Abstract
Spatial and temporal variations in physico-chemical properties of coastal waters play a major role in determining the density, diversity and occurrence of phytoplankton. The present study is conducted to assess spatio-temporal pattern of phytoplankton assemblage in the southern parts of the Caspian Sea (CS) which in turn can serve as an ecological health indicator in this region. Among 64 identified phytoplankton species, diatoms (37 species and 66.2%) and Dinophyceae (11 species and 18.2%) were dominant. Non-metric multidimensional scaling (NMDS) were employed to reveal Spatial and temporal distributions of phytoplankton assemblages. Four groups were established using hierarchical clustering based on species richness similarities which were absolutely represented the four seasons. The summer and spring samples were highly separated from the winter and autumn samples. The Linear Discriminant Analysis (LDA) model showed that temporal patterns of phytoplankton assemblages were mostly explained by chemical factors (silicate, phosphate, and nitrite) and temperature. In conclusion, results of this study suggested that spatio-temporal patterns of phytoplankton in the southern part of the CS are closely associated with seasonal variations in river flow and temperature. The temporal patterns are apparently dominant in this area and water nutrients are mainly responsible for seasonal changes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phytoplankton plays an important role in the primary production of the aquatic food chain and in global carbon dioxide fixation (Pal and Choudhury 2014). They are also considered to be bio-indicators in response to anthropogenic activities and climate change (Jaanus et al. 2009; Nehring 1998). Aquatic ecosystems are facing various nutrient-enrichment processes that affect nutrient limitation levels and diversity in phytoplankton assemblages (Hecky and Kilham 1988; Cloern 1999; Rudek et al. 1991). The close relationship between phytoplankton and the trophic state levels of marine surface water has been reported by many researchers (McCarthy and Goldman 1979; Pedersen and Borum 1996; Agawin et al. 2000; Burger et al. 2008; Zhu et al. 2010). However, seasonal cycles in weather conditions can greatly affect water nutrient levels in aquatic ecosystems (Berner and Berner 2012) and subsequently lead to variation in phytoplankton assemblages.
Spatio-temporal patterns of phytoplankton assemblages and their correlations with environments in coastal ecosystems have frequently been reported (May et al. 2003; Lopes et al. 2005; Barnes et al. 2006; Badylak and Phlips 2004). The nutrient enrichment of each coastal ecosystem is strongly influenced by the inherent physical properties of the area (Cloern 2001). Higher inputs of nitrogen and phosphorus into coastal waters due to anthropogenic activity have resulted in increases in the nitrogen/silicate and phosphorus/silicate ratios in these areas (Billen et al. 2001). This phenomenon has resulted in dominance of spatial pattern of phytoplankton in several coastal areas (Falkowski and Wilson 1992; Muylaert et al. 2006; Melo et al. 2007; Wang et al. 2016).
The climate around the southern part of the CS consists of four distinct seasons (Kosarev 2005). Approximately 130 rivers of various sizes drain into the sea, which has an annual freshwater inflow of roughly 300 km3 (Nasrollahzadeh et al. 2008). Hydrological regimes and circulation in the CS are affected by external factors, such as discharges and climate, due to its isolation from open seas (Tuzhilkin and Kosarev 2005). Therefore, various environmental conditions could cause apparent temporal and lower spatial variations in the composition of phytoplankton species due to changes in ambient nutrient ratios throughout the seasons in this region (Nasrollahzadeh et al. 2008). In recent years, several studies about phytoplankton assemblages in Iranian coastal ecosystems of the southwestern CS have been conducted. Roohi et al. (2010) found temporal changes in the phytoplankton community, with a higher number of species in summer. Ganjian et al. (2010) studied the seasonal and regional distribution of phytoplankton in the southern CS and reported both the spatial and temporal variation of phytoplankton as well. Although they did not specify domination of seasonal and regional in their study, they did state that the biomasses of Bacillariophyta, Cyanophyta, Chlorophyta, and Euglenophyta did not show significant differences between seasons. Ganjian Khenari et al. (2012) revealed that spatial variation in phytoplankton assemblages in the southern part of the CS is more apparent in spring and summer, whilst temporal variation is obvious in autumn and winter.
