Abstract
The Box tree pyralid Cydalima perspectalis Walker (Lepidoptera: Crambidae) is one of the accidentally introduced organisms that became invasive and established in Europe within a few years. Thus, eco-friendly preparations are required, which should be accessible, affordable, easy usable and suitable for the application in private and public areas. Therefore, the effects of the plant extract formulation NeemAzal®-T/S (active ingredient Azadirachtin A) and the commercial preparation Nemastar® (entomopathogenic nematode species Steinernema carpocapsae) were investigated on C. perspectalis larvae in laboratory bioassays and field trials. When NeemAzal®-T/S-treated leaf discs were consumed by larvae in the laboratory, a significant effect on mortality and feeding activity was noted after 14 days of exposure. At this time, 47–62% of the larvae had already died and less than 10% of larvae were still feeding. Application of different S. carpocapsae suspensions (10–200 EPN/100 μl, i.e. per larva) demonstrated a high susceptibility of both tested larval instars (2nd: 10–75% and 4th: 45–100% mortality). In field trials neither the application of Nemastar® nor the use of NeemAzal®-T/S caused mortality rates comparable to those generated by the treatment with plant protection products based on Bacillus thuringiensis. In conclusion, there is a higher variability in terms of the effects of the investigated agents compared to the constant effectiveness of B. thuringiensis. But their use would be possible if individual feeding damage and proper surveillance will be considered in order to be capable of repeating the application timely and a combination with further measures ought to take place.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Organisms are frequently brought into new environments by humans. After importing, the species are capable of reaching different levels of invasion success (introduced, established and pest) as defined by Williamson and Fitter (1996a). Just a small proportion of introduced species have the potential to become established, and only a small amount of these established species will become a serious pest (Williamson and Fitter 1996b). The Box tree pyralid Cydalima perspectalis (Walker 1859) is one of these accidentally introduced, therefore non-native and invasive organisms that have accomplished an establishment in Germany and Europe. Further, it can be classified as pest, because of causing negative economic effects as well. According to the law, trees belong to the owner of the land on which they grow. Their damage or destruction therefore affects the value of the land of which they form a part. Cemeteries and large park facilities with partially several-kilometre-long Buxus hedges are strongly affected by a reduction in economic value, depending on feeding damage caused by C. perspectalis (Leuthardt 2013). Moreover, C. perspectalis continuously spreads (Nacambo et al. 2014) and constantly causes serious damage on ornamental plantings and natural Buxus stands in Europe (John and Schumacher 2013).
Buxus is a traditionally important evergreen perennial shrub (Oberdorfer et al. 2001) of widespread use. It is planted in large numbers in private and public areas. It is also very popular on cemeteries for adorning graves and enclosing irrigation systems. Most of the plantings are structurally important subdivision elements. Especially in these public urban areas, the application of chemical insecticides often may be necessary, but is disliked or forbidden. Therefore, cemeteries are frequently becoming a source of infection for plants growing nearby, caused by renewed attacks. C. perspectalis develops exclusively on plants of the genus Buxus and herbivorous; competitors are lacking in Europe (Wan et al. 2014). Only a small amount of sucking organisms utilizes Buxus plants as feeding source, for instance the Boxwood psyllid (Psylla buxi), the Boxwood spider mite (Eurytetranychus buxi) and diverse scales (van Trier and Hermans 2007), but the damage caused is purely aesthetic and not as destructive as by C. perspectalis or fungal Buxus diseases.
Because of this pest characterisation, efficient pest management is required. Preparations based on Bacillus thuringiensis are eco-friendly and specifically effective against lepidopteran larvae. Many strains produce endotoxins as pre-proteins which accumulate in crystalline inclusions in the bacterium. After ingestion, these crystals are dissolved by proteases in the gut of the host insect producing active proteins which interfere with the metabolism of the target insect (Schnepf et al. 1998). Immediate feeding stop occurred, induced by rapid paralysis of mouthparts and gut, followed by gut and haemolymph poisoning, which leads to the death of the insect after a few days (Burges 1982; Schmutterer and Huber 2005). B. thuringiensis is highly effective against C. perspectalis and registered. However, two main problems arise: If treatment has to be done, public areas have to be closed for visitors until the spray deposit is completely dry (24 h). Furthermore, as the only available biological control measure, the risk of resistance formation exists with frequent application (Schnepf et al. 1998).
Thus, further eco-friendly preparations are needed which should be accessible, affordable, easy to use and suitable for the application in public areas, e.g. cemeteries, monasteries and public parks as well as house gardens. Since the beginning of biological control in the 1980s, numerous publications and reviews have been written regarding insect control and pointed out many advantages of Neem products and entomopathogenic nematodes (Schmutterer 1990; Kaya and Gaugler 1998; Schmutterer and Huber 2005). These agents are well investigated, commercially available and are used against various pests.
