Abstract
The efficacy of four bi- and tri-cyclic sesquiterpenes, namely inuloxins A, B and C and α-costic acid, extracted from aerial parts of Dittrichia viscosa collected in Algeria was assessed against the cowpea seed beetle Callosobruchus maculatus. The compounds were evaluated for their effect on adult mortality, oviposition and adult emergence. Three concentrations (100, 50 and 25 µg/ml) of each compound were tested with chickpea being used as the test plant. Complete adult mortality (100%) was achieved at only 1 day after exposure to inuloxins B and C and α-costic acid with LC50s less than 36 µg/ml. Lethal concentration values (LC50) were determined as 30.4, 35.2, 31.6 and 29.4 µg/ml, respectively, for inuloxins A, B and C and α-costic acid. Oviposition and F1 progeny emergence were significantly reduced (27% and 73%, respectively) after treatment with D. viscosa compounds. Our results also revealed that oviposition, adult emergence and sex ratio varied with the sex of the treated mating partner suggesting that the test compounds may have acted as male (or indirect female) chemosterilants resulting in reduced fecundity and fertility of untreated females that mated with treated males.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The benefits of growing grain legumes (pulses) are multiple and increasingly recognized. These staple crops have been domesticated as a food source since millennia. They are thought to have formed important dietary components of early civilizations in the Mediterranean and the Middle East (Smýkal et al. 2015). So far pulses continue to be an important component of the world agriculture to such an extent that the UN declared 2016 the International Year of Pulses to raise awareness about their important role in improving food security, alleviating poverty, combating malnutrition and promoting agricultural sustainability (FAO 2015).
However, intensification and yield improvement of pulses require the control of diseases and insect pests which are among the major constraints affecting crop productivity. Field-to-store chrysomelids can be particularly harmful. The cowpea seed beetle (CSB) also called the cowpea weevil, Callosobruchus maculatus Fabricius (Coleoptera: Chrysomelidae), is a cosmopolitan field-to-store pest that can inflict heavy damage to grain legumes. Sharma and Thakur (2014) considered that all legumes are vulnerable to the CSB attack both in field and storage. Cowpea and chickpea have been, however, reported as its elective hosts (Panzarino et al. 2011; Haouel-Hamdi et al. 2017). With cowpea, from a field infestation of only 1–2%, seed losses may reach up to 80% after 6–8 months storage (Youdeowei 1989 as cited in Sallam 2000). Total seed loss (100%) has also been reported to occur in a few months storage in West Africa (Lienard and Seck 1994). Likewise, C. maculatus was reported as a major storage pest of chickpea altering germination and nutritional properties of stored chickpea seeds (Sarwar 2010; Haouel-Hamdi et al. 2017).
The use of chemical insecticides and fumigants has helped to minimize the impact of stored-product insects. However, increased concerns about their adverse effects on human health, environment and food safety have spurred the search for more reliable and eco-friendly control measures.
In recent years, there was an increasing research effort focused on development of biological pesticides including plant-derived insecticides. Accordingly, a variety of plant species were assessed to control C. maculatus worldwide and the most investigated were Azadirachta indica, Aloe vera, Capsicum nigrum, Allium sativum, Mentha spp., Lantana spp., Eucalyptus spp. and Lavandula spp. Among the Mediterranean flora, Dittrichia viscosa L. Werner Rodolfo Greute 1973 (formerly Inula viscosa L. Aiton) is a remarkable plant species with potential active principles. Dittrichia viscosa is a sturdy wild perennial shrub widely distributed around the Mediterranean basin. In Algeria, this species, commonly called “Magramane”, is very common in the Tell region where it is frequently found in wastelands. Active ingredients of extracts of D. viscosa were successfully tested on a range of crop pests and diseases. Thus, chloroformic rinsates of D. viscosa leaves strongly inhibited colony-forming ability of several phytopathogenic bacteria (89–100% inhibition) including Pseudomonas syringae pv. garcae, and pv. syringae, P. syringae subsp. savastanoi and Xanthomonas campestris pv. pelargonii and also showed significant antifungal activity against disease-causing fungi such as Geotrichum candidum, Nectria haematococca var. cucurbitae and Ustilago maydis by inhibiting their mycelial radial growth within a range of 73–100% (Stavrianakou et al. 2010). Ethanol leaf extract of D. viscoa was assessed for aphicidal activity and caused 60% mortality of the green peach aphid, Myzus persicae Sulzer, 72 h after exposure (Madanat et al. 2016). In nematicidal tests conducted by Oka et al. (2001), almost no Meloidogyne javanica J2 was recovered from sand treated with a mixture of costic acid and isocostic acid extracted from leaves of D. viscosa at a concentration of 200 mg/kg. In pot trial, almost no galling (galling index = 0) was found in cucumber roots grown in sand treated with the mixture at any of the concentrations tested (50, 100 and 200 mg/kg). Organic extracts of D. viscosa showed allelopathic activity towards Peganum harmala L. and Silybum marianum L. and significantly affected their seed germination and seedling’s growth with leaves and flowers organic extracts being the most toxic (Omezzine et al. 2011). With regard to parasitic weeds, two of the presently tested metabolites (i.e. inuloxins A and C) extracted from aerial parts of D. viscosa caused up to 100% inhibition of seed germination of Orobanche crenata Forsk. and Cuscuta campestris Yuncker (Andolfi et al. 2013).