Previous phytoplankton studies of the southern part of the CS have limited spatial and temporal coverage. In addition, it is not well documented which factors contribute to spatial and/or temporal variation in this region. Therefore, in this study, we conducted tests to address the following research questions: 1) Do spatial or temporal patterns dominate? 2) Do physical or chemical factors have the potential to predict the patterns in this area? 3) Do anthropogenic nutrient sources affect phytoplankton dynamics and interrupt their cyclic variation in the southern part of the CS?
Materials and Methods
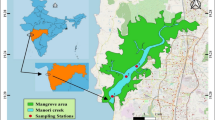
Eight sampling sites were established to take samples from the southwest to the southeast shores of the CS in Iranian water basins. The sites were parallel to the coasts of Astra (S1), Anzali (S2), Chamkhaleh (S3), Ramsar (S4), Sisangan (S5), Babolsar (S6), Amir Abad (67), and Khajeh Nafas (S8), and samples were taken from a depth of 1 m (three replicate in each site and 10 m apart for each replicate; Fig. 1). Phytoplankton samples were collected seasonally (midpoint of each season) from spring to winter of 2014.
We collected seawater in 3 L Nansen bottles, transferred the water into polyethylene bottles, and fixed the samples immediately with a formaldehyde solution (5%). Initial samples were concentrated by 24 h sedimentation to 80 ml, and all algae were counted using a 1 ml Sedgewick Rafter counting frame as described by Rashash and Gallagher (1995). Sampled were identified using a Nikon Eclipse Ti-S inverted microscope with 20, 40 and 100× magnification and illustrate keys (Sournia 1978; Carmelo 1997; Bilgrami and Saha 2002; Newell and Newell 1977). Species richness, species rank occurrence rate, and species indicator values were calculated for each site.
Temperature, salinity, dissolved oxygen, and pH were measured using portable multi-meters (HACH 51154, USA). Three replicate samples were collected at each sampling site for environmental data from the surface water. An additional water sample of 250 ml was filtered in situ and transferred to the laboratory to analyze total amounts of phosphate, silicate, nitrate, and nitrite.
Non-metric multidimensional scaling (NMDS) was used to classify the pattern of phytoplankton assemblages at the sites (Kruskal and Wish 1978). Hierarchical clustering was performed prior to NMDS analysis, and phytoplankton samples were classified into different groups. Bray–Curtis similarity was used as the distance measure, and the data were log transformed for treatment (Legendre and Legendre 2012). Linear Discriminant Analysis (LDA) was also employed for pattern recognition between groups.
The indicator value (IndVal) method proposed by Dufrêne and Legendre (1997) was employed to identify the indicator species among groups. The formula is as follows: IndValGroup k, Species j = 100 × Ak,j x Bk,j, where Ak,j = specificity and Bk,j = fidelity. In addition, correction for multiple testing was applied to obtain a corrected vector of p-values (Legendre and Legendre 1998). An analysis of variance (ANOVA) test was employed to assess the significance (p < 0.05) of variables with 1000 permutations.
All analyses were conducted in R version 3.3.2 (R Development Core Team 2016) using vegan (Oksanen et al. 2016), MASS (Venables and Ripley 2002), ggmap (Kahle and Wickham 2013), BiodiversityR (Kindt and Coe 2005), and labdsv (Roberts 2016) packages.
Results
Means (± SD) of environmental factors for all sites and seasons are given in Table 1. Maximum and minimum water temperatures were observed at S8 and S1, respectively. The highest value of dissolved oxygen was registered at site S4 in autumn, while the lowest value was registered at site S8 in summer. Water pH was equal at all sites during the different seasons. Maximum salinity was observed in summer at site S8. An analysis of inorganic nutrients showed clear temporal variation. Of all the sampling sites, S8 was distinct. High temperatures and low nitrates were obvious at this site. Minimum amounts of all nutrients were found in summer; maximum amounts of silicate, nitrite, and nitrate were found in autumn; and maximum amounts of phosphate were found in spring.
