Abstract
Computed tomography was used to study the burrowing activity of larvae of Sitotroga cerealella Olivier, 1789 (Lepidoptera: Gelechiidae) in damaged maize grain. The randomly selected cobs were shelled, and the damaged maize grains were measured. The volumes and locations of cavities formed by larvae were mapped by means of a new computer-based three-dimensional imaging method. The use of new technologies have greatly improved and facilitated the detailed investigation of injured grain. The results provided additional data for biological information to covertly developing S. Cerealella. Significant volume destructions were observed only in the highly perforated grains (P = 0.032). The embryos of the infested grain were destroyed independent of the number of larvae. The emerged holes and the larval cavities were found to be linked by larval passages, which have been prepared directly before the pupation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the case of the hidden lifestyles and covertly developing insects, the method and the rate of damage are a very important issue of plant protection. Previously these damages of stored product caused by pests can be roughly estimated, and the triggered injuries cannot be measured exactly in the majority of cases.
The Angoumois grain moth, Sitotroga cerealella is a typical example of these pests since the damage caused by the moth is hidden and difficult to assess. Its distribution is nearly global today, including essentially all of Europe as well as Australia, Africa, southern Asia, South Latin and North America [4]. This species is a serious primary pest that mainly attacks maize, wheat and sorghum, both in the field and in stores [9, 15]. Infestation with S. cerealella starts in the agricultural field as females lay their eggs, singly or in groups, on the surface of the grains. The emerged young larvae bore into the grain where they will complete their development. These small caterpillars spend their entire life inside an individual grain. Therefore, infestation is difficult to detect at this stage [6, 8]. The pupal stage lasts about 10 days. Adults leave a conspicuous emerging hole at one end of the kernel. The weight losses can be as much as 50 % for wheat and 24 % for corn. Its impact is greater in the tropics and subtropics where it attacks grain in the field as well as in storage [4].
The stored product pests are responsible for considerable economic losses to grains. Nowadays, the ensuring of the healthy and injury-free stored product is becoming a more and more important task with respect to practical plant protection and economic criterion [2, 4]. There is no method in practice, which is able to exactly measure the triggered, quantitative injuries of the hidden lifestyle insect pests. This study attempts to make up for this deficiency.
The purpose of this study was to determine accurately the decrease in the volume in maize grain caused by S. cerealella using computer-based three-dimensional reconstructions technology. The objective image analysis is expected to contribute to the knowledge of biological features of this serious pest. Besides the grain volume alteration, the possible involvement of the grain embryo and the ability of this insect to do damage would become more understood with the help of this technology.
Materials and methods
To determine the form and volume of the cavity of maize grain caused by S. cerealella, we collected damaged maize cobs from the classic maize drying shed. The cobs had been stored for 2 years originating from the surface of the plant product, where the cobs are more vulnerable to the damaging process. They were collected at Somogyszil (Somogy county; Hungary) on the 18 October 2013. The 10 randomly selected cobs were shelled, and the grain moisture was determined using a heat treating oven (Memmert UFE 400-800) (Memmert Ltd., www.memmert.com). The weight of samples was measured by a precision weighing balance (Ohaus Explorer Pro EP214CE) (Ohaus Corporation, http://balance.balances.com/scales/69). Furthermore, the damaged grains were classified into four groups based on the number and location of conspicuous emerged holes of adult moths: 1 h: one hole on the grain surface; 1 h-a: one hole on the top of the grain; 2 hs: two holes on the grain surface; 3 hs: three holes on the grain surface (Fig. 1). The number of replicates per treatment was 25.
Before the CT examination, the surface of the grains were glazed manually with a water base enamel paint (black colour, Unitop, Sygma Kalon Group) (Unitop, black colour paint, Trilak Ltd., www.trilak.hu) using a paint brush. After the drying period, this coat of paint made it possible to visualize the thin wall of the seed, damaged inside by the larvae. The CT measurements were performed covering the whole grains using a Siemens Somatom Sensation 16 Cardiac CT scanner (Siemens Ltd., Erlangen, Germany). The seeds were positioned in a 3 × 3 × 5 matrix in a polystyrene rack on the examination table. The following scanning parameters were used for data collection purposes: 120 kV, 230 mAs and spiral data collection. Axial scans were reconstructed every 0.1 mm with 0.6 mm slice thickness and 50 mm field of view.