From the organic extract of D. viscosa, three new bi- and tri-cyclic sesquiterpene lactones, named inuloxins A–C, were isolated together with α-costic acid (Fig. 1), another well-known sesquiterpene. The inuloxins A, B and C were characterized as new germacrenolide, eudesmanolide and seco-eudesmanolide lactones (Andolfi et al. 2013). As the absolute stereochemistry is very important for the biological activity of natural compounds (Evidente et al. 2011, 2013; Cimmino et al. 2017), the absolute configuration of inuloxins A and C was determined as 7R, 8R, 10S (Santoro et al. 2015) and 5S, 7S, 8S, 10S (Evidente et al. 2016; Johnson et al. 2018) by applying chiroptical [optical rotatory dispersion (ORD), electronic circular dichroism (ECD) and vibrational circular dichroism (VCD)] and computational methods.
Structures of inuloxins A–C (1–3) and α-costic acid (Andolfi et al. 2013)
To the best of our knowledge, no published report documenting the efficacy of compounds from D. viscosa towards the CSB is yet available. In the light of the results of the aforementioned works, the present study was designed to test the hypothesis that pure compounds from D. viscosa may also have insecticidal activity towards C. maculatus. A very recent research which revealed the efficiency of D. viscosa compounds, in particular α- and γ-isomers of costic acid, towards granary weevil Sitophilus granarius (L.) (Rotundo et al. 2019) might support our presumption. Here, we report on the extraction and purification of bioactive compounds from D. viscosa and the evaluation of their efficacy against the cowpea seed beetle under controlled conditions.
Materials and methods
Laboratory rearing of Callosobruchus maculatus
Callosobruchus maculatus (male and female adults) were placed in glass jars containing 250–300 g of healthy and untreated chickpea seeds (Cicer arietinum L.) and covered with muslin net. The jars were placed in an incubator set at a temperature of 28 ± 1 °C and 70 ± 5% relative humidity (RH) in the darkness. Under such conditions, C. maculatus develops a generation every 23–25 days. Newly emerged adults were recovered regularly for new infestations in new breeding jars to ensure a continuous supply of chrysomelids, and progenies (0–2-h-old males and females) were removed gradually and used for the different bioassays in the laboratory.
Plant material
Aerial parts of D. viscosa were collected during the growing season of 2014 around Algiers and identified. A voucher specimen of the plant is deposited at the herbarium of the Department of Botany of ENSA (Ecole Nationale Supérieure d’Agronomie), Algiers, Algeria. The leaves were air-dried in the shade in natural laboratory conditions and then finely grinded using a regular blender, and the resulting powder was stored in vacuum bags until use.
Metabolites extraction
General
The optical rotation was measured in chloroform (CHCl3) solution by a Jasco P-1010 digital polarimeter. Infrared (IR) spectra were recorded as deposit glass film on a Thermo Electron Corporation Nicolet 5700 FT-IR spectrometer, and ultraviolet (UV) spectra were measured in methanol (MeOH) on a Jasco V-530 spectrophotometer. Proton nuclear magnetic resonance (1H NMR) spectra were recorded at 600/125 mega-hertz (MHz) in deuterated chloroform on Bruker spectrometers. The same solvent was used as internal standard. Electrospray ionization mass spectroscopy (ESI–MS) spectra were recorded on Agilent Technologies 6120 Quadrupole liquid chromatography–mass spectrometry (LC/MS) instrument. Analytical and preparative thin-layer chromatography (TLC) was performed on silica gel (Kieselgel 60, F254, 0.25 and 0.5 mm, respectively, Merck, Darmstadt, Germany) plates. The spots were visualized by exposure to UV and/or by spraying first with 10% sulphuric acid (H2SO4) in methanol and then with 5% phosphomolybdic acid in ethanol, followed by heating at 110 C for 10 min. Column chromatography was performed using silica gel (Merck, Kieselgel 60, 0.063–0.200 mm) and a LIChroprep RP-18 column (40–63 μm, 10 × 240 mm) (Merck, Darmstadt, Germany).
Extraction and purification of inuloxins A–C and the α-costic acid
Extraction procedure was conducted as described by Andolfi et al. (2013). Briefly, the plant material (D. viscosa powder) was extracted in water–methanol [H2O–CH3OH (1:1), 1L], and the methanolic aqueous phase was in turn extracted three times with dichloromethane (CH2Cl2) (3 × 400 ml). The CH2Cl2 phases were combined, dried using sodium sulphate (Na2SO4) and evaporated under reduced pressure. The residue was purified by chromatography first on a silica gel column and then on TLC yielding inuloxins A–C (1–3) as homogeneous oil (with a yield of 0.026%, 0.042%, 0.0080%, for g of dried plant, respectively) as well as the α-costic acid with a yield of (0.11%). The identification of inuloxins A–C and α-costic acid was done by comparing their physis (ORD) and spectroscopic (1H NMR and ESI–MS) data with those of reference compounds (Andolfi et al. 2013).