Formulations with Azadirachtin, the most important active ingredient in seeds of the Neem tree (Azadirachta indica A. Juss (Meliacea)), were tested and successfully used against various insect pests. Benelli et al. (2017) reviewed the potential of Neem-based products and pointed out that on the basis of its properties like effective and eco-friendly features, including little non-target effects, multiple mechanisms of action, low cost and easy production, Neem-based products can serve as an advantageous option to build newer and safer arthropod control tools. It serves as insect growth regulator and feeding deterrent to many insects (Isman 2006) and there are anti-ovipositional, fecundity- and fitness-reducing properties as well (Schmutterer 1990).
Entomopathogenic nematodes of the families Steinernematidae and Heterorhabditidae are mutualistically associated with bacteria of the genus Xenorhabdus, serving as vectors. After penetrating a host, this complex causes a fast insect pathogenicity and can only be active against a specific host range (Schmutterer and Huber 2005). In many countries, they are considered as invertebrate beneficials and no registration is necessary.
The aim of this study was to assess the potential of other biological control agents as alternative or supplemental method to B.t., which are already commercially available on the market as well as non-target and bystander friendly to the greatest possible extent. Therefore, the biological control agents NeemAzal®-T/S and Nemastar® were investigated on their potential to affect larvae of C. perspectalis using laboratory bioassays and field trials.
Materials and methods
Biological control agents
The plant extract formulation NeemAzal®-T/S (NA), containing 1% of the active ingredient Azadirachtin A, oils and surface-active agents, was kindly provided by the company Trifolio-M GmbH (Lahnau, Germany). The commercial preparation Nemastar®, containing entomopathogenic nematodes (EPN) of the species Steinernema carpocapsae, as well as the wetting agent (wa) BREAK-THRU® and an adhesive agent (aa) were kindly provided from the company e-nema GmbH (Schwentinental, Germany). The commercially available Bacillus thuringiensis (BT) preparations XenTari®, containing the subspecies aizawai, and the product Dipel Es®, containing the subspecies kurstaki, were kindly donated by Cheminova Deutschland GmbH & Co. KG (Stade, Germany).
For conducting the field trials, treatments were made on-site following recommendations by the producers (Table 1). They were randomly assigned in a split plot design in four replications at the test area (Fig. 1). The EPN variant incorporated an adjuvant consisting of gel, dispersant and the wetting agent BREAK-THRU®, which was pre-dissolved in ethanol. It was ensured that EPN did not settle to the bottom by constant movement of the suspension in the laboratory and the sprayer in field. Treatments with water served as control. Applications were conducted using pressure sprayers (APV® Akku Roll: 55 l and 7 bar for EPN, filter was removed; Gloria® prima 5 (Type 39 TE): 5 l and 3 bar for NA and BT). Plants were sprayed with defined volumes (Table 1), and it was assured that they were dripping wet.
Buxus hedges (left) and experimental design (right) of field trials at the cloister in Seligenstadt conducted in the years 2012 and 2013, including four application sites per variant (coloured) and defined plots to assess the level of infestation before the application (a–h). For full names of variants see Table 1
Plant material
Plants of Buxus sempervirens L. var. arborescens L. were purchased from a Box tree nursery (Baumschule Atrops, Rheurdt, Germany). A part of the plants were potted and stored in the greenhouse until used for bioassays. Remaining plants had been planted on a testing field for conducting field trials. In addition, cut plant material (twigs of 30 cm length) of the large-leaf variant B. sempervirens var. rotundifolia was purchased and stored in a cooling chamber at 10 °C until cutting leaf discs for bioassays.
C. perspectalis larvae
C. perspectalis have been reared under laboratory conditions since 2010. Adult moths were kept in rearing cages (length 40 cm × width 36 cm × height 50 cm) with diluted sugar (5%) for feeding. Short Box tree twigs (15–20 cm) were regularly introduced into the cages for egg deposition. C. perspectalis egg masses (≤ 24 h old) were collected from the offered Box tree twigs. Hatched larvae were fed on watered Box tree twigs in transparent Perspex cylinders (40 cm height × 19.5 cm diameter) with textile covers. The different larval stages used for the laboratory bioassays (2nd, 3rd and 4th) were determined by the average time of moulting until the particular larval stage, initiated from egg masses of equal age. The duration of larval development was adapted from own previous investigations.
