Abstract
Biofilms are microbial communities that bind to surfaces resist adverse conditions. Increasing the survival of bacteria in biofilm structures compared to their planktonic form causes problems in drug treatment. On the other hand, drug resistance in the world is increasing and the need to discover and identify new compounds with the antimicrobial effect is felt. Marine sponges are adapted to unique marine environments and can fight pathogens of these ecosystems without having a dedicated defense system. This study aimed was to investigate the antimicrobial and anti-biofilm effects and also to identify the bioactive compounds of two samples of Psammocinia sp. and Hyattella sp. sponges. Six bacteria Pseudomonas aeruginosa, Acinetobacter baumanni, Klebsiella pneumonia, Escherichia coli, Staphylococcus aureus and Bacillus cereus were tested. The extract was first extracted using dichloromethane and methanol (DCM: MeOH) (1:1 v/v) solvents. The planktonic form was investigated using Disk diffusion and agar well diffusion methods. The minimum inhibitory concentration (MIC) was determined by the microdilution method and then the minimum bactericidal concentration (MBC). Gas Chromatography (GC) and Gas Chromatography-Mass Spectrometry (GC-MS) were performed to identify the compounds of each extract. No zone of inhibition (ZOI) was observed on the planktonic form of K. pneumonia due to both extracts. MIC have about 10 to 20 mg/ml and MBC in about 20 to 80 mg/ml was determined. The results showed that the effect of both extracts on the degradation of the biofilm formed by B. cereus was less than other bacteria. The results of GC-MS showed the presence of phenol, butanedioic acid, propanoic acid and Benzeneacetaldehyde compounds. This study showed that marine sponges at the Persian Gulf can be a good candidate for the extraction of bioactive compounds that use as antimicrobial agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biofilms are microbial communities attached to surfaces in an extracellular matrix (ESM) that have greater drug and antibiotic resistance than their planktonic form. Increasing drug resistance is one of the global concerns for the treatment of acute and chronic infections associated with the formation of microbial biofilms. Studies have shown that more than 90% of bacteria can form biofilms (Sadeghian et al. 2012; Li and Lee 2017; Lu et al. 2019; Masák et al. 2014). One of the new approaches that have been considered over the last two decades to prevent the formation of biofilms is the use of natural products (Lu et al. 2019; Tan and Vanitha 2004).
Each year, about 200 new molecules of marine sponges are reported, making them a vast and diverse source of natural compounds with medicinal and therapeutic properties over the past six decades. Among these features can be cited as antimicrobial properties (Ancheeva et al. 2017; Rane et al. 2014; Zhang et al. 2017).
Marine sponges from the phylum Porifera are metazoans that existed about 700 to 800 million years ago. Populations of them are found in tropical oceans, moderate waters, and freshwaters (Hentschel et al. 2002; Khoddami et al. 2018). Sponges have a high interaction with marine ecosystems and because they are attached to the solid seabed, they are not able to escape in the face of adverse conditions. For this reason, they fight predators and pathogens by producing certain compounds called secondary metabolites (Mahon et al. 2003; Mehbub et al. 2014; Paul et al. 2001; Mohammadi et al. 2019; Proksch 1994). Secondary metabolites are introduced as organic compounds that do not play a direct role in the growth and reproduction of living organisms (Mehbub et al. 2014; Tilviv 2004). Most of the secondary metabolites produced by sponges are very potent. So that they are still stable and active in saline and diluting ocean conditions (Abad et al. 2011; Mehbub et al. 2014).
Little research has been done on marine organisms in the Persian Gulf as a source of natural marine products (Seradj et al. 2012). The aim of this investigation was to recognize sponge with antimicrobial activity for chemical and pharmacological studies. Also, in this study, the antibiofilm activity of sponges extracts against some pathogenic bacteria was evaluated.
Material and Method
Techniques for investigating the anti-biofilm and antimicrobial effects, as well as the materials used, are listed below.
Collection and Identification of Marine Sponge
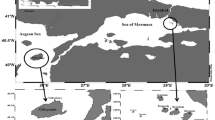
The marine sponges were collected at the Persian Gulf, Iran. These sponges were gathered from a depth of 10–12 meters in Lesser Tunb islands (26º14ʹ N-55º08ʹ E,68.8 km2) (Khoobdel et al. 2014) in November 2015. Three repetitions were collected for each marine sponge species. They were transported alive in seawater to the laboratory and maintained at 4ºC in a refrigerator before extraction. The collected sponges were identified according to the protocol of Hooper and Boury-Esnault (Boury-Esnault and Rützler 1997; Hooper 2000). Identification results have shown that these two sponge belongs: Psammocinia sp. and Hyattella sp. genus. Figure 1 show the macroscopic image of these sponges.