Mortality determination of C. maculatus
Filter paper (Whatman® GF/A) in Petri dishes (Ø = 9 cm) were impregnated with 1 ml of acetone in which the following amounts of the compounds (inuloxins A–C and α-costic acid) were separately diluted: 100, 50 and 25 µg. The control consisted in only acetone (1 ml per Petri dish). The solvent was allowed to evaporate for 10 min, and then, 20 C. maculatus adults (0–2 h old) were placed in each dish. Beetles exposed for 24 h to the compounds were subsequently transferred to new Petri dishes containing 50 g of untreated chickpea seeds and incubated (28 °C, 75% RH) in darkness. The insect mortality was assessed at 1, 3, 5 and 7 days after exposure, and the lethal concentration 50 (LC50) values were calculated by probit analysis. Seven days were considered to avoid natural mortality, knowing that C. maculatus adults (males and females) both have an average life time of 7 days under laboratory conditions (Fatima et al. 2016). The beetles were considered dead when they showed no response after being prodded gently with a pin. The experiment was conducted with five replicates.
Oviposition experiment, offspring production and sex ratio
Because of insufficient amounts of inuloxin B, only inuloxins A, C and α-costic acid could be tested in this set of experiments. Chickpea seeds (100 g) were placed in glass Petri dishes (Ø = 14 cm) and then treated with the compounds, applied separately, dissolved in acetone to dose rates corresponding to their LC50 values. Six millilitres were applied per dish, and the same amount of acetone without compounds was used for the control.
The chickpea seeds were thoroughly mixed to ensure uniform treatment with the compounds, and the solvent was allowed to evaporate for 10 min. Afterwards, 25 females (0–2 h old) were introduced into each dish. Dishes were then incubated (28 ± 1 °C and 70 ± 5% RH) in darkness. After 24 h of exposure, the females were removed from the dishes and transferred to new ones together with 100 g of untreated fresh chickpea seeds and 25 untreated virgin males. Four replications were set for each treatment in a complete randomized design.
The total number of eggs laid was counted, using a stereoscope microscope, on the 15th day when the maximum number of eggs was laid and no more oviposition occurred. The same experiment was performed under similar conditions except that only males were treated with the compounds. In summary, the following combinations were considered: treated females mated with untreated males (TF × UM); untreated females mated with treated males (UF × TM); untreated females mated with untreated males (control) (UF × UM).
To assess progeny production, a number of chickpea seeds were maintained at the end of both experiments, so as to keep only 20 eggs per dish considered as the potential number of adults expected to emerge in subsequent generation. The control dishes contained the same number of eggs, but coming from untreated pairs. Counts of the newly emerged beetles were made after 3 days, and percentage of progeny suppression in each treatment was evaluated as the difference between the number of eggs laid and the emerged adults. Sex ratio of the emerged adults was also determined.
Data analyses
All experiments were conducted in a complete randomized design with 5 replications for adult mortality test and 4 for others. Adult mortality data were corrected with Abbott (1925) formula. The compounds concentrations needed to induce 50% (LC50) mortality of C. maculatus were determined using a regression-probit method. Data analyses were performed using Statistica (V.8.5, 2014) software. Data were subjected to one-way ANOVA, and mean separations (at p ≤ 0.05) were determined by the Duncan’s test, where significant differences were found.
Results
Plant metabolites
Inuloxins A, B and C and α-costic acid were isolated as oily homogeneous compounds from the organic extract of D. viscosa as previously reported. They showed the same physis (ORD) and spectroscopic data (1H NMR and ESI–MS) identical to those reported in the literature (Andolfi et al. 2013).
Adult mortality
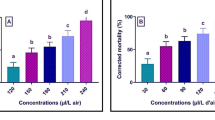
The cumulative mortality percentages presented in Fig. 2 show that response of C. maculatus adults to treatments by the D. viscosa compounds varied with increasing concentrations and periods after exposure. With the highest concentration (100 μg/ml), a total mortality of 100% was obtained at only 1 day after exposure to inuloxins B, C and α-costic acid, while a rate of 80.2% was obtained with inuloxin A which remained unchanged until the 7th day. With the two other concentrations (25 μg/ml and 50 μg/ml), adult mortality increased proportionally with the time after exposure. Three days after exposure resulted in a slight increase in mortality rates, not statistically different from the control, in all treatments with the compounds.