Laboratory bioassays
NeemAzal®-T/S (NA) bioassay with 3rd larval instar
The effect of NA was investigated on 3rd instar larvae by feeding them with treated leaf discs. Therefore, the required amount of NA was emulsified with distilled water to prepare different concentrations (0.1, 0.3 and 0.5%). Treatment with water served as control. Leaf discs were cut from B. sempervirens var. rotundifolia leaves using a cork borer (diameter 1 cm). They were dipped for 30 s into the particular emulsions and air-dried for 30 min. Treated leaf discs were placed in wells (d 3.5 cm) of multiwell plates in which 2 ml water agar (3%) had been filled in order to keep the leaf discs from drying. One larva was each added and 20 larvae were used per concentration. Multiwell plates were incubated at 25 °C, 16-/8-h light/dark condition (L/D), 50% relative humidity (RH). The trial was replicated three times. Larvae were further fed with freshly treated leaf discs two times a week. The vitality and feeding activity were also recorded two times a week. Bioassays were conducted for at least 2 weeks. Larvae were considered to be dead if they did not respond to prodding with forceps.
Entomopathogenic nematodes (EPN) bioassay with 2nd and 4th larval instars and pupae
Laboratory bioassays were conducted to define the susceptibility of young (2nd instar) and further developed (4th instar) C. perspectalis larvae to EPN of the species S. carpocapsae. A serial dilution of an EPN suspension was prepared to adjust different EPN concentrations (10, 20, 50, 100 and 200 EPN/100 μl). Therefore, product powder containing infective juveniles (IJs) of EPN was diluted in water (2 g in 1000 ml) and well stirred to prepare a stock suspension containing about 200 EPN/100 μl. To define the actual EPN concentration and be able to adjust the needed concentrations for the bioassays, a dilution and counting procedure was done following the method reported by Glazer and Lewis (2000). Single Buxus leaves were treated with 100 µl of EPN suspension and air-dried for 15 min. Treated leaves were placed in experimental vials (2 × 2 × 2 cm) in which 1 ml water agar (3%) had been filled in order to keep the leaves from drying. One larva was added per vial, and 15 larvae were included per concentration. Treatment with water served as control. Experimental vials were incubated at 25 °C, 16/8 h L/D, 50% RH. After 3 days, the mortality was determined, food (untreated) was replenished in the case of survived larvae, and vials were incubated for a further 4 days. Thus, the duration of the bioassays was 7 days and the trial was replicated two times per larval stage. Larvae were considered to be dead in the case of a brown coloration of the larvae or if they did not respond to prodding with forceps.
Laboratory bioassays were additionally conducted to define the susceptibility of fresh and matured C. perspectalis pupae on EPN of the species S. carpocapsae. Tested concentrations (10, 50, 200 EPN/100 μl) were prepared as mentioned before. Ten fresh and 10 matured pupae were included per concentration. They were individually placed in experimental vials (diameter 3.8 cm) and directly treated with 100 µl of EPN suspension. Treatment with water served as control. Experimental vials were incubated at 25 °C, 16/8 h L/D, 40% RH. Pupae were observed daily, and hatched moths were counted. After 14 days, all non-developed pupae were dissected to examine them for the presence of nematodes.
Field trials comparing commercial preparations (NA, EPN and BT)
Field trial on already infested Buxus hedges at Seligenstadt in 2012
In August 2012, a field trial was conducted on already infested Buxus hedges in the cloister of a public convent garden in Seligenstadt (50°2′38.75″N 8°58′31.25″E; South-Hessia, Germany) to compare the treatments EPN.12.wa, EPN.12.wa+aa with BTa.12 in the field (Table 1). The cloister (Fig. 1) consisted of low planted Buxus hedges, symmetrically arranged in four rectangles. Before the treatment, the current level of infestation was assessed. Therefore, eight plots of 0.25 m2 (2/replicate) were defined (Fig. 1a–h) and C. perspectalis larvae were searched within these plots and counted. Five days after the treatment (DAT5), living and dead larvae were counted on the total area of treated hedges (about 18 m2) for 10 min per replicate to evaluate the mortality directly in field. In addition, treated twigs (5/replicate) were collected at the application day (DAT0). The 5 samples per replicate, thus 20 per treatment, were cut and transferred to single vials. In the laboratory, one C. perspectalis larva obtained from own rearing was added to each vial. Vials were incubated in a climate chamber (25 °C, 16/8 h L/D, 50% RH) for a further 14 days, and mortality was determined.
Field trial on already infested Buxus hedges at Seligenstadt in 2013
In June 2013, a second field trial was conducted on already infested box hedges in the cloister (Fig. 1) of a public convent garden in Seligenstadt (50°2′38.75″N 8°58′31.25″E; South-Hessia, Germany) to compare treatments NA.13, EPN.13.adj and BTk.13 in the field (Table 1). Before the treatment, the current level of infestation was assessed. Therefore, eight plots of 0.25 m2 (2/replicate) were defined (Fig. 1a–h) and C. perspectalis larvae were searched within this plots and counted. Seven days after the application, living and dead larvae were counted on the total area of treated hedges for 10 min per replicate to evaluate the mortality directly in field. In addition, treated twigs (5/replicate) were collected three times at DAT0, DAT7 and DAT14. The 5 samples per replicate, thus 20 per treatment, were cut and transferred to single vials. In the laboratory, one C. perspectalis larva obtained from own rearing was added to each vial. Vials were incubated in a climate chamber (25 °C, 16/8 h L/D, 50% RH) for a further 14 days, and mortality was determined.