Preparation of Sponges Extracts
For extraction, 100 g of freeze-dried sponge samples were used. First, the samples were crushed into smaller pieces and placed in a polar and non-polar solvent of dichloromethane (DCM) and methanol (MeOH) in a ratio of 1: 1 v/v (Pech-Puch et al. 2020) in a shaker for 48 hours. The obtained extract was then filtered using Whatman No. 1 paper filter and thus large pieces of sponge were removed from the extract. To remove the solvent and concentrate the extract, after filtration, the obtained extract was incubated for 40 hours at 40 ° C. After the incubation period, the remaining extract was used to evaluate the antimicrobial and anti-biofilm effect (Hamayeli et al. 2019).
Bacteria
Six antibiotic-resistant pathogenic bacteria were used in this research includes: Pseudomonas aeruginosa (ATCC 27,853 ), Acinetobacter baumannii (ATCC 1611), Bacillus cereus (ATCC 1298), Klebsiella pneumoniae (ATCC 700,603), Escherichia coli (ATCC 35,218), Staphylococcus aureus (ATCC 1189).
Disk Diffusion Method
Microbial susceptibility was determined by Kirby-Bauer method (Hudzicki 2009) using paper blank disks with a diameter of 6.4 mm. Initially, a concentration of 100 mg/ml of each extract was prepared in DCM: MeOH (1:1 v/v) solvent. Blank disks were placed at this concentration for 1 h and then at room temperature for 30 min. Microbial culture of each of the studied bacteria with turbidity equivalent to 0.5 McFarland was performed on Mueller-Hinton Agar (MHA) medium and the disks were placed on the culture medium. The plates were incubated for 18 h at 37 ° C and then the diameter of the zone of inhibition (ZOI) was measured and reported in mm. Data were expressed as mean ± standard deviation. The blank disk containing DCM: MeOH (1:1 v/v) solvent was used as a control.
Agar Well Diffusion Method
Wells with a diameter of 6 mm were punched in plates containing MHA medium and microbial culture was performed with turbidity equivalent to 0.5 McFarland of each bacterium. Then 50 µl of the same solution prepared from the extracts was placed in the wells in the previous step. The plates were incubated at 37 ° C for 18 h and then ZOI was reported. Solvent-containing wells were considered as controls (Balouiri et al. 2016). Data were expressed as mean ± standard deviation.
Determination of the Minimum Inhibitory Concentration (MIC), and Minimum Bactericidal Concentration (MBC)
The broth microdilution method was followed according to the Clinical and Laboratory Standards Institute (CLSI) protocol supplement M100 (2017) (CLSI 2017; Reller et al. 2009) to determine the MIC. Initially, 100 mg of each extract in 1 ml of sterile nutrient broth medium was well vortexed and from this concentration of 100 mg/ml, 7 more dilutions (50, 25, 12.5, 6.25, 3.12, 1.56, 0.78 mg/ml) were prepared by serial dilution. In each well of the 96-well plate, 200 µl of the dilutions prepared were added, along with 50 µl of bacterial culture (0.5 McFarland) and 10 µl of sterile nutrient broth medium. Thus, the final concentration of each extract was 80, 40, 20, 10, 5, 2.5, 1.25 and 0.625 mg/ml in each well. Three control wells containing extracted nutrient broth culture medium, non-extracted broth nutrient medium, and microbial culture in broth medium (0.5 McFarland) were considered. The microplate was incubated for 18 h at 37 ° C. After this period, optical density (OD) was determined and recorded at 630 nm using an ELISA reader (Biotek ELx800). The lowest concentration of the extract in which bacterial growth was inhibited was determined as MIC. 100 µl of the well designated as MIC was applied to MHA medium to determine MBC (Mohsenipour and Hassanshahian 2016).