A total of 64 species belonging to six classes were identified in this study: Bacillariophyceae, Chlorophyceae, Dinophyceae, Cyanophyceae, Euglenophyceae, and Prymnesiophyceae. Bacillariophyceae was the predominant group with 37 species making up 66.2% of the total number. This was followed by Dinophyceae (11 species and 18.5%), Cyanophyceae (9 species and 9.4%), Chlorophyceae (4 species and 3.2%), Euglenophyceae (1 species and 1%) and Prymnesiophyceae (2 species and 1.9%). Furthermore, average cell densities ±standard deviation was as follows: Bacillariophyceae 1998 ± 2859, Dinophyceae 1480 ± 1642, Cyanophyceae 1170 ± 1069, Chlorophyceae 900 ± 1267, Euglenophyceae 68 ± 348 and Prymnesiophyceae 17 ± 55. The annual relative abundance of all taxa is shown in Table 2. This table shows that Euglena sp., Pseudo-nitzschia sp., and Thalassionema nitzschioides are the most abundant species in the community, making up 11.7%, 9.2%, and 9% of the total community, respectively.
The results of the NMDS showed that samples taken in the four seasons are distinct from each other and that the four groups (G1 = Autumn, G2 = Spring, G3 = Summer, G4 = Winter) created absolutely based on a Bray–Curtis dissimilarity matrix and cluster analysis represented the four seasons (Fig. 2).
The species richness and density of the phytoplankton in each group differ significantly (Fig. 3; Kruskal–Wallis test, p < 0.001). G1 and G4 show similar values for both species richness and density, which are apparently higher than the values in G2 and G3 (Fig. 3a1, b1). In terms of species richness, the proportion of different taxonomical group is similar in each group, with a predominance of diatoms (Fig. 3a2). However, the density proportion of G3 is different from that of the other groups, as the proportion of diatoms is apparently lower (Fig. 3b2).
The summer and spring samples were highly separated from the winter and autumn samples. Higher temperatures and salinity and lower nutrient values were observed in summer. The phosphate in spring samples was higher than in samples taken in the other seasons. The winter and autumn samples had similar nutrient values and physical parameters, except for temperature (Table 1).
Discriminant function analysis and principal component analysis were used to predict correlations between the four groups and environmental factors. Three discriminant functions were generated, and a Kappa test showed they were highly significant (p < 0.001). A two-dimensional figure based on F1 × F2 was generated, with a corresponding distribution of water quality parameters.
Two axes (F1 and F2) accounted for 68% and 28% of the between-group variability, respectively (Fig. 4). Environmental factors could predict the groups with 68.8% accuracy, and the prediction success rate was 62.5%, 50.0%, 87.5%, and 75.0% for groups 1–4, respectively.
G3 is opposite to G2 along the horizontal axis in the opposite direction (Fig. 4a), and the groups can be distinguished by temperature (Figs. 4a, b). G4 is ordered at the positive direction of the vertical axis (Fig. 4a) and is more linked to chemical factors (silicate, phosphate, and nitrite) (Fig. 4b). G1 is ordered around the center, and its linkage with environmental variables is unclear.
Table 3 shows the results of the IndVal analysis and the species tolerance rates. According to the results, eight indicator species were identified that belonged to G3 (seven species) and G2 (one species). The indicator species of G3 belonged to Dinophyceae, Bacillariophyceae, and Euglenophyceae. G2 had one species from the Bacillariophyceae class.
Discussion
Phytoplankton Community Structure
Our results indicated that diatoms dominated in terms of both species richness and density, similar to previous studies of the CS (Ganjian et al. 2010; Nasrollahzadeh et al. 2008; Roohi et al. 2010). Several diatom genuses, including Thalassionema, Pseudo-nitzschia, Rhizosolenia, Cerataulina, and Nitzschia; exhibited high relative abundance in this study, which were also reported as high abundance genus by Tas (2017) in the Sea of Marmara, Turkey. Euglena sp. was the most abundant species in this study. However, it ranked fourth in terms of species density in previous studies. Euglena belongs to the limnetic species, and the high richness of this genus reflects the fact that many river tributaries flow into the southern part of the CS. The existence of limnetic species in coastal areas coincides with significant riverine drainage (Pandiyarajan et al. 2014; Varona-Cordero et al. 2010). Stonik and Selina (2001) linked the high density of euglenoids in Zolotoi Rog Bay with discharges from the Vtoraya Rechka River. Therefore, the predominance of Euglena in the southern part of the CS mainly depends on river flows into this area. The predominance of Thalassionema nitzschioides and Pseudo-nitzschia sp. was another reason for significant freshwater inflow by rivers in this study. The predominance of these species has also been reported as typical of coastal waters (Caroppo et al. 2006; Li et al. 2013; Bresnan et al. 2015; Aktan 2011; Fehling et al. 2012). Bagheri et al. (2012a, b) found that freshwater inflow releases high nutrient