Conversely, significant increases were obtained on the 5th and the 7th days. The most notable mortality increases were recorded on the 7th day with a better efficiency of concentration of 50 μg/ml. Thus, at 2-day interval (the 5th to the 7th days), mortality rates increased from 21 to 93% (with inuloxin A), from 35 to 79% (with inuloxin C), from 58 to 91% (with α-costic acid) and from 27 to 42% with inuloxin B. With the lowest concentration (25 μg/ml), the same trend was observed, but with less efficacy and a better performance of inuloxin C. Thus, the mortality rates from the 5th to the 7th day went from 20 to 57% (with inuloxin C), from 12 to 39% (with α-costic acid), from 8 to 32% (with inuloxin A) and from 24 to 41% with inuloxin B. Not more than 2% mortality was observed in the control treatment. Furthermore, probit analysis showed that LC50 values of inuloxins A, B, C and α-costic acid against adults of C. maculatus were 30.4, 35.2, 31.6 and 29.4 µg/ml, respectively.
Effect on oviposition, progeny production and sex ratio
Effect on oviposition
Results shown in Table 1 indicate that oviposition performance varied with the sex of the treated mating partner. When treated females mated with untreated males (TF × UM), apart from inuloxin C, the other two compounds had no effect on the number of eggs laid since no significant differences were detected between treatments with inuloxin A and α-costic acid and the untreated control, whereas inuloxin C caused an oviposition value almost double compared to that of the control (97% increase, p < 0.001).
Conversely, when untreated females mated with treated males (UF × TM), all the three compounds (inuloxins A and C and α-costic acid) significantly reduced the number of eggs laid compared to the untreated control (p < 0.001) with oviposition deterrence almost equal ranging from 23 to 27%.
Adult offspring production
From results displayed in Table 2, it can be seen that adult offspring production was significantly affected whatever the sex of the treated mating partner. When treated females mated with untreated males (TF × UM), treatments with all the compounds resulted in significantly lower numbers of F1 adults emerged (p < 0.001) compared to the untreated control. Progeny suppression rate was the highest with α-costic acid and the lowest with inuloxin C. When untreated females mated with treated males (UF × TM), significantly much lower numbers of adults emerged were obtained with inuloxin C and α-costic acid (p < 0.001) with progeny suppression rates of 70% and 65%, respectively. Contrarily, inuloxin A had no significant effect.
Sex ratio
The results displayed in Table 3 show that adult offspring population sex ratio varied in response to variation in the sex of the treated mating partner. Sex ratio was not affected when females treated with all of the compounds mated with untreated males. Conversely, lower sex ratio was found when untreated females mated with males treated with α-costic acid and inuloxin C, although significant difference (p < 0.001) was detected only for the latter.
Discussion
Control of stored-product insects using efficient and safe methods that preserve product quality and consumer health is increasingly sought after. The present study was conducted with the aim to evaluate sesquiterpenes extracted from D. viscosa, a widespread Mediterranean plant, for their insecticidal activity towards the cowpea seed beetle, C. maculatus. To the best of our knowledge, no published record exists on toxicity of compounds from D. viscosa towards this chrysomelid. Consequently, comparative data are not available. Several studies, however, documented other Dittrichia spp.—stored-product insects combinations (Kima et al. 2003; Chandel and Singh 2017; Rotundo et al. 2019).
In our study, the efficacy of D. viscosa compounds as protectant of chickpea seeds against C. maculatus was assessed considering adult mortality, oviposition and F1 progeny emergence. Adult mortality is a key parameter for assessing the efficacy of a given stored-seed protectant. Mortality prevents adults from mating and laying eggs and thereby limits the population build-up of C. maculatus in seed storage. Our results showed that the test compounds (inuloxins B and C and α-costic acid) acted rapidly causing complete (100%) adult mortality only 1 day after exposure to the highest concentration (100 µg/ml). In contact toxicity tests conducted by Rotundo et al. (2019), n-hexane extract from D. viscosa aerial parts (leaves and flowers) proved highly bioactive towards the granary weevil, Sitophilus granarius (L.). GC–MS analysis of active fractions strongly suggested costic acid and, in particular, α- and γ-isomers, as the compound responsible for the contact toxicity of n-hexane extract against adults of granary weevil. Accordingly, an increasing insect mortality was observed in response to fractions containing increasing amounts of α- and γ-costic acid isomers. The calculated dose of α- and γ-costic was, respectively, 3.40 and 9.57 μg/adult for fraction 7 (mortality 100%), 2.36 and 6.42 μg/adult for TLC subfraction #5 (mortality 60%), and 0.94 and 2.57 μg/adult for TLC subfraction #4 (mortality 20%).
Compared to our findings, other species from the genus Inula (Dittrichia) showed less efficacy towards several stored-product pests even when tested with higher concentrations. Hence, root extract from Inula helenium L., used at a dose of 50 mg/100 µl of methanol, caused 0.0% mortality of Sitophilus oryzae L. adults within the first 3 days after exposure and resulted in 37% mortality on the 4th and last day of mortality assessment (Kima et al. 2003). In the same study, mortality rates on Callosobruchus chinensis L. were 27% and 30% at 1 and 2 days after treatment, respectively, which might suggest that C. chinensis was more sensitive. Extract from aerial parts of Inula racemosa Hook. achieved 66.59% mortality of C. chinensis L. infesting stored chickpea seeds in Uttar Pradesh in India after one-day exposure to the test material applied at up to 2% dose rate (Chandel and Singh 2017). Furthermore, the Neem, Azadirachta indica A. Juss., known for its high toxicity to C. maculatus, required higher concentrations and more time to achieve 100% adult mortality. For instance, neem leaf extracts applied at a concentration of 3.8 g/l achieved complete adult mortality of C. maculatus on the 3rd day after treatment (Paranagama et al. 2003). Similarly, chrysomelid mortality reached 65–100% at 3–5 days after surface treating cowpea seeds with neem seed oil (Ivbijaro 1990).