Inhibition of Biofilm formation
The formation of microbial biofilm in plate microtiter and its staining with crystal violet (CV) is described by Masumipour and Hassanshahian (2016). Then, 100 µl of each microbial suspension with turbidity equivalent to 1 McFarland and 100 µl of each of the extracts prepared by serial dilution were placed in 96-well polystyrene microplate wells. The final concentration of extracts in each well was three dilutions of 12.5, 6.25, and 3.12 mg/ml was estimated. Three control wells were considered as microbial suspension wells from each bacterial strain, wells containing sterile culture medium and wells containing an equal amount of extract and culture medium. The microplates were then incubated at 37 ° C for 24 h in a stationary state. The formed biofilms were stained by CV and at the end, 160 µl of glacial acetic acid 33% (v/v) were added to the wells (Stepanović et al. 2000). OD was recorded at 630 nm by the ELISA reader (Biotek ELx800) (e Silva et al. 2017).
Disruption of an Established Biofilm
Biofilm formation was established using microbial suspension (1 McFarland) in 96-well polystyrene microplate for 24 h at 37 ° C under static conditions. At this stage, two control wells containing culture medium and microbial suspension were also considered. After this time, the wells were gently drained under aseptic conditions and washed twice to remove planktonic cells. Then 100 µl of extract and 100 µl of culture medium were added to each well. The concentrations used in the extracts were the same as in the previous step (12.5, 6.25, and 3.12 mg/ml). Since the extracts had color, the control well containing equal amounts of the extract and culture medium was placed. The microplates were incubated for 24 h at 37 ° C and then CV was stained and an OD of 630 nm was recorded (e Silva et al. 2017).
Statistical Analysis
Differences for individual parameters between control and treated groups were tested with Duncan’s test by analysis of variance (ANOVA) using SPSS version 16.0 for Windows. Differences were considered significant if the P value less than 0.01, 0.05 and 0.001. All experiments were performed in triplicate and repeated three times.
Gas Chromatography (GC) and Gas Chromatography-Mass Spectrometry (GC-MS)
The marine sponge extracted with DCM: MeOH and investigated using GC Agilent Technologies CP 3800 GC with Split/Splitless Inlet United States (2004), CP-SIL 5CB column (30 m, 0.1 mm and ID 0.32 µm) equipped with Flame Ionization Detector (FID), as well as GC-MS Agilent Technologies, Varian Saturn 2000 United States (2004), the HP-5MS column (60 m, 0.25 mm, 0.25 µm). The specification of the detector was a GC/MS/MS and Mass range from 10 to 650 amu. equipped with Electron Impact Ionization (EI) ion source and NIST MS Library V.2.0.1. The oven was programmed a primary temperature 70ºC (hold for 2 min) to the terminal temperature 300ºC at the rate 10 C/min (hold for 10 min, 35 min in total) (Hassanshahian et al. 2019). H2 at the rate of 30 ml/min was used as the carrier gas in constant flow mode.
Results
The results obtained from the effect of extracts on the planktonic form and biofilm of bacteria as well as the analysis of GC-MS are reported below.
Antimicrobial Effect of Extracts on Planktonic Form
The ZOI of two sponge extracts that assayed by disc diffusion and agar-well plate methods against bacteria were illustrated in Table 1. As shown in this table the ZOI obtained by the agar-well plate was higher than ZOI obtained by the disc diffusion method. The most sensitive bacteria to the antibacterial effect of Psammocinia sp. and Hyattella sp. extract was B. cereus and P. aeruginosa respectively. The MIC and MBC results were presented in Table 1. According to this table, the values of MIC and MBC were different for each bacteria.
Anti-biofilm Effect of Extracts on Bacteria
The effect of sponge extracts on the inhibition of biofilm formation by pathogenic bacteria was studied. The results were shown in Figs. 2 and 3. As shown in this figure the maximum biofilm inhibition by two sponge extracts related to P. aeruginosa (90.86%). These extracts had the lowest inhibitory effect against biofilm formation of S. aureus (36.74%). Also, it can be concluded from this figure that with an increase in extract concentration the biofilm formation was more inhibited. For the destruction of biofilm structure, the Psammocinia sp. extract had the best antibiofilm activity against K. pneumoniae biofilm (90.32%) and Hyattella sp. extract had the optimum antibiofilm activity on E. coli biofilm (94.33%). The most resistant biofilm structure between studied bacteria was B. cereus (39.20%) (Fig. 4).
Statistical Analysis
The effect of bacteria genus and different concentration of sponge extracts on biofilm formation and destruction were analyzed statistically by Duncan’s test. The results were presented in Table 2. This table confirmed that for biofilm inhibition and destruction Hyattella sp. extract was significant. Also for biofilm destruction type of bacteria were significant with Psammocinia sp. extract (P < 0.05).
