol J 23:97–102. https://doi.org/10.5423/ppj.2007.23.2.097
Li YT, Hwang SG, Huang YM, Huang CH (2018) Effects of Trichoderma asperellum on nutrient uptake and Fusarium wilt of tomato. Crop Protect 110:275–282. https://doi.org/10.1016/j.cropro.2017.03.021
Lokanadhan S, Muthukrishnan P, Jeyaraman S (2012) Neem products and their agricultural applications. J Biopestic 5:72–76
López AC, Alvarenga AE, Zapata PD, Luna MF, Villalba LL (2019) Trichoderma spp. from Misiones, Argentina: effective fungi to promote plant growth of the regional crop Ilexparaguariensis St. Hil. Mycology 10:210–221. https://doi.org/10.1080/21501203.2019.1606860
Macías-Rodríguez L, Guzmán-Gómez A, García-Juárez P, Contreras-Cornejo HÁ (2018) Trichoderma atroviride promotes tomato development and alters the root exudation of carbohydrates, which stimulates fungal growth and the biocontrol of the phytopathogen Phytophthora cinnamomi in a tripartite interaction system. FEMS Microbiol Ecol 94:1–11. https://doi.org/10.1093/femsec/fiy137
Madhavi M, Kumar PC, Reddy DRR, Singh TVK (2006) Integrated management of wilt of chilli incited by Fusarium solani. Indian J Plant Prot 34:225–228
Miller AS, Rowe RC, Riedel RM (1996) Fusarium and verticillium wilts of tomato, potato, pepper, and eggplant. The Ohio State University, Columbus, pp 1–3
Mishra A, Ratan V, Trivedi S, Dabbas MR, Shankar K, Singh AK, Dixit S, Srivastava Y (2018) Survey of anthracnose and wilt of chilli: a potential threat to chilli crop in central Uttar Pradesh. J Pharmacogn Phytochem 7:1970–1976
Monte E, Llobell A (2003) Trichodermain organic agriculture. In: Proceeding V World Avocado congress (Actas V Congreso Mundial Del Aquacade), Granada-Malaga, Spain, pp 725–733
Morid B, Hajmansoor S, Kakvan N (2012) Screening of resistance genes to Fusarium root rot and Fusarium wilt diseases in tomato (Lycopersico nesculentum) cultivars using RAPD and CAPs markers. Euro J Exp Biol 2:931–939
Mukherjee M, Mukherjee PK, Horwitz BA, Zachow C, Berg G, Zeilinger S (2012) Trichoderma-plant-pathogen interactions: advances in genetics of biological control. Indian J Microbiol 52:522–529. https://doi.org/10.1007/s12088-012-0308-5
Pandey G, Verma KK, Singh M (2014) Evaluation of phytochemical, antibacterial and free radical scavenging properties of Azadirachta indica (neem) leaves. Int J Pharm Pharm Sci 6:444–447
Panse VG, Sukhatme PV (1985) Statistical methods for agricultural workers. Indian council of agricultural research, New Delhi
Raghu S, Benagi VI, Nargund VB, Jayalakshmi K (2018) Effect of chemicals, bio control agents and soil amendments integrated in different management modules on plant growth, yield and incidence of Fusarium wilt of chilli in Karnataka. Int J Chem Stud 6:870–877
Rahman MM, Rahman SMM, Akter A (2011) Comparative performance of some insecticides and botanicals against chilli fruit borer (Helicoverpa armigera). J Exp Sci 2:27–31
Rajani VV, Parakhia AM (2009) Management of root rot disease (Macrophomina phaseolina) of castor (Ricinus communis) with soil amendments and biocontrol agents. J Mycol Plant Pathol 39:290–293
Reena A, Anitha M, Aysha OS, Valli S, Nirmala P, Vinothkumar P (2013) Antagonistic activity of Trichoderma viride isolate on soil-borne plant pathogenic fungi. Int J Bioassays 2:294–297
Riker AJ, Riker RS (1936) Introduction to research on plant diseases. John S Swift, St. Louis
Rizvi R, Ansari RA, Iqbal A, Ansari S, Sumbul A, Mahmood I, Tiyagi SA (2015a) Dynamic role of organic matter and bioagent for the management of Meloidogyne incognita–Rhizoctonia solani disease complex on tomato in relation to some growth attributes. Cogent Food Agric 1:1068523. https://doi.org/10.1080/23311932.2015.1068523
Rizvi R, Singh G, Safiuddin ARA, Tiyagi SA, Mahmood I (2015b) Sustainable management of root-knot disease of tomato by neem cake and Glomus fasciculatum. Cogent Food Agric 1:1008859. https://doi.org/10.1080/23311932.2015.1008859
Saba H, Vibhash D, Manisha M, Prashant KS, Farhan H, Tauseef A (2012) Trichoderma: a promising plant growth stimulator and biocontrol agent. Mycosphere 3:524–531. https://doi.org/10.5943/mycosphere/3/4/14
Sadre NL, Deshpande VY, Mendulkar KN, Nandal DH (1983) Male antifertility activity of Azadirachta indica in different species. In: Natural pesticides from Neem tree (Azadirachta indica A. Juss) and other tropical plants. Deutsche Gesellschaft for Technische Zusammenarbeit (GTZ), Eschborn, Germany, pp. 473–482.