Field trial on intentionally infested Buxus plots at Darmstadt in 2014
In August 2014, a field trial was conducted on Buxus plants which were planted on an experimental field in Darmstadt (49°51′53″N 8°40′6″E; Hesse, Germany) to compare treatments NA.14, EPN.14.adj and BTk.14. An experimental area was created, consisting of 16 plots in four lines, with one plot comprising four Buxus plants (0.25 m2). Each plot was infected with 10 larvae (4th larval instar) obtained from the laboratory. Within the 4 days before the application, they were allowed to feed and to produce their typically loose webs in the plant. To minimize the impact of solar irradiation on nematodes, the trial was conducted late in the evening (8:00 pm) as recommended by the producers. Four days after the treatment (DAT4), the deployed larvae were collected from the treated plots. Dead larvae were counted, and living larvae were further incubated in the laboratory to determine the cumulative mortality at 3, 7 and 10 days of further incubation (DFI). In addition, samples were collected four times after the treatment (DAT0, 4, 7 and 11) from the plots to assess the persistence of applicated variants in the field. Therefore, 40 twigs per variant (10 each plot) were cut, transferred into vials and offered to single C. perspectalis larvae obtained from the laboratory. Vials were incubated in a climate chamber (25 °C, 16/8 h L/D, 50% RH) for a further 14 days, and field mortality of these larvae was determined.
Statistical analyses
For statistical analyses, the scientific data analysis software Sigma Plot Version 13.0 was used. The effect of treatment and concentration of NA preparations on feeding and mortality as well as the effect of treatment and concentration of EPN preparations on mortality of young and further developed larvae were assessed via one-way ANOVA (α = 0.05). If significant, pairwise multiple comparisons were conducted using Tukey tests. To analyse the effect of EPN concentrations on fresh and matured pupae, proportions were compared using a χ2-test. Other results are presented in bar graphs, if possible as mean values with standard deviations.
Results
Laboratory trials
Effect of NeemAzal®-T/S (NA) on 3rd larval instars
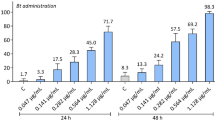
Investigation on the effect of NA on C. perspectalis demonstrated susceptibility of 3rd instar larvae. After 6 days feeding on treated leaf discs, the amount of vital and still feeding larvae was high and ranged between rates of 60 and 80% of exposed larvae (n = 20/concentration in each trial). No significant impact of treatments on feeding activity could be determined (F = 2.174, df = 3, P = 0.169). Regarding mortality after 6 days, only a few larvae died and no significant impact of treatments was detected (F = 1.065, df = 3, P = 0.416). After 14 days, feeding activity was significantly reduced to less than 10% in all treatments (F = 202.872, df = 3, P < 0.001). Mortality increased significantly to a maximum of 62% after 14 days of providing leaf discs treated with 0.3% or 0.5% NA (F = 7.318, df = 3, P < 0.05) (Fig. 2).
Mean proportion [%] of C. perspectalis 3rd instar larvae (n = 20/concentration) still feeding or dead after 6 and 14 days of providing Buxus leaf discs treated with different NeemAzal®-T/S (NA) concentrations (n = 3 trials). Different letters indicate significant differences (ANOVA, Tukey Test, α = 0.05)
EPN bioassays with larvae and pupae
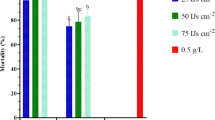
Both tested larval instars (2nd and 4th) were highly susceptible to EPN. After 3 days of exposure, mortality was significantly lower for the young larvae (F = 18.337, df = 11, P < 0.001) ranging between 10 and 75% in comparison with further developed larvae where mortality rates ranged between 45 and 100%. The mortality of young larvae was significantly affected after 3 days (F = 24.279, df = 5, P < 0.001) by higher concentrations (100 and 200 EPN/larva, P < 0.05), while the 4th instar larvae showed already a significant susceptibility to lower concentrations (20 and 50 EPN/100 μl (F = 12.65, df = 5, P = 0.004) at the same time. After 7 days of exposure, mortality was significantly affected by all treatments equal to or higher than 10 EPN/larva regarding both tested larval stages (2nd: F = 129.672, df = 5, P < 0.001; 4th: F = 85.641, df = 5, P < 0.001). A mortality of 100% could be determined after 7 days for all larvae which had been exposed to higher nematode concentrations (50, 100 and 200 EPN/larva) (Table 2).