The densities in the CT images were normalized into the range and thresholded at level 0.5 to differentiate the voxels of the grains from the background. Next, the coordinates of those grain voxels which were adjacent to other grain were extracted and considered to be the coordinates of the surface points of the grains. Based on these 3D point clouds, the surfaces of individual seeds were reconstructed through triangularization. Due to the finite resolution of the acquisition process, the 3D models were still noisy; thus, manual post-processing was applied to remove spikes and unexpected artefacts of the surfaces. The holes of the 3D objects were filled in by manually adjusting the 3D model, and the surface and volume of the damaged and reconstructed grains were computed.
The volume and the weight data were analysed by SAS software using one-way ANOVA (PROC ANOVA), with cavity as the response variable and location and the number of the emerged holes as the main effects. Post hoc tests were performed using the Tukey (HSD) test, with the significance level set to P < 0.05.
Results
The average weight of healthy maize grain was 0.354 ± 0.095 g (mean ± SE). The weights of damaged maize grains were the following: 1 h: 0.335 ± 0.025; 1 h-a: 0.333 ± 0.018; 2 hs: 0.332 ± 0.022; 3 hs: 0.326 ± 0.035 (P = 0.032). The moisture content was calculated to be 12.21 ± 0.34 %. The weight losses increased with the number of holes produced.
The volumes of the examined maize grain parallel with the weight results were measured by CT. The volume of the examined maize grain was 498.47 ± 26.74 mm3. The changing volume of the damaged maize grain was between 5.31 and 7.71 %. The cavities increased with the number of holes (1 h: 27.19 ± 2.08 mm3; 1 h-a: 28.44 ± 2.42 mm3; 2 hs: 32.01 ± 2.26 mm3; 3 hs: 35.51 ± 1.42 mm3). A significant difference in the cavity could be observed between the 1 h and 3 hs samples. Significant differences were not detected between the other damaging groups (P ≤ 0.098).
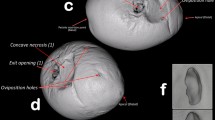
The shape and location of the larval cavities are shown in Fig. 2. The pericarp always remained intact. The destruction of the maize embryo, which is located in the ventro-apical part of the grain, can be evaluated based on the location of apparent cavity by CT (Fig. 3). The endosperm content was consumed by the caterpillar mostly that parts are near to the embryo.
Computer-based three-dimensional reconstructions of the damaged maize grain by S. cerealella as a function of the main structure of maize grain. a Endosperm; b pericarp; c embryo [1]
Discussion
The use of new technologies especially computer-based three-dimensional reconstructions and CT has greatly improved and facilitated the detailed investigation of injured grain, other plant parts assays and insect anatomy. This technology has not yet been used in applied plant protection. However, burrowing behaviour of insects has already been studied using CT technology [7, 10].
The results provided by CT can be used for both entomology and plant physiology as well. This method ensures additional data for biological and ecological information studying the hidden lifestyles of covertly developing insects.
The newly emerged larvae of S. cerealella bore into the apical part of grain where they will complete their development [9]. CT clearly showed the cavities of the burrowing larvae and revealed that, following their exit from infested maize, the fully developed larvae burrow to the crown end of the grain. In this way, the insect can develop in the apical part of grains in optimal and protected conditions. This phenomenon has already known in the case of other agricultural and stored product pests, e.g. Bruchophagus roddi Gusakovski [3], Ostrinia nubilalis Hübner [14], Acanthoscelides obtectus Say [13].
The emerging holes are situated in the crown end of the grain. The emerged holes and the larval cavities are linked by larval passage. Most likely, the larvae only moved next to the emerging hole directly before its pupation [5, 15].