Sarawaneeyaruk S, Krajangsang S, Pringsulaka O (2015) The effects of neem extract and azadirachtin on soil microorganisms. J Soil Sci Plant Nutr 15:1071–1083. https://doi.org/10.4067/s0718-95162015005000075
Shahid S, Khan MR (2016) Biological control of root-rot on mung bean plants incited by Macrophomina phaseolina through microbial antagonists. Plant Pathol J 15:27–39. https://doi.org/10.3923/ppj.2016.27.39
Sharma P, Sharma M, Raja M, Shanmugam V (2014) Status of Trichoderma research in India: a review. Indian Phytopathol 67:1–19
Shetty SA, Prakash HS, Shetty HS (1989) Efficacy of plant extracts against seed-born infection of Triconiella padwickii in paddy (Oryza sativa). Can J Bot 67:1956–1958. https://doi.org/10.1139/b89-248
Singh JK, Kumar M, Kumar S, Kumar A, Mehta N (2017) Inhibitory effect of botanicals on growth and sporulation of fusarium oxysporum inciting wilt of Chilli (Capsicum annuum L.). J Pharmacogn Phytochem 6:2199–2204
Soesanto L, Utami DS, Rahayuniati RF (2011) Morphological characteristics of four Trichoderma isolates and two endophytic Fusarium isolates. Can J Sci Ind Res 2:294–306
Srivastava DK, Yadav HL (2008) Antifungal activity of some medicinal plants against Fusarium oxysporum f. sp. lycopersici. Indian Phytopathol 61:99–102
Whitehead MD (1957) Sorghum grain a medium suitable for the increases of inoculum for studies of soil-borne and certain other fungi. Phytopathology 47:450
Windham K, Allen MC, Haenselor CM (1986) Antagonistic action of Trichoderma on Rhizoctonia and other soil fungi. Phytopathology 25:1244
Woo SL, Ruocco M, Vinale F, Nigro M, Marra R, Lombardi N, Pascale A, Lanzuise S, Manganiello G, Lorito MM (2014) Trichoderma-based products and their widespread use in agriculture. Open Mycol J 8:71–126. https://doi.org/10.2174/1874437001408010071
Yelmame MG, Mehta BP, Deshmukh AJ, Patil VA (2010) Evaluation of some organic extracts in in-vitro to control Fusarium solani causing chilli wilt. Int J Pharma Bio Sci 1:1–4
Acknowledgements
We are grateful to the Department of Plant Protection, Aligarh Muslim University, Aligarh, for providing us all the available necessary facilities required to complete this work and University Grants Commission, New Delhi, India, for funding our research work.
Funding
This study was funded by University Grants Commission, India (Grant Number: F1-17.1/2013–14/MANF-2013–14-MUS-UTT-20789/(SA-III/Website).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by AJ. The first draft of the manuscript was written by AJ, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
This article does not contain any studies with human participants or animals performed by the author.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jamil, A., Musheer, N. & Ashraf, S. Antagonistic potential of Trichoderma harzianum and Azadirachta indica against Fusarium oxysporum f. sp. capsici for the management of chilli wilt. J Plant Dis Prot 128, 161–172 (2021). https://doi.org/10.1007/s41348-020-00383-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-020-00383-1