concentrations—particularly high levels of silicate—in the southwestern CS that results in the dominance of diatoms. In fact, diatoms are more successive in coastal waters and mixing areas due to their heavier shape and because they are better competitors for dissolved inorganic nitrogen due to their larger specific storage volume (Trevisan et al. 2010; Dauchez et al. 1996; Kormas et al. 2002; Yurkovskis 2004). However, Kosarev and Yablonskaya (1994) reported that the most abundant and widespread group throughout the CS are diatoms. Wehr and Descy (1998) found that the most successful algal groups in large rivers are Bacillariophyceae. Indeed, diatom and dinoflagellates taxa have a competitive edge and grow rapidly in favorable conditions, especially in terms of temperature (Furnas 1990; Lomas and Glibert 1999). Diatoms are dominant in the colder waters of the eastern CS, contrary to dinoflagellates are prevalent in the warmer waters (Kideys et al. 2005). In the present study, high diatom densities were observed, except during the summer period (Fig. 3). Furthermore, the LDA analysis results revealed that environmental factors could predict phytoplankton assemblages with 68.8% accuracy. Bagheri et al. (2012a, 2012b) found that diatom abundance had a strong positive correlation with dissolved silicate and nitrogen. Lower values of water nutrients (nitrite, nitrate, silicate, and phosphate) and higher temperatures were found in summer (Table 1), while maximum and minimum of these two factors were found in autumn and winter as rainy seasons in the CS region, respectively. Therefore, it could be suggested that the variations in the water nutrient and temperature values could be the main reasons for changes in the density and species richness of diatoms throughout the seasons. Prorocentrum praximum, which exhibited high abundance, is native to the CS. However, Prorocentrum is a well-known genus that is widespread, results in harmful algae, and is an indicator of eutrophication in coastal waters worldwide (Laza-Martinez et al. 2011; Hernández-Becerril et al. 2000; Edwards et al. 2006). The high relative abundance of other species may be due to their potential in terms of distribution and successful reproduction or their ecological properties. For instance, Oscillatoria sp. has a high S/V ratio and high resistance to water mixing species, which allows it to predominate in favorable conditions. Bagheri et al. (2012a, 2012b) reported that Oscillatoria sp. was dominant in the CS during summer due to the increase in surface temperature. In addition, lower values of silicate in summer can help Oscillatoria sp. better compete with diatoms.
Predicting Phytoplankton Assemblage Patterns
Based on the NMDS and clustering analysis, four groups of seasonal data were identified in this study. However, spatial variations were observed, and dissimilarities between the sites were weak, particularly in autumn and winter (Fig. 2). Although other researchers have reported a wide range of spatial variations in the southern CS (Bagheri et al. 2014; 2012a, b; Ganjian Khenari et al. 2012), the results of this study indicate that temporal variations are more distinguishable. It is believed that local drainage may be a main impacting factor in the spatial differences of water quality (Lu et al. 2009). Therefore, variation in the fresh water inflow that has high nutrient content in different parts of the CS could be the major reason for spatial changes in phytoplankton patterns across space. Winter and autumn are known to be flood seasons in the CS basin, while summer and spring (mainly summer) are known to be drought seasons. According to Fig. 3, the phytoplankton patterns of G4, G1, and G2 are similar, and they are different from those of G3. The many Iranian rivers flowing into the southern CS have created a lengthy estuary zone (Mehdipour and Gearmi 2016). The amount of discharge is different at various sites. According to Zakeri (1997), 864 small and large rivers with a catchment of 193,161 km2 drain into the CS yearly. However, the water flow in the brackish ecosystems, such as the southern CS drainage basin, is highly seasonal and dependent on annual rainfall (Lara-Lara et al. 1980). However, recent drought has also caused a water deficit in the CS basin, especially for the Sefidrud River, which has the maximum drainage (Koushali et al. 2015) and consequently scant water flow in summer. Therefore, it could be suggested that the great amount of water in flood seasons distinguished G3 from G1, G4, and G2. LDA analysis revealed that G3 had a correlation with temperature. There was a decreasing trend in the surface water temperature from summer to winter (Table 1). According to LDA analysis, G2 and G3 were distinguished by temperature. It is well known that water temperature directly affects the growth of algae (Eppley 1972) and most indicator species in G3 are temperature preference. For instance, G3 had high indicator values of Euglena sp., which has a preference for high temperatures and low nutrients. Only one indicator species occurred in G2, Pseudosolenia calcar-avis, which is a successful species in mid-temperatures compared to other algae species in this study.