The Chemical Composition of Sponge Extract
The bioactive compounds of each sponge extracts were revealed by GC-MS. The results were presented in Table 3. Also, the chromatographs for each sponge extract were illustrated in Figs. 5 and 6. These figures show that phenolic and benzoic aromatic compounds are predominant in the two sponge extracts.
Discussion
A drug called Hymendin is a type of marine sponge for the treatment of tuberculosis in preclinical trials (Masoumipour et al. 2018). So far, other drug compounds with anti-viral and anti-cancer properties, such as Vidarabine and Cytarabine from sea sponges have been able to obtain Food and Drug Administration (FDA) approval (Mayer et al. 2010).
In February 2017, the World Health Organization (WHO) published a document outlining a list of pathogens that are a priority for research and development of new antibiotics. Based on this, bacteria such as A. baumannii, P. aeruginosa, and S. aureus resistant to antibiotics are a priority (WHO 2017).
Govinden-Soulange et al. (2014) in investigating the antimicrobial effect of Biemna tubulosa and Stylissa spp. sponges, mention the reason for the high level of MIC as the use of crude extracts, which contain a mixture of active and inactive compounds (Govinden-Soulange et al. 2014). The use of crude DCM: MeOH extracts of Psammocinia sp. and Hyattella sp. sponges, as shown in Table 1, was estimated at 10 to 20 mg/ml. It can probably be concluded that the use of refined extracts or any component of the compound alone can reduce the MIC (De and James, 2002).
Carneiro et al. (2019) identified a type of lectin from Aplysina fulva sponge that, although not effective in inhibiting the planktonic growth of bacteria, significantly reduced the formation of E. coli and S. aureus biofilms (Carneiro et al. 2019). A similar result was observed in the present study of K. pneumoniae. As in the disk diffusion and agar well plate tests, no ZOI was observed for both Hyattella sp. and Psammocinia sp. extracts, but the extracts were able to inhibit and destroy the biofilm of this bacterium. In another study of Callyspongia sp. sponge with solvent, the extraction method was similar to the observed ZOI for this bacterium of 8 mm (Hamayeli et al. 2018). From all the results of this research, it can be concluded that the types of marine sponges exhibit a diverse range of antimicrobial and anti-biofilm activities.
Hamayeli et al. (2017) reported three species of Dysidea sp. sponges from the Persian Gulf that each extract had a different antimicrobial effect on human pathogens, whose findings could be due to the production of bioactive compounds or symbiotic microorganisms. According to their report, at a concentration of 12.5 mg/ml of Dysidea sp. extract, the inhibition of B. cereus biofilm was at a maximum. Also, the ZOI observed in the disk-diffusion assay for A. baumannii was about 9 to 12 mm (Hamayeli et al. 2017).
Kaplan (2011) states that concentrations subMIC in some antibiotics can induce the agonistic effect of biofilm formation in vitro (Kaplan 2011). Therefore, concentrations lower than MIC (according to Table 1) specified for the biofilm test (3.12, 6.25 and 12.5 mg/ml) were used in this study. Also, Cepas et al. (2019) by examining the relationship between drug resistance of three gram-negative bacteria and biofilm formation, states that antibiotic-resistant acquisition in some gram-negative bacteria can encourage or inhibit the formation of microbial biofilm. However, multidrug-resistant bacteria do not tend to produce more biofilms than non-resistant bacteria (Cepas et al. 2019). Therefore, it is thought that the presence or absence of multiple drug resistance (MDR) of the bacteria in this study does not have a significant effect on the analysis of their biofilm formation. However, the relationship between the two is still unclear.
Carefully in Fig. 3, it appears that with increasing the concentration of Psammocinia sp. extract, the inhibition of E. coli biofilm formation has decreased. This is also seen in Fig. 4 for the degradation of the biofilm formed by the Hyattella sp. extract on the biofilm of E. coli, S. aureus and K. pneumonia. Perhaps the reduction of the anti-biofilm effect of the extracts on the mentioned bacteria with the Eagle effect can be explained. In the Eagle phenomenon, the antimicrobial effect of the compound decreases with increasing concentration (Prasetyoputri et al. 2019).