EPN were much less effective against C. perspectalis pupae. Between 70 and 100% of all treated pupae hatched within 14 days. Only a small amount of the pupae did not hatch (16%), including pupae of the untreated control. There was no significant difference in the proportions of dead fresh and matured pupae, and mortality was not related to the tested EPN concentration (χ2 = 1.333, df = 2, P = 0.513) (Table 2). Only one matured pupa treated with 200 EPN contained nematodes, indicating a successful penetration into the insect body. No EPN were detected in the remaining dissected pupae.
Field trials
Field trial on naturally infested Buxus hedges at Seligenstadt in 2012
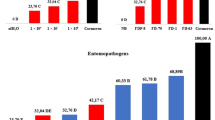
The number of C. perspectalis found in the Buxus hedges before the application of treatments EPN.12.wa, EPN.12.wa+aa and BTa.12 was 191 individuals in total on 8 m2 (32 × 0.25 m2 plots), i.e. 24 C. perspectalis/m2 (including larvae and a small amount of pupae). 5 days after the application, the estimated number of C. perspectalis was 421 including 102 dead larvae on the total area of treated hedges (18 m2), i.e. 23 C. perspectalis/m2, including 6 dead larvae/m2. The mean number of dead larvae was high in the BTa.12 treatment where only a few vital larvae were found. In contrast, in the EPN treatments the number of vital larvae was high and only a few dead larvae were found (Fig. 3).
Mean number of C. perspectalis found at already infested Buxus hedges (n = 4 sites/treatment) before (grey bars, all vital) and 5 days after (black bars) the application of different variants at the cloister at Seligenstadt in 2012 (see Fig. 1). For full names of tested biological control agents see Table 1
The application of EPN.12 and BT.12 caused significant differences in field mortality among the treatments 5 days after the application in 2012 (ANOVA, F = 81.245, df = 3, P < 0.001). BTa.12 (96%) significantly differed from EPN.12.wa (11%), EPN.12.wa+aa (16%) and the control (P < 0.001). These rates were supported in parallel by the mortality rates achieved in the laboratory, after treated twigs were collected at DAT0 and offered to larvae from the laboratory: EPN.12.wa (55%), EPN.12.wa+aa (80%) and BTa.12 (95%). BT was the most effective agent (Table 3).
Field trial on naturally infested Buxus hedges at Seligenstadt in 2013
In total, 60 larvae/8 m2 (32 × 0.25 m2 plots), i.e. 7.5 larvae/m2, were found in the Buxus hedges before the application of NA.13, EPN.13.adj and BTk.13 in 2013. Seven days after the application, the counted number of C. perspectalis did not differ, but also pupae were found on the total area of treated hedges. The number of C. perspectalis found in the different variants did not decrease after the treatment but two dead larvae were found in the EPN.13.adj-treated area. In the BTk.13-treated hedges, no larvae or pupae were found after the treatment at all (Fig. 4).
Mean number of C. perspectalis found at already infested Buxus hedges (n = 4 sites/treatment) before (grey bars, all vital) and 7 days after (black bars) the application of different variants at the cloister at Seligenstadt in 2013 (see Fig. 1). For full names of tested biological control agents see Table 1
The persistence in field differed between the variants. Exposure to treated twigs of all tested variants caused dead larvae in the laboratory if they were collected at the application day: NA.13 (30%), EPN.13.adj (55%) and BTk.13 (75%). Larvae exposed to twigs collected on DAT7 and DAT14 with NA.13 or EPN.13.adj were not affected in contrast to the BTk.13 treatment. BT was the most effective agent, and dead larvae were found in 2013 also on DAT7 and DAT14 (10–15%) (Table 3).
Field trial on intentionally infested Buxus plots at Darmstadt in 2014
Not all deposited larvae were retrieved from Buxus plots 4 days after the treatment (Fig. 5, numbers in parentheses). At this day (DAT4) 66% of retrieved larvae, which were exposed to BTk.14, and 30% of larvae exposed to EPN.14.adj were dead. In contrast, all collected larvae from C and NA.14 were alive. Mortality rates of the field collected larvae further increased with the days of further incubation (DFI3, 7 and 10) of the surviving larvae in the laboratory. Thus, the mortality of BTk.14-treated larvae rose to 100% at DFI3. Mortality of EPN.14.adj-treated larvae reached the maximum of 63% at DFI3. In addition larvae treated with NA.14 showed an increased mortality, which reached 54% at DFI10 (Fig. 5). These results suggest that larvae already have been affected in field.