The lower temperatures in the winter caused diatoms to be dominant throughout the seasons (Figs. 3 a2, b2). G4 showed a correlation with water nutrients in this study. This might be due to the high amounts of discharged nutrients in the coastal waters of the CS in winter. According to Bagheri et al. (2012a, b), high precipitation in winter increases the nutrients in the coastal waters of the CS. Therefore, this might have contributed to increases in the number of phytoplankton and to changes in phytoplankton composition in winter (Fig. 3). In addition, about 90% of the silicate in the global marine system is estimated to come from rivers (Sommer 1994; Eker and Kideys 2003; Humborg et al. 2004; Moncheva and Carstensen 2005; Nasrollahzadeh et al. 2008). It is therefore predictable that the amount of silicate would increase in CS coastal waters due to river flooding in winter and autumn. Moreover, species richness and density were lower in G3 than in G4, which could be related to the huge difference in river discharges between summer and winter in the region.
G1 had greater density than G2 and G3, but this seems uncorrelated with the present environmental variables. Kiørboe (1993) found that turbulence in surface water limits nutrient uptake and light intensity for phytoplankton assemblages. Although the availability of water nutrients is greater in winter than in autumn, surface water is turbid and turbulent in the coastal waters of the CS and can limit phytoplankton growth in this season.
In conclusion, the spatial-temporal patterns of phytoplankton in the southern part of the CS are closely associated with seasonal variations in river flow and temperature. However, anthropogenic warming has increased the risk of drought in the CS basin, and decreased river drainage has made these temporal patterns dominant in this area.
References
Agawin NS, Duarte CM, Agusti S (2000) Nutrient and temperature control of the contribution of picoplankton to phytoplankton biomass and production. Limnol Oceanogr 45(3):591–600
Aktan Y (2011) Large-scale patterns in summer surface water phytoplankton (except picophytoplankton) in the Eastern Mediterranean. Estuar Coast Shelf Sci 91(4):551–558
Badylak S, Phlips EJ (2004) Spatial and temporal patterns of phytoplankton composition in subtropical coastal lagoon, the Indian River lagoon, Florida, USA. J Plankton Res 26(10):1229–1247
Bagheri S, Mansor M, Turkoglu M, Makaremi M, Wan Omar WO, Negarestan H (2012a) Phytoplankton species composition and abundance in the southwestern CS. Ekoloji 21(83):32–43
Bagheri S, Mansor M, Turkoglu M, Makaremi M, Babaei H (2012b) Temporal distribution of phytoplankton in the south-western CS during 2009–2010: a comparison with previous surveys. J Mar Biol Assoc U K 92(06):1243–1255
Bagheri S, Turkoglu M, Abedini A (2014) Phytoplankton and nutrient variations in the Iranian waters of the CS (Guilan region) during 2003-2004. Turk J Fish Aqua Sci 14(1):231–245
Barnes DK, Fuentes V, Clarke A, Schloss IR, Wallace MI (2006) Spatial and temporal variation in shallow seawater temperatures around Antarctica. Deep-Sea Res PT II: Top Stud Oceanogr 53(8):853–865
Berner EK, Berner RA (2012) Global environment: water, air, and geochemical cycles. Princeton, Princeton University Press
Bilgrami KS, Saha LC (2002) A textbook of algae. CBS publication, new Dehli. Biology of the Indian Ocean. Springer-Verlag, berlin
Billen G, Garnier J, Ficht A, Cun C (2001) Modeling the response of water quality in the seine river estuary to human activity in its watershed over the last 50years. Estuaries 24:977–993
Bresnan E, Kraberg A, Fraser S, Brown L, Hughes S, Wiltshire KH (2015) Diversity and seasonality of Pseudo-nitzschia (Peragallo) at two North Sea time-series monitoring sites. Helgoland Mar Res 69(2):193–204
Burger DF, Hamilton DP, Pilditch CA (2008) Modelling the relative importance of internal and external nutrient loads on water column nutrient concentrations and phytoplankton biomass in a shallow polymictic lake. Ecol Model 211(3):411–423