Phenol the highest compound detected in both extracts was detected by GC-MS analysis. Other compounds with the highest percentage of Psammocinia sp. extract included propanoic acid and benzene acetaldehyde. Indraningrat et al. (2016) in their article, refer to the production of phenolic compounds by marine sponges and their microorganisms (Indraningrat et al. 2016). Shaala et al. (2020) also reported the antimicrobial effect of chlorinated propanoic acid derived a actinomycete strain from the Callyspongia siphonella sponge against E. coli and S. aureus bacteria (Shaala et al. 2020). Rajasabapathy et al. (2020) also reported an actinomycete strain isolated from Orina sagittaria sponge that had the ability to inhibit methicillin resistant S. aureus. The results of GC-MS showed 10 volatile organic compounds of ethyl acetate and hexane extracts of this strain (Rajasabapathy et al. 2020). In the present study, GC-MS analysis identified hexadecanoic acid, methyl ester in Psammocinia sp. extract and the results of agar well plate on E. coli and S. aureus bacteria with ZOI were 30 and 21 mm, respectively.
Marine animals are a good candidate for the extraction of new antimicrobial agents. The biological properties of marine extracts from Persian Gulf sponges have been less studied and identified. Overall, the results of the present study confirmed that both sponge extracts cause sufficient antibacterial effects against planktonic and biofilms typically forms. Both extracts had low destruction effects on B. cereus biofilm. Further research in this area can lead to the discovery of new antimicrobial drugs in the future.
References
Abad M, Bedoya L, Bermejo P (2011) Marine compounds and their antimicrobial activities. Science against microbial pathogens: communicating current research technological advances 51:1293–1306
Ancheeva E, El-Neketi M, Song W, Lin W, Daletos G, Ebrahim W, Proksch P (2017) Structurally unprecedented metabolites from marine sponges. Curr Org Chem 21(5):426–449
Balouiri M, Sadiki M, Ibnsouda SK (2016) Methods for in vitro evaluating antimicrobial activity: A review. J Pharm Anal 6(2):71–79. https://doi.org/10.1016/j.jpha.2015.11.005
Boury-Esnault N, Rutzler K (1997) Thesaurus of sponge morphology. Smithsonian contributions to zoology. Washington DC. pp 65
Carneiro RF, Viana JT, Torres RCF, da Silva LT, Andrade AL, de Vasconcelos MA,.. . Sampaio AH (2019) A new mucin-binding lectin from the marine sponge Aplysina fulva (AFL) exhibits antibiofilm effects. Arch Biochem Biophys 662:169–176. https://doi.org/10.1016/j.abb.2018.12.014
Cepas V, López Y, Muñoz E, Rolo D, Ardanuy C, Martí S,.. . Soto SM (2019) Relationship between biofilm formation and antimicrobial resistance in gram-negative Bacteria. Microb Drug Resist 25(1):72–79. https://doi.org/10.1089/mdr.2018.0027
CLSI (2017) Performance standards for antimicrobial susceptibility testing. 27th ed. CLSI supplement M100. Clinical and Laboratory Standards Institute, Wayne
De N, James N (2002) Antibacterial spectrum of extracts Of· Ocimum Gratissimum L.{BASIL) and Xylopia Aetiopica A. Rich (DUNAL). Niger J or Basic Appl Sci 11:165–175
e Silva S, Carvalho J, Aires C, Nitschke M (2017) Disruption of Staphylococcus aureus biofilms using rhamnolipid biosurfactants. J Dairy Sci 100(10):7864–7873. https://doi.org/10.3168/jds.2017-13012
Govinden-Soulange J, Marie D, Kauroo S, Beesoo R, Ramanjooloo A (2014) Antibacterial properties of marine sponges from Mauritius waters. Trop J Pharm Res 13(2):249–254. https://doi.org/10.4314/tjpr.v13i2.13
Hamayeli H, Hasanshahian M, Shoshtari N, Askari Hesni M (2017) Study the antimicrobial effect of three marine sponges (Dysidea sp.) collected at Persian Gulf on some pathogenic bacteria in planktonic and biofilm forms. Iran J Med Microbiol 11(4):45–56
Hamayeli H, Hassanshahian M, Mohammadi M (2018) The anti-biofilm effects of sponge (Callyspongia sp.) and two sea anemones (Zoanthus sansibaricus and Cerianthus lloydii) collected from the Persian Gulf. J Kerman Univ Med Sci 25(6):493–504
Hamayeli H, Hassanshahian M, Hesni MA (2019) The antibacterial and antibiofilm activity of sea anemone (Stichodactyla haddoni) against antibiotic-resistant bacteria and characterization of bioactive metabolites. Int Aquat Res 11(1):85–97. https://doi.org/10.1007/s40071-019-0221-1
Hassanshahian M, Saadatfar A, Masoumipour F (2019) Formulation and characterization of nanoemulsion from Alhagi maurorum essential oil and study of its antimicrobial, antibiofilm, and plasmid curing activity against antibiotic-resistant pathogenic bacteria. J Environ Health Sci Eng. https://doi.org/10.1007/s40201-020-00523-7.