Cumulative mortality [%] of retrieved C. perspectalis larvae, collected in 2014 from Buxus plots in field (n = 4) 4 days after treatment (DAT). Mortality was assessed after 3, 7 and 10 days of further incubation (DFI). Numbers in parentheses show number of larvae (retrieved/deployed). For full names of tested biological control agents see Table 1
In addition, the persistence in field differed between the variants. When exposing larvae to twigs collected at DAT0, 93% of EPN.14.adj-treated larvae and 78% of BTk.14-treated larvae died. BTk.14 was the most persistent variant and caused high mortality rates (> 90%) of larvae exposed to twigs collected at DAT4 and 11. The persistence of EPN.14.adj fluctuated and rapidly decreased in field, but after 7 days larvae were infected by nematodes again as conferred by dissection. In contrast, larvae exposed to twigs treated with water or NA.14 were not affected (Fig. 6).
Persistence of potential biological control agents in the field. Mortality [%] of C. perspectalis larvae (n = 40/treatment) fed with treated Box twigs collected in 2014 from Buxus plots in field at 0, 4, 7 and 11 days after treatment (DAT) after application of different variants in the field. For full names of tested biological control agents see Table 1
Discussion
This is the first report on the susceptibility of European C. perspectalis populations to the biological control agents Nemastar® (EPN) and NeemAzal®-T/S (NA). The results demonstrate clearly the potential of both tested agents to control C. perspectalis, but also the challenges of application on Buxus plants in the field.
The vulnerability of C. perspectalis larvae (3rd instar) to NA was assessed by the potential impact on feeding activity and survival of larvae after repeated ingestion of treated leaf discs. In a preliminary trial, the impact on young (2nd instar) and further developed (4th instar) larvae was tested based on uniquely ingestion of single treated Buxus leaves. The responding time was highly extended, and larvae were starving many weeks until all of them died (up to seven weeks). Only minor differences occurred in the reaction of the tested larval stages (Table 4). In the present study, the method of investigation was improved to a more realistic situation. Third instar larvae had been fed continuously with freshly treated leaf discs. There was only little variation in the response on the different tested NA concentrations (1–5 ml/l). None of the tested concentrations led to mortality earlier than 6 days after first ingestion of treated leaf discs. This kind of time delay is well known regarding investigations on the impact of NA to several pests. Most insect pests continue to feed on the treated plants for some time, but as a rule, the amount of food ingested by them is considerably reduced, due to the influence of the “secondary” anti-feedant effect (Schmutterer 1990). For example Efil et al. (2005) as well noted a duration of 6 days until larval mortality occurred, when leaf discs had been treated with concentrations of 0.5–2.5 ml/l before offering them to larvae of the Beet armyworm Spotoptera exiqua Hübner (Lepidoptera: Noctuidae). Application rates used in our study complied with the usual applications of 1–7 ml/l NA for bioassays and recommended rates of 250 ml/100 l water against lepidopteran pests in the field (Dammini Premachandra et al. 2005; Erler et al. 2010). It can be concluded that C. perspectalis larvae are susceptible to ingested NA at common concentrations (preferably 3–5 ml/l), causing feeding stop and larval mortality within two weeks in laboratory bioassays.
The presented results suggest that also EPN affected C. perspectalis larvae. In preliminary investigations by the authors, three EPN species were examined on their impact on C. perspectalis larvae. The species Heterorhabditis bacteriophora showed the lowest effect on larval mortality (8–15%) in comparison with S. feltiae (46–100%) and S. carpocapsae (85–100%) at concentrations of 25–200 EPN/larva (Göttig 2012). Shannag and Capinera (1995) compared five EPN species against the Melonworm moth Diaphania hyalinata Linnaeus (Crambidae), a close relative to C. perspectalis, and determined highest infectiousness of S. carpocapsae as well. Furthermore, D. hyalinata was significantly less susceptible at first instar larval stage and pupae than older larvae, which was also evident in this study. In contrast, Choo et al. (1991) reported that there was no difference in infection on Japanese C. perspectalis larvae by H. bacteriophora and S. carpocapsae achieving 100% mortality in all treatments ranging between 10 and 80 EPN/larva. Our investigation on the infectivity of different S. carpocapsae concentrations (10–200 EPN) on C. perspectalis demonstrated a high susceptibility of both tested larval instars (2nd instar: 10–75% and 4th instar: 45–100%). Infection of further developed larvae was faster in comparison with young larvae, regarding the pathogenicity after 3 days. It is known that insect size as well as nematode size can influence the potential of EPN species to invade potential host species, because they are most frequently penetrated via body orifices (Bastidas et al. 2014). Further developed larvae have bigger orifices or probably fed more of the leaves or had more contact to the surface. It was found that pupae were less vulnerable to EPN, even if adult nematodes were found in one of 13 dead and dissected pupae in our study. This could be observed in the case of treated winter cocoons of C. perspectalis larvae (usually 3rd instar larvae), too (data not shown). In contrast, infection of cocooned codling moth larvae (Cydia pomonella L., Lepidoptera: Tortricidae) was high and mortality ranged between 30 and 100% after application of S. carpocapsae (0.5–5.0 million/tree) in the field (Unruh and Lacey 2001).