Our results showed also that oviposition and F1 progeny emergence were significantly reduced (up to 27% and 73%, respectively) after treatment with D. viscosa compounds which is consistent with several previous studies that reported significant reductions in the number of eggs laid and F1 offspring produced following treatments of C. maculatus with different plant extracts or essential oils (Raja et al. 2001; Boeke et al. 2004; Rahman and Talukder 2006; Demnati and Allache 2014). Obviously, under the effect of the test material, most of the eggs laid failed to complete their life cycle, hence leading to a reduced progeny production. Raja et al. (2001) linked reduction in adult emergence to low egg hatchability and assumed that oil vapours of the plants they tested probably diffused into eggs and affected the physiological and biochemical process associated with embryonic development. Compounds used in the present study are bi- and tri-cyclic sesquiterpene lactones, named inuloxins A–C and α-costic acid (Andolfi et al. 2013), isolated from the aerial parts of the Asteraceae member, Dittrichia (= Inula) viscosa. Sesquiterpene lactones are one of the most prevalent and biologically significant classes of secondary metabolites and are most prevalent in the Asteraceae (Chadwick et al. 2013). Sesquiterpenes have been shown to exert a wide range of effects on stored-product insect pests including contact toxicity, growth alteration, repellent, deterrent and antifeedant activities, as well as reproduction inhibition by ovicidal and larvicidal effects (Tripathi et al. 2000; García et al. 2003; Nawrot and Harmatha 2012; Aliyu et al. 2014; Russo et al. 2015; Wei et al. 2018).
Most striking finding of the present study is the difference between the responses of males and females of C. maculatus with males exhibiting greater sensitivity to treatment with the test compounds. Support for this statement was found when the oviposition was significantly reduced only when untreated females mated with treated males (UF × TM), but not in the opposite case. Also, significantly much lower F1 progeny emergence could be obtained from eggs laid by untreated females that mated with males treated with 2 out of the 3 compounds tested (inuloxin C and α-costic acid). Moreover, sex ratio was only affected when untreated females mated with treated males, with globally a female-biased offspring sex ratio.
All these data suggest that the compounds tested may have induced male sterility resulting in reduced fecundity and fertility of untreated females that mated with the treated males. Several plant extracts and essential oils have been reported to act as chemosterilants against insects (Saxena et al. 1977; Singh 2017) by reducing insect oviposition, egg hatchability, postembryonic development and progeny production. In addition to direct toxicity, chemosterilization by natural products can be an attractive method of control by mass-producing sterile males for release. Another explanation could be an indirect effect of treated males on the female ovaria. We can hence assume that sesquiterpenes present on the aedeagus of the treated males may be introduced into the female body cavity upon mating and thus cause ovicidal effects that are stronger than when the females were treated alone. Further investigations are needed for a better understanding of this phenomenon and its use for control purposes.
Findings of the present study clearly suggest that effective protection of stored chickpea seeds can be expected through the combined action of reduced egg laying, high adult mortality and low emergence rates. They also demonstrated that metabolites extracted from D. viscosa exerted a significant toxic activity on different developmental stages of C. maculatus under controlled conditions. Therefore, the use of these metabolites or the whole plant of D. viscosa could help protect stored chickpea seeds or other pulses.
As a prerequisite for their use in practice for control of stored-product pests, botanical insecticides must meet a number of criteria and hence must mainly exhibit low mammalian toxicity and low (or nil) residues on foods, have low adverse effects on grain handling and quality properties, pose no environmental hazards, be easy to extract and formulate, have slow degradation of active ingredients, require only limited skills to use and be acceptabile in terms of price (Kis-Tamas, 1990 as cited in Peter et al. 1999; Okonkwo, 2005; Rozman, 2015). Among these requirements, safety-related aspects are of utmost importance.
Despite the lack of evidence-based information on safety, it is often assumed that “natural” plant extracts are safe and hence less hazardous to beneficial and non-target organisms, including man, and to environment compared to synthetic chemicals (Peter et al. 1999, Guleria and Tiku, 2009, Adeyemi 2010, Rozman 2015). Such assumption might come from the fact, as stated by Peter et al. (1999), that most of the plant species that have been studied are those used locally as culinary spices or in traditional medicine, and therefore, some researchers infer that the material is safe to use as an insecticide. Many authors, however, urged to carry on toxicological testing if plant fragments or extracts are to be promoted for use as stored foodstuffs protectants (Peter et al. 1999; Okonkwo 2005).