Hentschel U, Hopke J, Horn M, Friedrich AB, Wagner M, Hacker J, Moore BS (2002) Molecular evidence for a uniform microbial community in sponges from different oceans. Appl Environ Microbiol 68(9):4431–4440. https://doi.org/10.1128/AEM.68.9.4431-4440.2002
Hooper JN (2000) Sponguide: guide to sponge collection and identification. Queensland Museum, Brisbane
Hudzicki J (2009) Kirby-Bauer disk diffusion susceptibility test protocol. American Society for Microbiology, Washington, D.C.
Indraningrat AAG, Smidt H, Sipkema D (2016) Bioprospecting sponge-associated microbes for antimicrobial compounds. Mar Drugs 14(5):87. https://doi.org/10.3390/md14050087
Kaplan JB (2011) Antibiotic-induced biofilm formation. Int J Artif Organs 34(9):737–751. https://doi.org/10.5301/ijao.5000027
Khoddami M, Sheikh Hosseini M, Hassanshahian M (2018) Antibacterial activity of (Essential Oil) against pathogenic bacteria and determination of chemical composition of essential oils by gas chromatography–mass spectrometry analysis in four regions of Kerman. J Diet Suppl 16(5):530–540
Khoobdel M, Tavassoli M, Salari M, Firozi F (2014) The stinging Apidae and Vespidae (Hymenoptera: Apocrita) in Iranian islands, Qeshm, Abu–Musa, Great Tunb and Lesser Tunb on the Persian Gulf. Asian Pac J Trop Biomed 4:S258–S262. https://doi.org/10.12980/APJTB.4.2014C1153
Li X-H, Lee J-H (2017) Antibiofilm agents: A new perspective for antimicrobial strategy. J Microbiol 55(10):753–766. https://doi.org/10.1007/s12275-017-7274-x
Lu L, Hu W, Tian ZR, Yuan DD, Yi GJ, Zhou YY, Li MX (2019) Developing natural products as potential anti-biofilm agents. Chin Med 14:17. https://doi.org/10.1186/s13020-019-0232-2
Mahon AR, Amsler CD, McClintock JB, Amsler MO, Baker BJ (2003) Tissue-specific palatability and chemical defenses against macropredators and pathogens in the common articulate brachiopod Liothyrella uva from the Antarctic Peninsula. J Exp Mar Biol Ecol 290(2):197–210. https://doi.org/10.1016/S0022-0981(03)00075-3
Masák J, Čejková A, Schreiberová O, Řezanka T (2014) Pseudomonas biofilms: possibilities of their control. FEMS Microbiol Ecol 89(1):1–14. https://doi.org/10.1111/1574-6941.12344
Masoumipour F, Hassanshahian M (2016) Antimicrobial activity of five medicinal plants on Candida albicans. Iran J Toxicol 10:65–77
Masoumipour F, Hassanshahian M, Jafarinasab T (2018) Antimicrobial activity of combined extracts of trachyspermum, thymus and pistachio against some pathogenic bacteria. J Kerm Med Uni 25:153–163
Mayer AMS, Glaser KB, Cuevas C, Jacobs RS, Kem W, Little RD,.. . Shuster DE (2010) The odyssey of marine pharmaceuticals: a current pipeline perspective. Trends Pharmacol Sci 31(6):255–265. https://doi.org/10.1016/j.tips.2010.02.005
Mehbub MF, Lei J, Franco C, Zhang W (2014) Marine sponge derived natural products between 2001 and 2010: trends and opportunities for discovery of bioactives. Mar Drugs 12(8):4539–4577. https://doi.org/10.3390/md12084539
Mohammadi M, Masoumipour F, Hassanshahian M, Jafarinasab T (2019) Study the antibacterial and antibiofilm activity of Carum copticum against antibiotic-resistant bacteria in planktonic and biofilm forms. Microb Pathog 129:99–105
Mohsenipour Z, Hassanshahian M (2016) Antibacterial activity of Euphorbia hebecarpa alcoholic extracts against six human pathogenic bacteria in Planktonic and Biofilm forms. Jundishapur J Microbiol 9(6):e34701