The volumes of the formed cavities and the weight of the maize grain were not influenced by the number of larvae. Significant volume destructions were observed only in the highly perforated grains (3 hs). The embryos of the infested grains were destroyed in all cases, independently of the number of larvae. This can be explained with the high α-tocopherol content of embryo, which is a very attractant component for the more herbivore insects [11]. Therefore, the damaged maize grain by S. cerealella cannot be later utilized as seed.
According to Pandey and Pandey [12], contents of protein, total and reducing sugars, starch, ash and oil of damaged grain and on losses caused by S. cerealella indicated that the chemical constituents of the grain were not related to losses. It is thought that the losses in the different varieties may be related to the combined effects of the chemical and physical properties of the grain.
References
Borrás L, Curá JA, Otegui ME (2002) Maize kernel composition and post-flowering source-sink ratio. Crop Sci 42:781–790
Bratspies RM (2004) Consuming (f) ears of corn: public health and biopharming. Am J Law Med 30:371–404
Brewer GJ, Horber E (1984) Field infestation and alfalfa seed chalcid (Hymenoptera: Eurytomidae) development in different Medicago clones. Environ Entomol 13:1157–1159
Bushra S, Aslam M (2014) Management of Sitotroga cerealella in cereal grains: a review. Arch Phytopath Plant Prot 47:2365–2376
CABI (2012) Sitotroga cerealella (grain moth). Invasive species compendium. Datasheets, maps, images, abstracts and full text on invasive species of the world. http://www.cabi.org/isc/datasheet/50238
Compton JAF, Sherington J (1999) Rapid assessment methods for stored maize cobs: weight losses due to insect pests. J Stored Prod Res 35:77–87
Friedrich F, Beutel RG (2008) Micro-computer tomography and a renaissance of insect morphology. Optical Engineering, Applications. International Society for Optics and Photonics. doi:10.1117/12.794057
Hamed M, Nadeem S (2012) Effect of cereals on the development of Sitotroga cerealella (Olivier)(Lepidoptera: Gelechiidae) and subsequent quality of the egg parasitoid Trichogramma chilonis (Ishii)(Hymenoptera: Trichogrammatidae). Pak J Zool 44:923–929
Hansen LS, Skovgaard H, Hell K (2004) Sitotroga cerealella (Lepidoptera: Gelechiidae), development parameters of a strain from maize stores in West Africa. Int Prot Stored Prod IOBC Bull 27:69–74
Harrison RD, Gardner WA, Tollner WE, Kinard DJ (1993) X-ray computed tomography studies of the burrowing behavior of fourth-instar pecan weevil (Coleoptera: Curculionidae). J Econ Entomol 86:1714–1719
Jermy T (1976) The host-plant in relation to insect behaviour and reproduction. Plenum Press, New York
Pandey V, Pandey ND (1978) Relation between the chemical constituents of damaged grains of maize varieties and losses caused by Sitotroga cerealella. Indian J Entomol 40:339–341
Parsons DMJ, Credland PF (2003) Determinants of oviposition in Acanthoscelides obtectus: a nonconformist bruchid. Physiol Entomol 28:221–231
Showers WB, Reed GL, Oloumisadeghi H (1974) Mating studies of female European corn borers: relationship between deposition of egg masses on corn and captures in light traps. J Econ Entomol 67:616–619
Weston PA, Rattlingourd PL (2002) Progeny production by Tribolium castaneum (Coleoptera: Tenebrionidae) and Oryzaephilus surinamensis (Coleoptera: Silvanidae) in maize previously infested by Sitotroga cerealella (Lepidoptera: Gelechiidae). J Econ Entomol 93:533–536
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keszthelyi, S., Kovács, G. & Donkó, T. Computer tomography-assisted imaging analysis in damaged maize grain caused by Sitotroga cerealella . J Plant Dis Prot 123, 89–92 (2016). https://doi.org/10.1007/s41348-016-0009-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-016-0009-0