Carmelo RT (1997) Identifying marine phytoplankton. Academic Press, San Diego. Paperback
Caroppo C, Turicchia S, Margheri MC (2006) Phytoplankton assemblages in coastal waters of the northern Ionian Sea (eastern Mediterranean), with special reference to cyanobacteria. J Mar Biol Assoc U K 86(05):927–937
Cloern JE (1999) The relative importance of light and nutrient limitation of phytoplankton growth: a simple index of coastal ecosystem sensitivity to nutrient enrichment. Aquat Ecol 33(1):3–15
Cloern JE (2001) Our evolving conceptual model of the coastal eutrophication problem. Mar Ecol Prog Ser 210:223–253
Dauchez S, Legendre L, Fortier L, Levasseur M (1996) Nitrate uptake by size-fractionated phytoplankton on the Scotian shelf (Northwest Atlantic): spatial and temporal variability. J Plankton Res 18:577–595
Dufrêne M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366
Edwards M, Johns DG, Leterme SC, Svendsen E, Richardson AJ (2006) Regional climate change and harmful algal blooms in the northeast Atlantic. Limnol Oceanogr 51(2):820–829
Eker E, Kideys AE (2003) Distribution of phytoplankton in the southern Black Sea in summer 1996, spring and autumn 1998. J Mar Sys 39:203–211
Eppley RW (1972) Temperature and phytoplankton growth in the sea. Fish Bull 70(4):1063–1085
Falkowski PG, Wilson C (1992) Phytoplankton productivity in the North Pacific ocean since 1900 and implications for absorption of anthropogenic CO 2. Nature 358(6389):741–743
Fehling J, Davidson K, Bolch CJ, Brand TD, Narayanaswamy BE (2012) The relationship between phytoplankton distribution and water column characteristics in North West European shelf sea waters. PLoS One 7(3):e34098
Furnas MJ (1990) In situ growth rates of marine phytoplankton: approaches to measurement, community and species growth rates. J Plankton Res 12(6):1117–1151
Ganjian Khenari AG, Ghasemnejad M, Roohi A, Omar RPWMW, Mansor M, Mirbagheri B, Ghaedi A (2012) Temporal and spatial variations of phytoplankton in the CS. Afr J Microbiol Res 6(20):4239–4246
Ganjian A, Wan Maznah WO, Yahya K, Fazli H, Vahedi M, Roohi A, Farabi SMV (2010) Seasonal and regional distribution of phytoplankton in the southern CS. Iran J Fish Sci 9(3):382–401
Hecky RE, Kilham P (1988) Nutrient limitation of phytoplankton in freshwater and marine environments: a review of recent evidence on the effects of enrichment. Limnol Oceanogr 33(4):796–822
Hernández-Becerril DU, Altamirano RC, Alonso RR (2000) The dinoflagellate genus Prorocentrum along the coasts of the Mexican Pacific. Hydrobiologia 418(1):111–121
Humborg C, Smedberg E, Blomqvist S (2004) Nutrient variations in boreal and subarctic Swedish rivers: landscape control of land–sea fluxes. Limnol Oceanogr 49:1871–1883
Jaanus A, Toming K, Hällfors S, Kaljurand K, Lips I (2009) Potential phytoplankton indicator species for monitoring Baltic coastal waters in the summer period. Hydrobiologia 629(1):157–168
Kahle D, Wickham H (2013) Ggmap: spatial visualization with ggplot2. The R Journal 5(1):144–161 http://journal.r-project.org/archive/2013-1/kahle-wickham.pdf
Kideys AE, Soydemir N, Eker E, Vladymyrov V, Soloviev D, Melin F (2005) Phytoplankton distribution in the CS during march 2001. Hydrobiologia 543:159–168
Kindt R, Coe R (2005) Tree diversity analysis. A manual and software for common statistical methods for ecological and biodiversity studies. World Agroforestry Centre (ICRAF), Nairobi. ISBN 92-9059-179-X
Kiørboe T (1993) Turbulence, phytoplankton cell size, and the structure of pelagic food webs. Adv Mar Biol 29:1–72
Kormas KA, Garametsi V, Nicolaidou A (2002) Size-fractionated phytoplankton chlorophyll in an eastern Mediterranean coastal system (Maliakos Gulf, Greece). Helgoland Mar Res 56:125–133
Kosarev AN (2005) Physico-geographical conditions of the CS. In: Kostianoy AG, Kosarev AN (eds) The CS environment. Springer, Germany, pp 5–31
Kosarev AN, Yablonskaya AE (1994) The Caspian Sea. SPB Academic Publishing, The Hague.