Despite the increasing interest in D. viscosa benefits and uses, very little is known about its toxicity. A few preliminary laboratory and field-based investigations suggest that the plant might be considered as toxicologically safe. Thus, flavonoid-rich acetone extract from D. viscosa aerial parts collected in Jordan showed no toxic effect on male rats particularly on their reproductive system (Abbas et al. 2017). In toxicity tests conducted by Ouahchia et al. (2017), methanolic extracts of leaves and flowers, collected in Algeria, orally administered to Swiss albinos mice showed no acute toxicity or sub-chronic toxicity at the doses of 400 mg/kg and 800 mg/kg. In a study conducted by Sofou et al. (2017), costic acid isolated from leaves of D. viscosa collected from five different areas of Crete was tested for its efficacy against Varroa destructor, an ectoparasite of the European honey bee. In field tests, costic acid as well as the total extract was active against the parasite but showed no toxic effect on the bees. Moreover, costic acid was also not cytotoxic to human umbilical vein endothelial cells (HUVEC) at concentrations of up to 230 micromolar (μM). The authors concluded that this compound could be used as a safe, environment friendly and low-cost efficient agent for controlling varroosis in Apis mellifera colonies.
Likewise, plant materials and their extracts intended to be used as stored-product protectants should not affect the quality, flavour, aroma or smell of the stored products. According to Okonkwo (2005), only a few investigations have done taste trials to ascertain if the stored product treated with an insecticidal plant is acceptable to consumers. This seems to be the case with D. viscosa. Thus, despite abundant literature regarding the use of D. viscosa essential oil or extracts as storage protectants, none of the papers examined so far have considered the effects that they may have on tainting and residues in stored food commodities.
Therefore, standardized laboratory tests need to be undertaken to quantify such effects, given for instance the fact that the strong smell of D. viscosa plant material is likely to impart odour to treated stored foodstuffs.
More significant use of plant products in practice requires the use of appropriate methods of application. Here too, information is very limited even at the farmer level. Peter et al. (1999) reported that very few systematic studies have been conducted to determine how farmers utilize plant protectants, the methods employed and their effectiveness. The same authors strongly recommend to conduct on-farm or simulated field trials and urged to facilitate and resource this area of research as a priority. In laboratory-based trials, plant material was used either as direct admixture with the commodity (mainly as dried powdered material) or as a solvent extracts or essential oil applied to seeds or to filter papers. Fumigant toxicity tests using essential oils from different plants were also conducted (Rajendran and Sriranjini 2007). Nonetheless, appropriate application methods and delivery systems need to be developed for practical use of plant-based insecticides for stored-product pests control.
References
Abbas MA, Hameed NA, Al-Khateeb EH, Al Haj RM-N (2017) Evaluation of toxicity and fertility effects of Inula viscosa aerial parts extract in male rats. Jordan J Biol Sci 11(2):147–154
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18:268–267. Reprinted in J Am Mosq Control Assoc 1987, 3(2):302–303
Adeyemi MMH (2010) The potential of secondary metabolites in plant material as deterents against insect pests: a review. Afr J Pure Appl Chem 4(11):243–246
Aliyu AB, Adeyemi MMH, Abdulkadir I, Dambatta MB, Amupitan JO, Oyewale AO (2014) Antifeedant activity of Vernonia oocephala against stored product pest Tribolium casteneum (Herbst). Bangladesh J Sci Ind Res 49:243–248
Andolfi A, Zermane N, Cimmino A, Avolio F, Boari A, Vurro M, Evidente A (2013) Inuloxins A–D, phytotoxic cyclic sesquiterpene lactones produced by Inula viscosa: potential for broomrapes and field dodder management. Phytochemistry 86:112–120
Boeke SJ, Barnaud C, van Loon JJA, Kossou DK, Van Huis A, Dicke M (2004) Efficacy of plant extracts against the cowpea beetle, Callosobruchus maculatus. Int J Pest Manag 50:251–258
Chadwick M, Trewin H, Gawthrop F, Wagstaff C (2013) Sesquiterpenoids lactones: benefits to plants and people. Int J Mol Sci 14:12780–12805
Chandel BS, Singh A (2017) Entomotoxicity of Chromolaena odorata, Tagetes minuta and Reichardia tingitana in suppressing oviposition and adult emergence of Callasobruchus chinensis (L.) infesting stored chickpea seeds in U.P. Int J Zool Stud 2:38–44