Although both biological control agents can affect C. perspectalis in the laboratory, limited success was achieved in field applications. In the presented field trials on naturally infested Buxus hedges (year 2012 and 2013), it was not possible to detect an exact difference in the occurring amount of C. perspectalis before and after the treatment. In general, the method of feeding treated leaf samples to laboratory reared larvae proved to be reliable in order to quantify effects even in field trials. In addition, field studies conducted on Buxus hedges intentionally infested with a defined number of larvae (year 2014) facilitated the quantitative recording of the effect. Thus, the method was suitable to assess field mortality.
In this study, the particular ranking of the effectiveness of NA, EPN and BT was comparable in each experiment. The application of NA resulted in a low response of treated larvae, and larval mortality constantly took a long time. This extensive duration until the larvae stopped feeding and died could be problematic for rapid efficiency in acute damage cases. But NA may be considered for applications on small larvae, as already mentioned for other targets, if and when a temporary feeding damage does not endanger the plants. The application would be of great advantage if other sucking pests are present on the Buxus plant or being part of applications combining more than one control agent. Impacts were proved on over 400 insect species, especially on lepidopteran species (> 138) (Schmutterer and Huber 2005).
Using EPN in field produced mortality rates of about 16–65% at a range of 2.5 million EPN/m2, but high fluctuations were observed in persistence tests. Mortality rose up to 80%, if field-treated twigs collected at the application day were fed to larvae in the laboratory. Field mortality was in the same range as results presented by Shannag and Capinera (1995), where infection rates of 52–55% were produced in field applications of 5 billion EPN per hectare (0.5 million/m2) on D. hyalinata on crookneck squash foliage. As invertebrate macroorganisms, the application of EPN requires no registration as plant protection product and in many countries there are no user restrictions. The rate of mortality in field and nematode success to penetrate host species is strongly related to weather conditions, the application technique and easy accessibility of the host on the plant foliage, in contrast to soil applications. Factors like the UV irradiation and temperature, as well as the relative humidity, play a decisive role in foliar application and the persistence of EPN in field (Glazer 1992; Smits 1996; Arthurs et al. 2004). Many advantages also apply to EPN. They are partially able to actively seek their host; they are almost harmless to non-target organisms, are easily mass produced, have a broad host range, and have the ability to kill their hosts rapidly (Shannag and Capinera 1995; Kaya and Gaugler 1998).
Because larvae of C. perspectalis are highly susceptible to BTk and BTa, the use of plant protection products based on Bacillus thuringiensis is currently the only alternative to chemical insecticides and available effective biological control methods. However, the requirements for proper application are high and pesticide resistance is becoming an increasingly important factor in the selection of suitable control agents, even for using BT against other lepidopteran species (Heckel et al. 2007; Kang et al. 2014). Resistance rapidly can change the available insecticides arsenal. Therefore, plant-derived natural products like NA as well as beneficials like EPN are considered to be valuable candidates to prevent resistance on common pesticides.
In conclusion, there is a higher variability in terms of the effects of the investigated agents Nemastar® and NeemAzal®-T/S compared to the constant effectiveness of Dipel ES® in the field. But their use would be possible if feeding damage and proper surveillance of the result will be considered in order to be capable of repeating the application timely. More frequent and timely applied treatments may improve efficacy and a combination with further measures ought to take place.