Koushali HP, Moshtagh R, Mastoori R (2015) Water resources modelling using system dynamic in Vensim. J Water Resource Hydraul Eng 4(3):251–256
Kruskal JB, Wish M (1978) Multidimensional Scaling. Sage Publications, Beverly Hills
Lara-Lara JR, Alvarez Borrego S, Small LF (1980) Variability and tidal exchange of ecological properties in a coastal lagoon. Estuar Coast Shelf Sci 11:613–637
Laza-Martinez A, Orive E, Miguel I (2011) Morphological and genetic characterization of benthic dinoflagellates of the genera Coolia, Ostreopsis and Prorocentrum from the south-eastern Bay of Biscay. Eur J Phycol 46(1):45–65
Legendre P, Legendre LFJ (1998) Numerical Ecology. Second English edition, Elsevier Science, Amsterdam
Legendre P, Legendre LFJ (2012) Numerical Ecology. Third English edition, Elsevier Science, Amsterdam
Li Y, Wang DR, Su J, Zhang J (2013) Impact of monsoon-driven circulation on phytoplankton assemblages near fringing reefs along the east coast of Hainan Island, China. Deep Sea Res PT II: Top Stud Oceanogr 96:75–87
Lomas MW, Glibert PM (1999) Interactions between NH+ 4 and NO− 3 uptake and assimilation: comparison of diatoms and dinoflagellates at several growth temperatures. Mar Biol 133(3):541–551
Lopes MRM, Bicudo CEDM, Ferragut MC (2005) Short term spatial and temporal variation of phytoplankton in a shallow tropical oligotrophic reservoir, southeast Brazil. Hydrobiologia 542(1):235–247
Lu FH, Ni HG, Liu F, Zeng EY (2009) Occurrence of nutrients in riverine runoff of the Pearl River Delta, South China. J Hydrol 376(1– 2):107–115
May CL, Koseff JR, Lucas LV, Cloern JE, Schoellhamer DH (2003) Effects of spatial and temporal variability of turbidity on phytoplankton blooms. Mar Ecol Prog Ser 254:111–128
McCarthy JJ, Goldman JC (1979) Nitrogenous nutrition of marine phytoplankton in nutrient-depleted waters. Science 203(4381):670–672
Mehdipour N, Gearmi MH (2016) Benthic communities on hard substrates and intra-community relation with environmental factors in mesohaline estuarine. J Fishe Sci Com 10(3):23
Melo S, Bozelli RL, Esteves FA (2007) Temporal and spatial fluctuations of phytoplankton in a tropical coastal lagoon, southeast Brazil. Braz J Biol 67(3):475–483
Moncheva OA, Carstensen SJ (2005) Long-term variability of vertical chlorophyll a and nitrate profiles in the open Black Sea: eutrophication and climate change. Mar Ecol Prog Ser 294:95–107
Muylaert K, Gonzales R, Franck M, Lionard M, Van der Zee C, Cattrijsse A, Sabbe K, Chou L, Vyverman W (2006) Spatial variation in phytoplankton dynamics in the Belgian coastal zone of the North Sea studied by microscopy, HPLC-CHEMTAX and underway fluorescence recordings. J Sea Res 55(4):253–265
Nasrollahzadeh HS, Din ZB, Foong SY, Makhlough A (2008) Trophic status of the Iranian CS based on water quality parameters and phytoplankton diversity. Cont Shelf Res 28(9):1153–1165
Nehring S (1998) Establishment of thermophilic phytoplankton species in the North Sea: biological indicators of climatic changes? ICES J Mar Sci: J Du Conseil 55(4):818–823
Newell GE, Newell RC (1977) Marine plankton: a practical guide, 5th edn. Hutchinson Educational, London
Oksanen FJ, Blanchet G, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Stevens MHM, Szoecs E, Wagner H (2016) Vegan: community ecology package. R Package Version 2:4–1 https://CRAN.R-project.org/package=vegan
Pal R, Choudhury AK (2014) An introduction to Phytoplanktons: diversity and ecology. Springer, India
Pandiyarajan RS, Shenai-Tirodkar PS, Ayajuddin M, Ansari ZA (2014) Distribution, abundance and diversity of phytoplankton in the inshore waters of Nizampatnam, south east coast of India. Ind J Geo-Mar Sci 43:348–356