Cimmino A, Masi M, Evidente M, Superchi S, Evidente A (2017) Application of Mosher’s method for absolute configuration assignment to bioactive plants and fungi metabolites. J Pharm Biomed Anal 144:59–89
Demnati F, Allache F (2014) Effect of Verbascum sinuatum (Scrophulariaceae) on oviposition of Callosobruchus maculatus (Bruchidae). J Crop Prot 3:327–334
Evidente A, Andolfi A, Cimmino A (2011) Relationships between the stereochemistry and biological activity of fungal phytotoxins. Chirality 23:674–693
Evidente A, Cimmino A, Andolfi A (2013) The effect of stereochemistry on the biological activity of natural phytotoxins fungicides, insecticides and herbicides. Chirality 25:59–78
Evidente M, Santoro E, Petrovic AG, Cimmino A, Koshoubu J, Berova N, Evidente A, Superchi S (2016) Absolute configurations of phytotoxic inuloxins B and C based on experimental and computational analysis of chiroptical properties. Phytochemistry 130:328–334
Fatima SM, Usman A, Sohail K, Afzaal M, Shah B, Adnan M, Ahmed N, Junaid K, Shah SRA, Inayat-ur-Rahman (2016) Rearing and identification of Callosobruchus maculatus (Bruchidae: Coleoptera) in chickpea. J Entomol Zool Stud 4(2):264–266
Food and Agriculture Organization of the United Nations [FAO] (2015) Action plan for the international year of pulses: nutritious seeds for a sustainable future. http://www.fao.org/3/a-bl213e.pdf. Accessed 27 Mar 2018
García M, Sosa ME, Donadel OJ, Giordano OS, Tonn CE (2003) Effects of some sesquiterpenes on the stored-product insect Tenebrio molitor (Coleoptera: Tenebrionidae). Rev Soc Entomol Argent 62:17–26
Guleria S, Tiku AK (2009) Botanicals in pest management: current status and future perspectives. In: Peshin R, Dhawan AK (eds) Integrated pest management: innovation-development process. Springer, Berlin, pp 317–329. https://doi.org/10.1007/978-1-4020-8992-3_12
Haouel-Hamdi S, Titouhi F, Boushih E, Dhraief MZ, Amri M, Mediouni-Ben Jemâa J (2017) Population demographic traits and reproductive parameters of the seed beetle Callosobruchus maculatus infesting stored lentil and chickpea commodities. Tunis J Plant Prot 12:67–81
Ivbijaro MF (1990) The efficacy of seed oils of Azadirachta indica A. Juss and Piper Guineense Schum and Thonn on the control of Callosobruchus maculatus F. Int J Trop Insect Sci 11:149–152
Johnson JL, Raghavan V, Cimmino A, Moeini A, Petrovic AG, Santoro E, Superchi S, Berova N, Evidente A, Polavarapu PL (2018) Chiroptical spectroscopic analyses of absolute configurations of chiral molecules with multiple stereogenic centers without prior knowledge of the relative configurations: a case study of inuloxin C. Chirality. https://doi.org/10.1002/chir.23013
Kima SI, Roha JY, Kima DH, Leeb HS, Ahna YJ (2003) Insecticidal activities of aromatic plant extracts and essential oils against Sitophilus oryzae and Callosobruchus chinensis. J Stored Prod Res 39:293–303
Lienard V, Seck D (1994) Review of control methods against Callosobruchus maculatus (F.) (Coleoptera: Bruchidae), a pest of grains of cowpea Vigna unguiculata (L.) Walp. in tropical Africa. Insect Sci Appl 15:301–311
Madanat MH, Al Antary TM, Abu Zarqa MH (2016) Toxicity of six ethanol plant extracts against the green peach aphid Myzus persicae Sulzer (Homoptera: Aphididae). Fresenius Environ Bull 25:706–718
Nawrot J, Harmatha J (2012) Phytochemical feeding deterrents for stored product insect pests. Phytochem Rev 11:543–566
Oka Y, Ben-Daniel BH, Cohen Y (2001) Nematicidal activity of powder and extracts of Inula viscosa. Nematology 3(8):735–742. https://doi.org/10.1163/156854101753625245
Okonkwo EU (2005) Plant materials used for controlling insect pests of stored products in Nigeria, Families Annonaceae, Piperaceae, and Rutaceae. J Herbs Spices Med Plants 11(1–2):47–69. https://doi.org/10.1300/j044v11n01_02
Omezzine F, Rinez A, Ladhari A, Farooq M, Haouala R (2011) Allelopathic potential of Inula viscosa against crops and weeds. Int J Agric Biol 13:841–849
Ouahchia C, Cherif HS, Hamaidi-Chergui F, Marzen L, Deradji S, Hemma R, Nouar N, Saidi F (2017) Acute and subacute toxicity of Inula viscosa L. (Dittrichia viscosa L.) methanolic extracts. AgroBiologia 7(2):562–573
Panzarino O, Bari G, Vernile P, De Lillo E (2011) Preliminary results on the preferences of Callosobruchus maculatus on Apulian germplasm of Cicer arietinum. Redia J Zool 94:45–52
Paranagama PA, Adhikari AACK, Abeywickrama KP, Bandara KANP (2003) Evaluation of volatile constituents of Neem (Azadirachta Indica A. Juss.) leaf extracts against Callosobruchus Maculatus (F.). J Natl Sci Found Sri Lanka 31:445–458