References
Arthurs S, Heinz KM, Prasifka JR (2004) An analysis of using entomopathogenic nematodes against above-ground pests. Bull Entomol Res 94(04):297–306
Bastidas B, Portillo E, San-Blas E (2014) Size does matter: the life cycle of Steinernema spp. in micro-insect hosts. J Invertebr Pathol 121:46–55
Benelli G, Canale A, Toniolo C, Higuchi A, Murugan K, Pavela R, Nicoletti M (2017) Neem (Azadirachta indica): towards the ideal insecticide? Nat Prod Res 31(4):369–386
Burges HD (1982) Control of insects by bacteria. Parasitology 84(4):79–117
Choo HY, Kaya HK, Lee SM, Kim TO, Kim JB (1991) Laboratory evaluation of enthomopathogenic nematodes Steinernema carpocapsae and Heterorhabditis bacteriophora against some forest insect pests. Korean J Appl Entomol 30(4):227–232
Dammini Premachandra WTS, Borgemeister C, Poehling H-M (2005) Effects of Neem and Spinosad on Ceratothripoides claratris (Thysanoptera: Thripidae), an important vegetable pest in Thailand, under laboratory and greenhouse conditions. J Econ Entomol 98(2):438–448
Efil L, Ozgen I, Yardim EN (2005) Effects of a commercial Neem insecticide (NeemAzalTM-T/S) on early and late developmental stages of the Beet armyworm Spodoptera exiqua (Hübner) (Lepidoptera: Noctuidae). PJBS 8(4):520–526
Erler F, Cetin H, Saribasak H, Serttas A (2010) Laboratory and field evaluations of some botanical pesticides against the cedar leaf moth, Acleris undulana. J Pest Sci 83(3):265–272
Glazer I (1992) Survival and efficacy of Steinernema carpocapsae in an exposed environment. Biocontrol Sci Technol 2(2):101–107
Glazer I, Lewis EE (2000) Bioassays of entomopathogenic nematodes. In: Navon A, Ascher KRS (eds) Bioassays of entomopathogenic microbes and nematodes. CAB International (CABI Publishing), Wallingford 2000, pp. 234
Heckel DG, Gahan LJ, Baxter SW, Zhao JZ, Shelton AM, Gould F, Tabashnik BE (2007) The diversity of Bt resistance genes in species of Lepidoptera. J Invertebr Pathol 95(3):192–197
Isman MB (2006) Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu Rev Entomol 51:45–66
John R, Schumacher J (2013) Der Buchsbaum-Zünsler (Cydalima perspectalis) im Grenzach-Wyhlener Buchswald—Invasionschronik und Monitoringergebnisse [The Box-Tree Pyralid (Cydalima perspectalis) in the Box-Tree Forest of Grenzach-Whylen]. Gesunde Pflanzen 65(1):1–6
Kang J, Huang F, Onstad DW (2014) Modeling evolution of resistance of sugarcane borer (Lepidoptera: Crambidae) to transgenic Bt corn. Environ Entomol 43(4):1084–1104
Kaya HK, Gaugler R (1998) Entomopathogenic Nematodes. Annu Rev Entomol 38:181–206
Leuthardt FLG (2013) Distribution, life history, food choice and chemical ecology of the box-tree pyralid Cydalima perspectalis. Dissertation, University of Basel
Nacambo S, Leuthardt FLG, Wan H, Li H, Haye T, Baur B et al (2014) Development characteristics of the box-tree moth Cydalima perspectalis and its potential distribution in Europe. J Appl Entomol 138(1–2):14–26
Oberdorfer E, Schwabe A, Müller T (2001) Pflanzensoziologische Exkursionsflora für Deutschland und angrenzende Gebiete. Eugen Ulmer, Stuttgart
Schmutterer H (1990) Properties and potential of natural pesticides from the neem tree, Azadirachta indica. Annu Rev Entomol 35:271–297
Schmutterer H, Huber J (2005) Natürliche Schädlingsbekämpfungsmittel. Eugen Ulmer GmbH & Co, Germany
Schnepf E, Crickmore N, van Rie J, Lereclus D, Baum J, Feitelson J et al (1998) Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol Mol Biol Rev 62(3):775–806
Shannag HK, Capinera JL (1995) Evaluation of entomopathogenic nematode species for the control of Melonworm (Lepidoptera: Pyralidae). Environ Entomol 24(1):143–148
Smits PH (1996) Post-application persistence of entomopathogenic nematodes. Biocontrol Sci Technol 6(3):379–388
Unruh TR, Lacey LA (2001) Control of codling moth, Cydia pomonella (Lepidoptera: Tortricidae), with steinernema carpocapsae: effects of supplemental wetting and pupation site on infection rate. Biol Control 20(1):48–56
van Trier H, Hermans D (2007) Buchs. Eugen Ulmer, Stuttgart
Walker F (1859) Part XVIII pyralides—list of specimens of lepidopterous insects in the collection of the British Museum (Vol. 18, pp. 509-798). London: British Museum (Natural History), Department of Zoology
Wan H, Haye T, Kenis M, Nacambo S, Xu H, Zhang F, Li H (2014) Biology and natural enemies of Cydalima perspectalis in Asia: is there biological control potential in Europe? J Appl Entomol 138(10):715–722
Williamson M, Fitter A (1996a) The characters of successful invaders. Biol Conserv 78:163–170
Williamson M, Fitter A (1996b) The varying success of invaders. Ecology 77(6):1661–1666
Acknowledgements
We would like to express our gratitude towards the Arthur and Aenne Feindt-Foundation (Hamburg), for the generous support of this work as a part of the project “Development of friendly methods for monitoring and regulating the Box tree pyralid, Cydalima perspectalis (Lepidoptera: Crambidae), an invasive pest in ornamentals”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Göttig, S., Herz, A. Susceptibility of the Box tree pyralid Cydalima perspectalis Walker (Lepidoptera: Crambidae) to potential biological control agents Neem (NeemAzal®-T/S) and entomopathogenic nematodes (Nemastar®) assessed in laboratory bioassays and field trials. J Plant Dis Prot 125, 365–375 (2018). https://doi.org/10.1007/s41348-018-0154-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-018-0154-8