Pedersen MF, Borum J (1996) Nutrient control of algal growth in estuarine waters. Nutrient limitation and the importance of nitrogen requirements and nitrogen storage among phytoplankton and species of macroalgae. Mar Ecol Prog Ser 142:261–272
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna https://www.R-project.org/
Rashash DMC, Gallagher DL (1995) An evolution of algal enumeration. Am Water Works Assn J 87:127–132
Roberts DW (2016) Labdsv: ordination and multivariate analysis for ecology. R Package Version 1.8–0. https://CRAN.R-project.org/package=labdsv
Roohi A, Kideys AE, Sajjadi A, Hashemian A, Pourgholam R, Fazli H, Khanari AG, Eker-Develi E (2010) Changes in biodiversity of phytoplankton, zooplankton, fishes and macrobenthos in the southern CS after the invasion of the ctenophore Mnemiopsis Leidyi. Biol Invasions 12(7):2343–2361
Rudek J, Paerl HW, Mallin MA, Bates PW (1991) Seasonal and hydrological control of phytoplankton nutrient limitation in the lower Neuse River estuary, North Carolina. Mar Ecol Prog Ser 75(2):133–142
Sommer U (1994) Are marine diatoms favoured by high Si/N ratios? Mar Ecol Prog Ser 115:309–315
Sournia A (1978) Phytoplankton manual, monographs on oceanographic methodology. UNESCO, Paris
Stonik IV, Selina MS (2001) Species composition and seasonal dynamics of density and biomass of euglenoids in Peter the Great Bay, sea of Japan. Russ J Mar Biol 27(3):174–176
Tas S (2017) Planktonic diatom composition and environmental conditions in the golden horn estuary (sea of Marmara, Turkey). Fundam Appl Limnol 189(2):153–166
Trevisan R, Poggi C, Squartini A (2010) Factors affecting diatom dynamics in the alpine lakes of Colbricon (northern Italy): a 10-year survey. J Limnol 69:199–208
Tuzhilkin VS, Kosarev AN (2005) Thermohaline structure and general circulation of the CS waters. In: Kostianoy AG, Kosarev AN (eds) The CS environment. Springer, Germany, pp 33–57
Varona-Cordero F, Gutiérrez-Mendieta FJ, del Castillo MEM (2010) Phytoplankton assemblages in two compartmentalized coastal tropical lagoons (Carretas-Pereyra and Chantuto-Panzacola, Mexico). J Plankton Res 32(9):1283–1299
Venables WN, Ripley BD (2002) Modern applied statistics with S, Fourth Edition. Springer, New York. ISBN 0-387-95457-0
Wang C, Li X, Wang X, Wu N, Yang W, Lai Z and Lek S (2016) Spatio-temporal patterns and predictions of phytoplankton assemblages in a subtropical river delta system. Fundamental and Applied Limnology/Archiv für Hydrobiologie 187(4):335–349
Wehr JD, Descy JP (1998) Use of phytoplankton in large river management. J Phycol 34(5):741–749
Yurkovskis A (2004) Long-term land-based and internal forcing of the nutrient state of the Gulf of Riga (Baltic Sea). J Mar Sys 50:181–197
Zakeri H (1997) Water catchment area of the Caspian Sea. Student Quarterly of the Water Engineering Faculty of Khajeh Nassirud-Din Tousi, Abangan, p 12. http://www.netiran.com/Htdocs/Clippings/Social/970700XXSO0 2.html
Zhu W, Wan L, Zhao L (2010) Effect of nutrient level on phytoplankton community structure in different water bodies. J Environ Sci 22(1):32–39
Acknowledgements
This research was conducted under the Iranian National Institute for Oceanography and Atmospheric Science foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None to declare.
Rights and permissions
About this article
Cite this article
Mehdipour, N., Wang, C. & Gerami, M.H. Spatio-Temporal Pattern of Phytoplankton Assemblages in the Southern Part of the Caspian Sea. Thalassas 33, 99–108 (2017). https://doi.org/10.1007/s41208-017-0027-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41208-017-0027-0