Peter G, Moss C, Dales M, Fidgen A, Evans J, Gudrups I (1999) The use of spices and medicinals as bioactive protectants for grains. Food and Agriculture Organization of the United Nations Rome and Natural Resources Institute Chatham Maritime Chatham, Kent UK. Fao Agricultural Services Bulletin No. 137. http://www.fao.org/3/x2230e/x2230e00.htm#con. Accessed 1 May 2019
Rahman A, Talukder FA (2006) Bioefficacy of some plant derivatives that protect grain against the pulse beetle, Callosobruchus maculatus. J Insect Sci 6:1–10
Raja N, Albert S, Ignacimuthu S, Dorn S (2001) Effect of plant volatile oils in protecting stored cowpea Vigna unguiculata (L.) Walpers against Callosobruchus maculatus (Coleoptera: Bruchidae) infestation. J Stored Prod Res 37:127–132
Rajendran S, Sriranjini V (2007) Plant products as fumigants for stored-product insect control. J Stored Prod Res 44:126–135
Rotundo G, Paventi G, Barberio A, De Cristofaro A, Notardonato I, Russo MV, Germinara GS (2019) Biological activity of Dittrichia viscosa (L.) Greuter extracts against adult Sitophilus granarius (L.) (Coleoptera, Curculionidae) and identification of active compounds. Sci Rep 9:6429. https://doi.org/10.1038/s41598-019-42886-4
Rozman V (2015) Control of stored products pests by natural products. IOBC-WPRS Bull 111:295–299
Russo S, Cabrera N, Chludil H, Yaber-Grass M, Leicach S (2015) Insecticidal activity of young and mature leaves essential oil from Eucalyptus globulus Labill. against Tribolium confusum Jacquelin du Val (Coleoptera: Tenebrionidae). Chil J Agric Res 75:375–379
Sallam MN (2000) Insect damage: damage on post-harvest. In: AGSI/FAO (ed) Insect damage: post-harvest operations, pp 1–38. http://www.fao.org/fileadmin/user_upload/inpho/docs/Post_Harvest_Compendium_-_Pests-Insects.pdf. Accessed 19 May 2018
Santoro E, Mazzeo G, Petrovic AG, Cimmino A, Koshoubu J, Evidente A, Berova N, Superchi S (2015) Absolute configurations of phytotoxins seiricardine A and inuloxin A obtained by chiroptical studies. Phytochemistry 116:359–366
Sarwar M (2010) Some possibilities on the effectiveness of plant powders as grain protectants against cowpea weevil, Callosobruchus maculatus (Fabricius) Walp. (Coleoptera: Bruchidae) infestation in chickpea. Int J Agron Plant Prod 1:45–50
Saxena BP, Koul O, Tikku K, Atal CK (1977) A new insect chemosterilant isolated from Acorus calamus L. Nature 270:512–513
Sharma S, Thakur DR (2014) Comparative developmental compatibility of Callosobruchus maculatus on cowpea, chickpea and soybean genotypes. Asian J Biol Sci 7:270–276
Singh S (2017) Natural plant products—as protectant during grain storage: a review. J Entomol Zool Stud 5:1873–1885
Smýkal P, Coyne CJ, Ambrose MJ et al (2015) Legume crops phylogeny and genetic diversity for science and breeding. Crit Rev Plant Sci 34:43–104
Sofou K, Isaakidis D, Spyros A, Büttner A, Giannis A, Katerinopoulo HE (2017) Use of costic acid, a natural extract from Dittrichia viscosa, for the control of Varroa destructor, a parasite of the European honey bee. Beilstein J Org Chem 13:952–959. https://doi.org/10.3762/bjoc.13.96
Stavrianakou S, Liakopoulos G, Miltiadou D, Markoglou AN, Ziogas BN, Karabourniotis G (2010) Antifungal and antibacterial capacity of extracted materiel from non-glandular and glandular leaf hairs applied at physiological concentrations. Plant Stress 4:25–30
Tripathi AK, Prajapati V, Aggarwal KK, Khanuja SPS, Kumar S (2000) Repellency and toxicity of oil from Artemisia annua to certain stored-product beetles. J Econ Entomol 93:43–47
Wei XM, Guo SS, Yan H, Cheng XL, Wei F, Du SH (2018) Contact toxicity and repellency of the essential oil from Bupleurum bicaule helm against two stored product insects. J Chem 2018:5830864
Acknowledgements
This work was partly supported by the General Directorate of Scientific Research and Technological Development (DGRSDT, Algeria) within the MEDILEG Project ARIMNet Call 2011. The first author mobility within a PhD program was supported by a Grant from the Algerian Ministry of Higher Education and Scientific Research (MESRS—Algeria).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gueribis, F., Zermane, N., Khalfi-Habess, O. et al. Bioefficacy of compounds from Dittrichia viscosa (Asteraceae) as protectant of chickpea seeds against the cowpea seed beetle Callosobruchus maculatus (Coleoptera: Chrysomelidae). J Plant Dis Prot 126, 437–446 (2019). https://doi.org/10.1007/s41348-019-00240-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-019-00240-w