Paul VJ, Cruz-Rivera E, Thacker R (2001) Chemical mediation of macroalgal-herbivore interactions: ecological and evolutionary perspectives. Marine chemical ecology. CRC Press, Boca Raton, pp 227–265
Pech-Puch D, Pérez-Povedano M, Gómez P, Martínez-Guitián M, Lasarte-Monterrubio C, Vázquez-Ucha JC, Bou G (2020) Marine organisms from the Yucatan Peninsula (Mexico) as a potential natural source of antibacterial compounds. Mar Drugs 18(7):369. https://doi.org/10.3390/md18070369
Prasetyoputri A, Jarrad AM, Cooper MA, Blaskovich MA (2019) The eagle effect and antibiotic-induced persistence: two sides of the same coin? Trends Microbiol 27(4):339–354. https://doi.org/10.1016/j.tim.2018.10.007
Proksch P (1994) Defensive roles for secondary metabolites from marine sponges and sponge-feeding nudibranchs. Toxicon 32(6):639–655. https://doi.org/10.1016/0041-0101(94)90334-4
Rajasabapathy R, Ghadi SC, Manikandan B, Mohandass C, Surendran A, Dastager SG,.. . James RA (2020) Antimicrobial profiling of coral reef and sponge associated bacteria from southeast coast of India. Microb Pathog 141:8. https://doi.org/10.1016/j.micpath.2020.103972
Rane R, Sahu N, Shah C, Karpoormath R (2014) Marine bromopyrrole alkaloids: Synthesis and diverse medicinal applications. Curr Topics Med Chem 14(2):253–273
Reller LB, Weinstein M, Jorgensen JH, Ferraro MJ (2009) Antimicrobial susceptibility testing: a review of general principles and contemporary practices. Clin Infect Dis 49(11):1749–1755. https://doi.org/10.1086/647952
Sadeghian I, Hassanshahian M, Sadeghian S, Jamali S (2012) Antimicrobial effects of Quercus Brantii fruits on bacterial pathogens. Jundi J Microbiol 5(3):465–469
Seradj H, Moein M, Eskandari M, Maaref F (2012) Antioxidant activity of six marine sponges collected from the Persian Gulf. Iran J Pharm Sci 8(4):249–255
Shaala LA, Youssef DT, Alzughaibi TA, Elhady SS (2020) Antimicrobial chlorinated 3-phenylpropanoic acid derivatives from the red sea marine actinomycete streptomyces coelicolor LY001. Mar Drugs 18(9):450. https://doi.org/10.3390/md18090450
Stepanović S, Vuković D, Dakić I, Savić B, Švabić-Vlahović M (2000) A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods 40(2):175–179. https://doi.org/10.1016/S0167-7012(00)00122-6
Tan BK, Vanitha J (2004) Immunomodulatory and antimicrobial effects of some traditional Chinese medicinal herbs: a review. Curr Med Chem 11(11):1423–1430. https://doi.org/10.2174/0929867043365161
Tilvi S, Rodrigues C, Naik C, Parameswaran P, Wahidhulla S (2004) New bromotyrosine alkaloids from the marine sponge Psammaplysilla purpurea. Tetrahedron 60(45):10207–10215. https://doi.org/10.1016/j.tet.2004.09.009
WHO (2017) WHO Priority Pathogens List for R&D of New Antibiotics. Available online: http://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/. Accessed Aug 2017
Zhang HW, Dong ML, Chen JW, Wang H, Tenney K, Crews P (2017) Bioactive secondary metabolites from the marine sponge genus agelas. Mar Drugs 15(11):29. https://doi.org/10.3390/md15110351
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hamayeli, H., Hassanshahian, M. & Askari Hesni, M. Identification of Bioactive Compounds and Evaluation of the Antimicrobial and Anti-biofilm Effect of Psammocinia sp. and Hyattella sp. Sponges from the Persian Gulf. Thalassas 37, 357–366 (2021). https://doi.org/10.1007/s41208-020-00268-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41208-020-00268-y